Abstract
Arsenic trioxide (As2O3) has recently been successfully used to treat all-trans retinoic acid (ATRA) resistant relapsing acute promyelocytic leukemia. However, its molecular mechanisms of action are poorly understood. In the present study, we used the human leukemia (HL-60) cell line as a test model to study the cellular and molecular mechanisms of anti-cancer properties of As2O3. We hypothesized that As2O3-induced expression of stress genes and related proteins may play a role in the cellular and molecular events leading to cell cycle modulation in leukemic cells. To test this hypothesis, we performed Western blot analysis to assess the expression of specific cellular response proteins including p53, c-fos, RARE, Cyclin A, and Cyclin D1. Densitometric analysis was performed to determine the relative abundance of these proteins. Western Blot and densitometric analyses demonstrated a strong dose-response relationship with regard to p53 and RARE expression within the dose range of 0-8μg/mL. Expression of c-fos was slightly up-regulated at 2μg/mL, and down-regulated within the dose-range of 4-8 μg/mL. A statistically significant down-regulation of this protein was detected at the 6 and 8 μg/mL dose levels. No statistically significant differences (p>0.05) in Cyclin D1 expression was found between As2O3-treated cells and the control. Cyclin A expression in As2O3-treated HL-60 cells was up-regulated at 6μg/mL, suggesting that it is required for S phase and passage through G2 phase in cell cycle progression. Taken together, these results indicate that As2O3 has the potential to induce cell cycle arrest through activation of the 53-kDa tumor suppressor protein and repression of the c-fos transcription factor. Up-regulation of RARE by As2O3 indicates that its cytotoxicity may be mediated through interaction/binding with the retinoic acid receptor, and subsequent inhibition of growth and differentiation.
Keywords: As2O3, HL-60 cells, cyclin A, cyclin D1, RARE, c-fos, p53, APL
Introduction
Recent studies have shown that arsenic trioxide (As2O3) can induce a clinical remission in patients with acute promyelocytic leukemia (APL). Arsenic-containing compounds have been reported to induce apoptosis in leukemic cells both in vivo and in vitro [1]. Many studies on APL-derived cell lines and transgenic mice carrying the PML/RARα fusion proteins indicate that As2O3 induces degradation of both PML/RARα and native PML from the nuclei of the malignant cells [2, 3]. This process allows partial differentiation of leukemia population to proceed. In vitro studies on APL-derived leukemia cells have also indicated that As2O3 causes disappearance of both mutant PML/RARα and wild type PML from the nucleus, eliminating its dominant negative oncogenic effect and leading to terminal maturation of the malignant cells [3].
Recent studies have also indicated that arsenic induces neoplastic cell transformation and apoptosis in tumor cells, by significantly affecting specific signal transduction pathways and by activating the expression of AP-1 and nuclear factor kappa B (NF-/B) in JB6 cells [4, 5]. Other laboratory studies using myeloid leukemia cell lines that do not express PML-RARα have shown that melarsoprol and As2O3 inhibit cell growth, downregulate Bcl-2 protein, and induce apoptosis [6].
Although As2O3 in vitro influences signal transduction pathways in tumor cells, its specific molecular mechanisms of action remain to be elucidated. In the present study, we hypothesized that As2O3-induced expression of stress genes and related proteins plays a role in the molecular events leading to cell cycle modulation in leukemic cells. To test this hypothesis, we performed the western blot and densitometric analyses to assess the expression and relative abundance of specific cellular proteins including p53, c-fos, RARE, cyclin A, and cyclin D1 in human leukemia cells exposed to As2O3.
Materials and Methods
Chemicals and test media
Arsenic trioxide (As2O3), CASRN 1327-53-3, MW 197.84, with an active ingredient of 100% (w/v) arsenic in 10% nitric acid was purchased from Fisher Scientific in (Houston, Texas, U.S.A). Growth medium RPMI 1640 containing 1 mmol/L L-glutamine was purchased from Gibco BRL products (Grand Island, NY). Ninety-six well plates were obtained from Costar (Cambridge, MA). Fetal bovine serum (FBS), antibiotics (penicillin G and streptomycin), and phosphate buffered saline (PBS) were obtained from Sigma Chemical Company (St. Louis, MO).
Tissue culture
The HL-60 promyelocytic leukemia cell line was purchased from the American Type Culture Collection –ATCC (Manassas, VA). This cell line has been derived from peripheral blood cells of a 36-year old Caucasian female with acute promyelocytic leukemia (APL). The HL-60 cells grow as a suspension culture. The predominant cell population consists of neutrophilic promyelocytes [7].
In the laboratory, cells were stored in the liquid nitrogen until use. They were next thawed by gentle agitation of their containers (vials) for 2 min in a water bath at 37°C. After thawing, the content of each vial of cell was transferred to a 25 cm2 tissue culture flask, diluted with up to 10 mL of RPMI 1640 containing 1 mmol/L L-glutamine (GIBCO/BRL, Gaithersburg, MD) and supplemented with 10% (v/v) fetal bovine serum (FBS), 1% (w/v) penicillin/streptomycin. The 25 cm2 culture flasks (2 × 106 viable cells) were observed under the microscope, followed by incubation in a humidified 5% CO2 incubator at 37°C. Three times a week, they were diluted under same conditions to maintain a density of 5 × 105/mL, and harvested in the exponential phase of growth. The cell viability was assessed by the trypan blue exclusion test (Life Technologies), and manually counted using a hemocytometer.
Western blot and densitometric analyses
Western blot analysis was conducted to determine specific cellular response proteins including p53, Cyclin A, Cyclin D1, RARE, and c-fos at 24 h of arsenic trioxide (As2O3) exposure. HL-60 cells were grown in 96 well polystyrene tissue plates. Briefly, 200 μL cells (5 × 105/mL) were added to each well of 96 tissue culture plates and treated with 2, 4, 6 and 8 μg/mL of As2O3 for 24 h. These doses were selected based on the results of previous experiments in our laboratory indicating that As2O3 is cytotoxic to HL-60 cells, showing a 24 hr LD50 of 6.4 ± 0.7 μgmL [8]. Control well plates were also made without As2O3. After the incubation period, cells were centrifuged at 800 rpm for 5 min, the supernatant was carefully aspirated, and the cells were washed twice with PBS. The total protein was measured by the Bradford method at 600 nm using a microtiter plate reader [9]. Twenty μL of native sample buffer (0.2 mol/L Tris, pH 6.8, 1% SDS, 30% glycerol, 7.5% mercaptoethanol, 0.1% bromophenol blue) were added to each plate well and the cells were mechanically collected into micro-centrifuge tubes. Cellular protein lysates (15μg/mL) from human leukemia HL-60 cells containing an equal volume of sample buffer were heated at 100°C for 10 min. Appropriate amounts of total cellular protein were loaded onto 10% SDS polyacrylamide gels and electrophoresed at 100 V constant voltage for 1 hr. Samples were transferred onto a nitrocellulose membrane on ice and the membrane was blocked (Tris buffer saline with 5% nonfat dry milk, 0.1 Tween 20) for 24 hr at 4°C. Detection of membrane-bound proteins was carried out using specific primary antibodies for the proteins of interest (c-fos 15:1000, p53 1:1000, RARE 1:500, Cyclin A 1:500, and Cyclin D1 1:750) (Santa Cruz Biotechnology Inc., Santa Cruz, CA). Equal lane loading was assessed using α-tubulin mouse monoclonal primary antibody (Figure 1). Subsequently, the reaction was probed with a 1:750 dilution of alkaline conjugated anti-mouse IgG secondary antibody. NBT/BCIP color substrate was incorporated to develop protein bands. Immunoblot 1-D protein bands were assessed for relative abundances using Total Lab-Image computer software (Nonlinear USA Inc. Durham, NC).
Figure 1.
Expression of α-tubulin in human leukemia (HL-60) cells exposed to arsenic trioxide. HL-60 cells were treated with different doses of arsenic trioxide, and Western blot analysis of α-tubulin expression was performed as indicated in the Materials and Methods.
Statistical analysis
To determine the lysate volumes to be loaded on the gels, protein samples collected from controls and As2O3-treated cells were measured using the Bradford method based of the optimal density readings at 600 nm. After electrophoresis, the expression levels of specific cellular proteins were photographed using the Gel Documentation System (Nucleotech Corporation, Inc, San Mateo CA). The experiments were performed three or more times to ensure reproducibility. Densitometric analysis was performed using the Gel Documentation System (Nucleotech Corporation, Inc, San Mateo CA) to determine the relative abundance of protein expression. Comparison of protein levels between control cells and As2O3-treated HL-60 cells was performed using one way analysis of variance (ANOVA) for multiple samples and Student's t-test for paired sample sets. All p-values <0.05 were considered to be significant.
Results
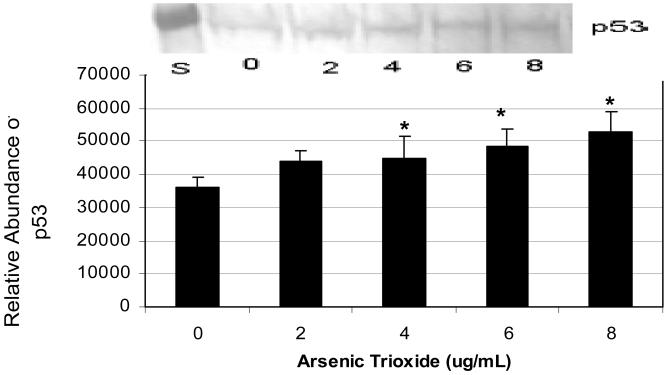
The expression and relative abundance levels of p53 in human leukemia (HL-60) cells exposed to arsenic trioxide (As2O3) are represented in Figure 2. According to ANOVA Dunnett's test (p < 0.05), western blot and the densitometric analyses demonstrated a significant increase of p53 expression in As2O3-treated HL-60 cells showing a gradual increase in protein expression with increasing doses of As2O3.
Figure 2.
Expression and relative abundance of p53 in human leukemia (HL-60) cells exposed to arsenic trioxide. HL-60 cells were treated with different doses of arsenic trioxide, and Western blot and densitometric analyses of p53 expression were performed as indicated in the Materials and Methods. α-tubulin expression was used to assess equal lane loading. Inset shows a representative Western Blot analysis. Bars represent p53 relative abundance. Each point represents the mean value and the standard deviation of three experiments. * Significantly different from control (0 μg/mL), p < 0.05.
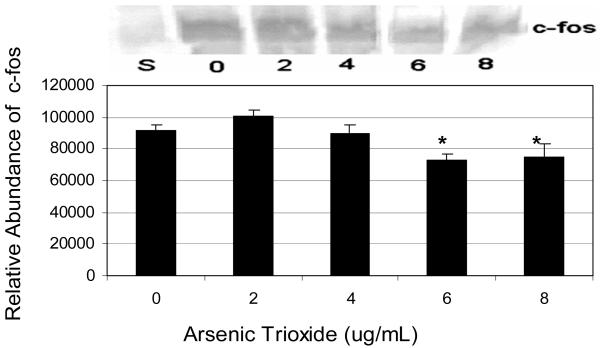
The relative level of c-fos expression was slightly up-regulated at 2μg/mL, and down-regulated within the dose-range of 4-8 μg/mL. Statistically significant down-regulation of this protein was detected at the 6 and 8 μg/mL dose levels. Hence, c-fos expression data indicated a somewhat biphasic response that encompasses up-regulation at 2 μg/mL level of exposure and down-regulation at higher doses of exposure (Fig 3).
Figure 3.
Expression and relative abundance of c-fos in human leukemia (HL-60) cells exposed to arsenic trioxide. HL-60 cells were treated with different doses of arsenic trioxide, and Western blot and densitometric analyses of c-fos expression were performed as indicated in the Materials and Methods. α-tubulin expression was used to assess equal lane loading. Inset shows a representative Western Blot analysis. Bars represent c-fos relative abundance. Each point represents the mean value and the standard deviation of three experiments. * Significantly different from control (0 μg/mL), p < 0.05.
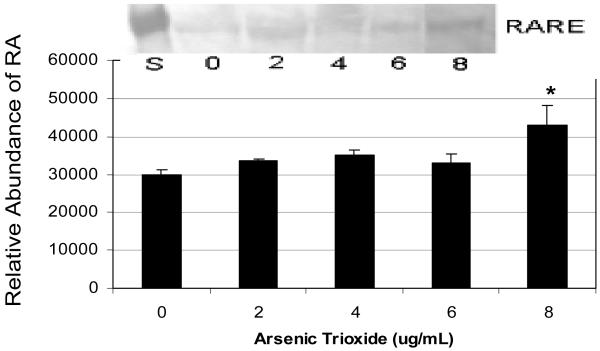
The data of RARE expression in As2O3-treated cells is represented in Figure 4. As shown in this figure, the expression of RARE in HL-60 cells exposed to As2O3 at 2, 4, and 6 μg/mL dose levels are not significantly different (p > 0.05) compared to the control. However, an up-regulation of this protein was observed in As2O3-treated cells with a statistically significant increase at 8 μg/mL compared to the control.
Figure 4.
Expression and relative abundance of RARE in human leukemia (HL-60) cells exposed to arsenic trioxide. HL-60 cells were treated with different doses of arsenic trioxide, and Western blot and densitometric analyses of RARE expression were performed as indicated in the Materials and Methods. α-tubulin expression was used to assess equal lane loading. Inset shows a representative Western Blot analysis. Bars represent RARE relative abundance. Each point represents the mean value and the standard deviation of three experiments. * Significantly different from control (0 μg/mL), p < 0.05.
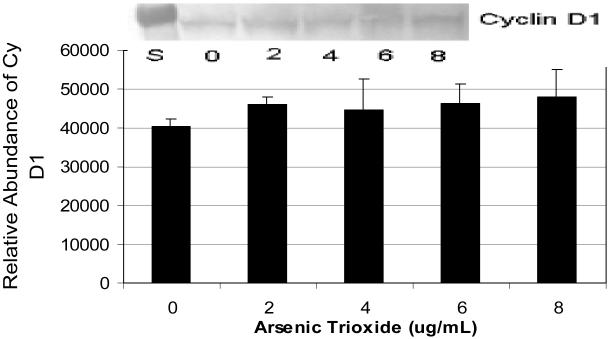
Western blot analysis showed a slight increase in cyclin D1 expression in As2O3-treated HL-60 cells. However, the statistical analysis based on the densitometric analysis did not show any significant differences (p > 0.05) between As2O3-treated cells and the control (Fig 5).
Figure 5.
Expression and relative abundance of Cyclin D1 in human leukemia (HL-60) cells exposed to arsenic trioxide. HL-60 cells were treated with different doses of arsenic trioxide, and Western blot and densitometric analyses of Cyclin D1 were performed as indicated in the Materials and Methods. α-tubulin expression was used to assess equal lane loading. Inset shows a representative Western Blot analysis. Bars represent Cyclin D1 relative abundance. Each point represents the mean value and the standard deviation of three experiments.
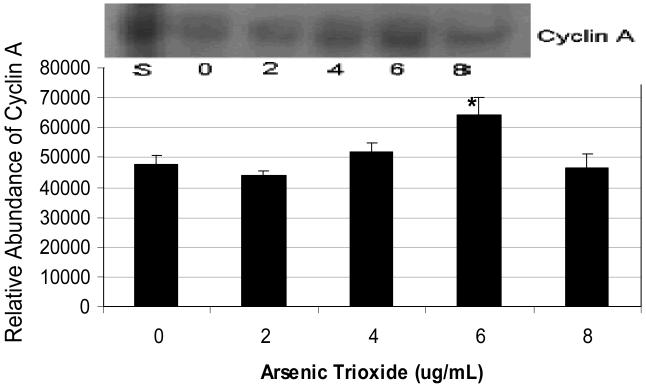
Data on cyclin A expression and its relative abundance in As2O3-treated HL-60 cells are represented in Figure 6. As shown in this figure, there was no statistically significant difference between the control and As2O3-treated cells up to 4 μg/mL dose levels. An up-regulation of this protein was detected at 6 μg/mL As2O3, suggesting that it is likely required for S phase and passage through G2 phase in cell cycle progression. However, at 8 μg/mL of As2O3 treatment, the expression of this protein was similar to that of the control.
Figure 6.
Expression and relative abundance of Cyclin A in human leukemia (HL-60) cells exposed to arsenic trioxide. HL-60 cells were treated with different doses of arsenic trioxide, and Western blot and densitometric analyses of Cyclin A were performed as indicated in the Materials and Methods. α-tubulin expression was used to assess equal lane loading. Inset shows a representative Western Blot analysis. Bars represent Cyclin A relative abundance. Each point represents the mean value and the standard deviation of three experiments. * Significantly different from control (0 μg/mL), p < 0.05.
Discussion
The present study was designed to investigate the influence of arsenic trioxide (As2O3) treatment on the expression of p53, c-fos, RARE, cyclin A, and cyclin D1 in human leukemia (HL-60) cells. Data from western blot and densitomentric analyses show a gradual increase of p53 expression in HL-60 cells with increasing doses of As2O3. This increased expression of the 53 KDa tumor suppressor protein detected in the As2O3-treated HL-60 cells suggests the potential of As2O3 to cause either G1 cell cycle arrest and/or apoptosis in HL-60 cells. However, despite the ability of As2O3 to induce cell cycle arrest, little is known concerning the precise role of this agent in cell cycle progression and the function of p53 in this process. The increase in p53 expression in As2O3-treated cells may be indicative of a cellular response to oxidative and DNA damage. A series of recent studies have demonstrated DNA damage [10], oxidative stress [11, 12], UV irradiation [13, 14], and transcriptional blockade [15] induce translocation of p53 to the mitochondria. Research has suggested that arsenic induces DNA damage, such as chromosome aberration [16], and sister chromatid exchange [17]. Because p53 mutations are common in most human cancers and apoptosis plays a key role in the bioactivity of most chemotherapeutic agents, As2O3 seems to be very useful for the treatment of APL and certain human cancers. This notion is supported by the findings that p53 is the most commonly mutated tumor suppressor gene, and the lack of p53 activation or expression is associated with an increased risk of tumor formation [18-20]. Previous studies in our laboratory have demonstrated that As2O3 is able to transcriptionally induce the expression of p53, c-fos, and HSP70 in human liver carcinoma (HepG2) cells [21]. The p53 transcription factor has been shown to mediate apoptosis through its direct action at the mitochondria [22, 23].
The result of c-fos expression shows a somewhat biphasic response that encompasses a slight up-regulation at lower doses of exposure, and down-regulation at higher doses of exposure. The inhibition of c-fos expression at higher level of As2O3 exposure is in agreement with the activation of p53 expression, suggestive of cell cycle arrest at G1 check point of the cell cycle. p53 has been shown to repress regulators of cell proliferation, such as c-fos and c-jun which are early-response nuclear oncogenes [24]. Many researchers have reported that c-fos and p53 share similar function and similar transcriptional regulatory pathways in apoptosis following excitotoxic stimulation [25, 26]. Finding from the present study implies that c-fos repression may play an important role in HL-60 cells apoptosis induced by As2O3. c-fos is known to be highly induced in many human cancer cell lines in response to stimilus. This proto-oncogene (c-fos) plays a role in cell proliferation, differentiation, and may contribute to tumor promotion. In other cell lines, As2O3 has been reported to activate the expression of c-fos through numerous signal transduction pathways at low dose of exposure. For example, in a human bladder epithelial cell line, arsenic increased cell proliferation and AP-1 DNA binding [27].
In mammalian cells, cyclins D, E, and A are key cyclins involved in G1 to S phase transition. Cyclin D assembles with cdk4/6 in early G1; cyclin E combines with cdk2 later in G1, and cyclin A associates with cdk2 at the beginning of S phase [28, 29]. In the present study, the data of western blot and densitometric analyses show a slight increase of Cyclin D1 expression in As2O3-treated HL-60 cells. However, there were no statistically significant differences (p >0.05) in cyclin D1 expression between As2O3-treated cells and the control. The lack of a statistically significant difference in cyclin D1 expression in As2O3-treated cells compared to the control is in agreement with the activation of the p53 tumor suppressor protein, and the repression of the c-fos proto-oncogene, indicative of cell cycle arrest at the G1/S checkpoint. Cyclin D1 is a 36 kDa nuclear protein. The cell cycle in eukaryotic cells is mediated by the formation, activation, and deactivation of complexes containing cyclin-dependent kinases (CDK) and cyclins. Few studies have demonstrated that cyclin D1 over-expression does not correlate with the proliferation rate in rat mammary tumors or in human tumors [30, 31].
Data generated from the present study shows that As2O3 doesn't significantly affect progression from G0/G1 into S phase. This transition is, in part, controlled by the activity of G1 cyclin-cdk's which include D cyclins associated with either cdk4 or cdk6 and E cyclins associated with cdk2 [32, 33], suggesting that the expression of cyclin D1 may be an early event in chemical tumorigenesis, causing an increase in cell proliferation.
Recent publications have accumulated evidence showing an increased expression of cyclin D1 in tumor cell lines [34-36]. Studies have shown that arsenite exposure is able to activate the PI-3K/Akt pathway and induce cyclin D1 expression in mouse epidermal Cl41 cells [37]. It has been reported that mitogen-activated protein kinase (MAPK) cascades are involved in the modulation of cyclin D1 expression [38]. Other studies have revealed that ectopic expression of cyclin D1 can shorten the G1 phase, whereas inhibition of cyclin D1 expression blocks G1-S transition. It has been demonstrated that carcinogenic compounds can induce cyclin D1 expression, which in turn promote tumor cell proliferation [39].
Data generated from the present study have demonstrated a positive expression of cyclin A in As2O3-treated HL-60 cells showing an up-regulation at 6 μg/mL of As2O3 exposure. Cyclin A is required in more than one phase of the cell cycle. It is one of the first cyclins to be identified and is believed to function between that of cyclin E and cyclin B. Generally, it is expressed in late S and G2 phase and degraded during mitosis just prior to metaphase. It associates with two cyclin dependent kinases: cdk2 in the S phase of the cell cycle and Cdc2 in the G2/M phase. These associations are required for both DNA replication and mitosis [40-42]. Cyclin A is required for S phase and passage through G2 phase in cell cycle progression. Interestingly, our results indicated that treatment of HL-60 cells with As2O3 may lead to cell cycle progression at the G2 checkpoint as demonstrated by the significant increase of cyclin A expression at 6 μg/mL of As2O3 exposure. The activation of cyclin A expression at higher level of As2O3 exposure is in agreement with slight increase in cyclin D1 expression; suggestive that exposure of human leukemia HL-60 cells to As2O3 may result in an increase in the proportion of cells in mitosis.
Up-regulation of the retinoic acid response element (RARE) in HL-60 cells by As2O3 suggests that its toxicity may be mediated through interaction or binding with the retinoic acid receptor, and subsequent inhibition of growth and differentiation. Substantial data showed that As2O3 exposure induces remission in patients with APL at least in part through a mechanism that results in the degradation of the aberrant PML-retinoic acid receptor-αfusion protein. In the early 1980s, it was noted that all trans retinoic acid (ATRA) could induce differentiation of myeloid cell lines such as HL-60 [43], and of primary cells from patients with APL [44]. Retinoids, a group of structural and functional analogues of vitamin A, are known to mediate cellular signals critical for embryonic morphogenesis, cell growth, and differentiation. The use of retinoids to suppress tumor development has been evaluated in several animal models of carcinogenesis, including models of skin, breast, oral cavity, lung, hepatic, gastrointestinal, prostatic, and bladder cancers [45]. Clinically, retinoids are able to reverse premalignant lesions and inhibit the development of primary tumors [46, 47].
Conclusions
The present study provides new insights into the biochemical effects of arsenic trioxide (As2O3) in HL-60 cells and the potential of As2O3 as a chemotherapeutic agent for the treatment of acute promyelocytic leukemia. Using western blot and densitometric analyses, our data provide evidence that As2O3 performs key functions of an anti cancer agent against human leukemia; it is antiproliferative and apoptotic at high doses (p53 activation and c-fos repression), and induces cellular differentiation at low doses (cyclins D1 and A expression). These results are consistent with previous studies reporting that As2O3 (an effective drug for the treatment of APL) exerts dose-dependent dual effects in APL cells by triggering apoptosis and inducing partial differentiation [48- 51].
Taken together, we have demonstrated in the present study that As2O3 serves as an activator of the p53, RARE, cyclin A and cyclin D1, and a repressor of c-fos in HL-60 cells. It is evident that As2O3 exposure places HL-60 cells under a degree of toxic stress that evokes response acting at different levels on the cell cycle. This toxic action of As2O3 induces transcription of specific genes that affect mitogen response, cell cycle progression, and programmed cell death. Findings from our studies suggest that activation of cyclin D1, cyclin A, and RARE by As2O3 in HL-60 cells may be an important part of the multistep process involved in cell cycle progression, whereas the activation of the p53 tumor suppressor protein and repression of the c-fos transcription factor at higher level of exposure may be involved in cell cycle arrest and apoptosis. Further in vitro studies are underway to refine the molecular mechanisms of action and to determine whether signal transduction pathways vary according to the cell type, form of arsenic, or other factors.
Acknowledgments
This research was financially supported by a grant from the National Institutes of Health (Grant No. 2G12RR13459), through the RCMI-Center for Environmental Health at Jackson State University. The authors thank Dr. Ronald Mason: President and Dr. Abdul Mohamed: Dean Emeritus of College of Science, Engineering & Technology at Jackson State University, for their technical support of this research.
References
- 1.Chen GQ, Zhu J, Shi XG, Ni HJ, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, Shen ZX, Sun GL, Ma J, Zhang P, Zhang TD, Gazin C, Naoe T, Chen SJ, Wang ZY, Chen Z. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: arsenic trioxide induces NB 4 cell apoptosis with down-regulation of bcl-2 expression and modulation of PML-RARα/PML proteins. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- 2.Rego EM, He LZ, Warrell RP, Jr, Wang ZG, Pandolfi PP. Retinoic acid (RA) and As2O3 treatment in transgenic models of acute promyelocytic leukemia (APL) unravel the distinct nature of the leukemiamogenic process induced by the PML-RARapha and PLZF-RARalpha oncoproteins. Proc Natl Acad Sci USA. 2000;97:10173–10178. doi: 10.1073/pnas.180290497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu J, Koken MHM, Quignon F, Chelbi-Alix MK, Degos L, Wang ZY, Chen Z, de Thé H. Arsenic-induced PML targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leukemia. Proc Natl Acad Sci USA. 1997;94:3978–3983. doi: 10.1073/pnas.94.8.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bode A, Dong Z. Apoptosis induction by arsenic: mechanisms of action and possible clinical applications for treating therapy-resistant cancers. J Drug Resist Updat. 2000;3:21–29. doi: 10.1054/drup.2000.0114. [DOI] [PubMed] [Google Scholar]
- 5.Bode AM, Dong Z. The paradox of arsenic: molecular mechanisms of cell transformation and chemotherapeutic effects. Crit Rev Oncol/Hematol. 2002;42:5–24. doi: 10.1016/s1040-8428(01)00215-3. [DOI] [PubMed] [Google Scholar]
- 6.Wang ZY, Chen Z. Differentiation and apoptosis induction therapy in acute promyelocytic leukemia [review] Lancet Oncol. 2000;1:101–106. doi: 10.1016/s1470-2045(00)00017-6. [DOI] [PubMed] [Google Scholar]
- 7.Freshney RI. A manual of basic techniques. Alan Liss Inc (University Library); 1983. Culture of animal cells. [Google Scholar]
- 8.Yedjou CG, Tchounwou PB. In-vitro cytotoxic and genotoxic effects of arsenic trioxide on human leukemia (HL-60) cells using the MTT and alkaline single cell gel electrophoresis (Comet) assays. Mol Cell Biochem. 2007;301:123–130. doi: 10.1007/s11010-006-9403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradford MM. A rapid, sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 10.Erster S, Mihara M, Kim RH, Petrenko O, Moll UM. In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation. Mol Cell Biol. 2004;24:6728–6741. doi: 10.1128/MCB.24.15.6728-6741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonini P, Cicconi S, Cardinale A, Vitale C, Serafino AL, Ciotti MT, Marlier LN. Oxidative stress induces p53-mediated apoptosis in glia: p53 transcription-independent way to die. J Neurosci Res. 2004;75:83–95. doi: 10.1002/jnr.10822. [DOI] [PubMed] [Google Scholar]
- 12.Endo H, Kamada H, Nito C, Nishi T, Chan PH. Mitochondrial translocation of p53 mediates release of cytochrome c and hippocampal CA1 neuronal death after transient global cerebral ischemia in rats. J Neurosci. 2006;26:7974–7983. doi: 10.1523/JNEUROSCI.0897-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumont P, Leu JI, Della Pietra AC, III, George DL, Murphy M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet. 2003;33:357–365. doi: 10.1038/ng1093. [DOI] [PubMed] [Google Scholar]
- 14.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 15.Arima Y, Nitta M, Kuninaka S, Zhang D, Fujiwara T, Taya Y, Nakao M, Saya H. Transcriptional blockade induces p53-dependent apoptosis associated with translocation of p53 to mitochondria. J Biol Chem. 2005;280:19166–19176. doi: 10.1074/jbc.M410691200. [DOI] [PubMed] [Google Scholar]
- 16.Patlolla AK, Tchounwou PB. Cytogenetic evaluation of arsenic trioxide in Sprague-Dawley rats. Mutation Research. 2005;587:126–133. doi: 10.1016/j.mrgentox.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Volgelstein B, Kinzler KW. p53 function and dysfunction. Cell. 1992;70:523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- 18.Hollstein M, Rice K, Greenbalt MS, Soussi T, Fuchs R, Sorlie T, Hovig E, Smith-Sorenson B, Montesano R, Harris CC. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994;22:3551–3555. [PMC free article] [PubMed] [Google Scholar]
- 19.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr., Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumors. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 20.Malkin D, Li FP, Strong LC, Fraumeni JF, Jr, Nelson CE, Kim DH, Kassel J, Gryka MA, Bischoff FZ, Tainsky MA, Friend SH. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 21.Tchounwou PB, Yedjou CG, Dorsey WC. Arsenic trioxide induced transcriptional activation and expression of stress genes in human liver carcinoma cells (HepG2) Cellular and Molecular Biology™. 2003;49(7):1071–1079. [PubMed] [Google Scholar]
- 22.Erster S, Moll UM. Stress-induced p53 runs a transcription-independent death program. Biochem Biophys Res Commun. 2005;331:843–850. doi: 10.1016/j.bbrc.2005.03.187. [DOI] [PubMed] [Google Scholar]
- 23.Schuler M, Green DR. Transcription, apoptosis and p53: catch-22. Trends Genet. 2005;21:182–187. doi: 10.1016/j.tig.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Kley N, Chung RY, Fay S, Loeffler JP, Seizinger BR. Repression of the basal c-fos promoter by wild-type p53. Nucleic Acids Res. 1992;20:4083–4087. doi: 10.1093/nar/20.15.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lackinger D, Kaina B. Primary mouse fibroblasts deficient for c-Fos, p53 or for both proteins are hypersensitive to UV light and alkylating agent-induced chromosomal breakage and apoptosis. Mutat Res. 2000;457:113–123. doi: 10.1016/s0027-5107(00)00133-0. [DOI] [PubMed] [Google Scholar]
- 26.Nango R, Chieko T, Tsukamoto I. Jun N-terminal kinase activation and upregulation of p53 and p21 in selenite-induced apoptosis of regenerating liver. Eur J Pharmacol. 2003;47:1–8. doi: 10.1016/s0014-2999(03)01764-3. [DOI] [PubMed] [Google Scholar]
- 27.Daum G, Pham J, Deou J. Arsenite inhibits Ras-dependent activation of ERK but activates ERK in the presence of oncogenic Ras in baboon vascular smooth muscle cells. Mol Cell Biochem. 2001;217:131–136. doi: 10.1023/a:1007276812824. [DOI] [PubMed] [Google Scholar]
- 28.Draetta GF. Mammalian G 1 cyclins. Curr Opin Cell Biol. 1994;6:842–846. doi: 10.1016/0955-0674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 29.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 30.Sgambato A, Han EK, Zhang YJ, Moon RC, Santella RM, Weinstein IB. Deregulated expression of cyclin D1 and other cell cycle-related genes in carcinogen-induced rat mammary tumors. Carcinogenesis. 1995;16:2193–2198. doi: 10.1093/carcin/16.9.2193. [DOI] [PubMed] [Google Scholar]
- 31.Weinstat-Saslow D, Merino MJ, Manrow RE, Bluth RF, Wittenbel KD, Simpson JF. Overexpression of cyclin D mRNA distinguishes invasive and in situ breast carcinomas from nonmalignant lesions. Nat Med. 1995;1:1257–1260. doi: 10.1038/nm1295-1257. [DOI] [PubMed] [Google Scholar]
- 32.Hunter T, Pines J. Cyclins and cancer. II: cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 33.Grana X, Reddy EP. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs) Oncogene. 1995;11:211–219. [PubMed] [Google Scholar]
- 34.Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Lin SC. Molecular characterization of the cyclin-dependent kinase inhibitor p27 promoter. Biochim Biophys Acta. 1997;1353:307–311. doi: 10.1016/s0167-4781(97)00063-8. [DOI] [PubMed] [Google Scholar]
- 36.Lee RJ, Albanese C, Stenger RJ, Watanabe G, Inghirami G, Haines GK, 3rd, Webster M, Muller WJ, Brugge JS, Davis RJ, Pestell RG. 60(v-src) induction of cyclin D1 requires collaborative interactions between the extracellular signal-regulated kinase, p38, and Jun kinase pathways. A role for cAMP response element-binding protein and activating transcription factor-2 in pp60(v-src) signaling in breast cancer cells. J Biol Chem. 1999;274:7341–7350. doi: 10.1074/jbc.274.11.7341. [DOI] [PubMed] [Google Scholar]
- 37.Ouyang W, Li J, Ma Q, Huang C. Essential roles of PI-3K/Akt/IKK {beta}/NF {kappa} B pathway in cyclin D1 induction by arsenite in JB6 Cl41 cells. Carcinogenesis. 2006;27(4):864–873. doi: 10.1093/carcin/bgi321. [DOI] [PubMed] [Google Scholar]
- 38.Lavoie JN, L'Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Puebla ML, Robles AI, Conti CJ. ras activity and cyclin D1 expression: an essential mechanism of mouse skin tumor development. Mol Carcinog. 1999;24(1):1–6. [PubMed] [Google Scholar]
- 40.Girard F, Strausfeld U, Fernandez A, Lamb NJ. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- 41.Pagano MR, Pepperkok F, Verde W, Ansorge, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO (Eur Mol Biol Organ) J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 43.Breitman T, Collins S, Selonick S. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci USA. 1980;77:2936. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breitman T, Collins SJ, Keene B. Terminal differentiation of human promeylocytic cells in culture in response to retinoic acid. Blood. 1981;57:1000. [PubMed] [Google Scholar]
- 45.Evans TRJ, Kaye SB. Retinoids present role and future potential. Br J Cancer. 1999;80:1–8. doi: 10.1038/sj.bjc.6690312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong WK, Lippman SM, Itri LM, Karp DD, Lee JS, Byers RM, Schuntz SS, Kramer AM, Lotan R, Peters LL, Dimery TW, Brown BW. Goepfert: Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323:795–801. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- 47.Lippman SM, Lee JJ, Sabichi AL. Cancer chemoprevention: progress and promise. J Natl Cancer Inst. 1998;85:1492–1498. doi: 10.1093/jnci/90.20.1514. [DOI] [PubMed] [Google Scholar]
- 48.Zhang P, Wang SY, Hu XH. Arsenic trioxide treated 72 cases of acute promyelocytic leukemia. Chin J Hematol. 1996;17:58–60. [Google Scholar]
- 49.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qin QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou C, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY. Use of arsenic trioxide in the treatment of acute promyelocytic leukemia (APL): II clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 50.Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, deBlasio A, Gabrilove J, Scheinberg DA, Pandolfi PP, Warrell RP., Jr Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. New Engl J Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 51.Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM, Liu MM, He KL, Niu C, Ma J, Zhang P, Zhang TD, Paul P, Naoe T, Kitamura K, Miller W, Waxman S, Wang ZY, de The H, Chen SJ, Chen Z. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]