Abstract
Neural transplantation offers the potential of treating Parkinson’s disease by grafting fetal dopamine neurons to depleted regions of the brain. However, clinical studies of neural grafting in Parkinson’s disease have produced only modest improvements. One of the main reasons for this is the low survival rate of transplanted neurons. The inadequate supply of critical neurotrophic factors in the adult brain is likely to be a major cause of early cell death and restricted outgrowth of fetal grafts placed into the mature striatum. Glial derived neurotrophic factor (GDNF) is a potent neurotrophic factor that is crucial to the survival, outgrowth and maintenance of dopamine neurons, and so is a candidate for protecting grafted fetal dopamine neurons in the adult brain. We found that implantation of adeno-associated virus type 2 encoding GDNF (AAV2-GDNF) in the normal monkey caudate nucleus induced over-expression of GDNF that persisted for at least 6 months after injection. In a 6-month within-animal controlled study, AAV2-GDNF enhanced the survival of fetal dopamine neurons by 4-fold, and increased the outgrowth of grafted fetal dopamine neurons by almost 3-fold in the caudate nucleus of MPTP-treated monkeys, compared with control grafts in the other caudate nucleus. Thus, the addition of GDNF gene therapy to neural transplantation may be a useful strategy to improve treatment for Parkinson’s disease.
Keywords: associated adenoviral vector (AAV), dopamine, fetal tissue, glial derived neurotrophic factor (GDNF), graft, monkey, MPTP, Parkinson’s disease, striatum, transplantation
Introduction
Neural transplantation offers the potential of treating Parkinson’s disease by grafting fetal dopamine (DA) neurons to depleted regions of the host brain, providing those DA-deficient regions with a regulated source of DA. This strategy promises to provide long-term amelioration of parkinsonian signs, which available treatments are unable to achieve. Animal studies in the rodent (reviewed by Brundin et al., 1994), and in the primate (Annett, 1994; Bankiewicz et al., 1993; Elsworth et al., 1996; Fine et al., 1988; Taylor et al., 1991) have shown that grafts of fetal DA neurons can lead to reversals in biochemical and behavioral indices of DA deficiency. However, in clinical studies the improvements in Parkinsonism have been variable, and generally rather modest (Freed et al., 1992; Freed et al., 2001; Lindvall and Hagell, 2001; Olanow et al., 2003; Redmond, 2002; Spencer et al., 1992; Widner et al., 1992).
The death of the majority (~90–95%) of transplanted DA neurons from the fetal ventral mesencephalon (VM) soon after grafting in rat (Brundin et al., 2000) and human (Olanow et al., 1996) can serve to limit the success of the neural transplantation treatment strategy for Parkinson’s disease. An important contributor to this poor survival appears to be the environment of the adult host brain, which may be less than optimal for the survival and growth of grafted immature neurons. In particular, the inadequacy of critical growth factors in the adult brain may be a major cause of early cell death and restricted outgrowth of fetal grafts placed into the mature striatum. Thus, providing additional neurotrophic support for donor fetal neurons may reduce the problem of poor survival of neural grafts in Parkinson’s disease.
Glial derived neurotrophic factor (GDNF) is a potent neurotrophic factor that is crucial to the development, survival, and outgrowth of DA neurons (Airaksinen and Saarma, 2002; Lin et al., 1993), and so it is a good candidate for protecting and maintaining grafted fetal DA neurons in the host brain. GDNF is highly expressed in the developing rat striatum, yet its concentration is relatively low in the adult brain (Choi-Lundberg and Bohn, 1995; Schaar et al., 1993; Stromberg et al., 1993). Several studies in the 6-hydroxydopamine (6-OHDA) lesioned striatum of rats have demonstrated improvements in survival and outgrowth of grafted fetal DA neurons when central injections of GDNF have been administered, or when GDNF overexpressing cells have been co-grafted to the striatum (Ahn et al., 2005; Espejo et al., 2000; Rosenblad et al., 1996; Sautter et al., 1998; Sinclair et al., 1996; Sullivan et al., 1998; Wilby et al., 1999; Yurek, 1998). These effects of GDNF on grafted VM were evaluated 1 to 8 weeks after transplantation. Gene therapy with a recombinant adeno-associated virus offers a strategy for longer-term delivery of GDNF to circumscribed regions of the brain in a relatively safe and non-invasive manner (Grieger and Samulski, 2005).
Interestingly in the rodent overexpression of GDNF in the intact striatum leads to an initial increase in DA turnover, followed by a selective down-regulation of TH at times longer than 6 weeks (Georgievska et al., 2004b). However, in this rat study the effect on TH was not accompanied by alteration in DA synthesis or content, indicating that the GDNF-induced changes in TH are a compensation for the initial overactivity of the DA system. Likewise in rats with a lesioned nigrostriatal DA system and intrastriatal grafts of fetal dopaminergic neurons, overexpression of GDNF results in an increase in survival of fetal dopaminergic neurons at 4 weeks, with an eventual down-regulation of TH in the grafted neurons (Georgievska et al., 2004a). Despite these rodent data with lentivirus vector delivery of GDNF, we were sufficiently encouraged by our studies with recombinant adeno-associated viral vectors (AAV) (Sondhi et al., 2005) and with GDNF-secreting macrocapsules (Redmond et al., 2002) to investigate the potential enhancement of fetal DA neuron survival and outgrowth in the striatum of MPTP-treated monkeys co-implanted with a recombinant AAV harboring the GDNF gene.
Materials and Methods
Young adult male St Kitts green (vervet) monkeys (Chlorocebus sabaeus) at the St Kitts Biomedical Research Foundation (St. Kitts, West Indies) were used. As the subjects were feral monkeys, their exact ages were not known, but they were all mature with a mean weight (± standard deviation) of 6.4 ± 0.6 kg. All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care & Use Committee.
Striatal delivery of GDNF by AAV2-GDNF in normal monkeys
Production and purification of recombinant AAV serotype 2 vector (AAV2) is described elsewhere (Grieger et al., 2006). For the present study, the vector plasmid contained the human GDNF cDNA, driven by a chicken beta-actin promoter with a cytomegalovirus immediate-early enhancer, flanked by AAV2 inverted terminal repeats (Fig. 1). The head of the right caudate nucleus of 10 monkeys was injected by stereotactic surgery with 12 microliters of AAV2-GDNF at a concentration of 2.3 × 109 particles per microliter, delivered at 1 microliter per minute by a microperfusion pump (Stoelting Instruments, Wood Dalle, IL). Animals were euthanized by pentobarbital overdose at 1 month (mean time ± S.D., 36 ± 1 day), 3 months (95 ± 4 days) or 6 months (185 ± 4 days) after treatment. Brains were perfused with saline, followed by 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4), as before (Sladek et al., 1995). After postfixing overnight, brains were stored in 0.1M phosphate buffer containing 0.1% sodium azide (pH 7.4). Subsequently, 50 micrometer-thick serial Vibratome sections were cut coronally through the caudate nucleus. Every fourth section was double-labeled for GDNF-immunoreactivity, using goat anti-GDNF (BAF-212 at 1:250, R&D Systems, Minneapolis, MN). GDNF-immunoreactivity was visualized using biotinylated horse anti-goat IgG (BA-9500 at 1:250, Vector Laboratories, Burlingame, CA), and the ABC technique with nickel-intensified 3,3′-diaminobenzidine (DAB) as chromagen. Quantification comprised unbiased stereological counts of GDNF-ir cells in the caudate nucleus together with the volume of tissue occupied by GDNF-ir. These measures were made using StereoInvestigator 7 software (MicroBrightField Inc., Williston, VT).
Fig. 1.
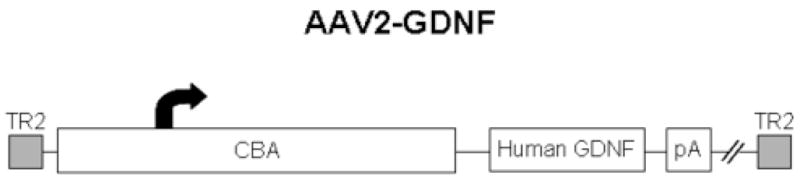
The AAV2 vector contained the human GDNF cDNA, driven by a chicken beta-actin promoter with a cytomegalovirus immediate-early enhancer (CBA promoter), and a SV40 polyadenylation site, flanked by AAV2 inverted terminal repeats.
Implantation of AAV2-GDNF and fetal ventral mesencephalon in MPTP monkeys
As before (Elsworth et al., 2000), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (MPTP; Sigma-Aldrich Corp., St. Louis, MO) was administered by intramuscular injection 4 times over 5 days, so that the total dose of MPTP given to each monkey was 2.0 mg/kg. The behavior of each monkey was rated by observers, blinded to experimental conditions, twice daily, 5 days a week, for the first month after MPTP treatment, to classify severity of parkinsonian symptoms (Taylor et al., 1994; Taylor et al., 1997). The four MPTP-treated monkeys used in this part of the study were asymptomatic or mildly symptomatic. MPTP-treated monkeys in these categories have substantial striatal dopamine depletion (>50%, Elsworth et al., 2000), which is relevant as outgrowth of grafted fetal dopamine neuron is inhibited in the non-lesioned striatum (Doucet et al., 1990). Two months after MPTP treatment (75 ± 1 days) implantation of embryonic ventral mesencephalon (VM) with or without AAV2-GDNF was performed.
Three monkeys each received bilateral solid tissue grafts of VM in the head of the caudate nucleus with a 10-microliter injection of either AAV2-GDNF (left) or saline (right) 4 mm anterior to the VM deposit in the caudate nucleus. Implants were made by using stereotactic surgery with a direct vertical approach (Sladek et al., 1995). Appropriate donor tissue for implantation was derived from embryonic monkeys and identified by ultrasonography of pregnant monkeys (crown-rump length 16–20 mm, estimated fetal age 45–48 days). VM was dissected, then divided along the midline, and dissected further to isolate the developing DA neurons of the substantia nigra. The 2 dissected halves of a donor VM were transplanted to the left and right caudate nucleus of one host monkey (Sladek et al., 1995). One additional MPTP-treated monkey received a unilateral graft of half a fetal VM (crown-rump length 17 mm, estimated fetal age 46 days) with an adjacent AAV2-GDNF injection, of the same volume from the same vector stock at the same coordinates as the other monkeys. Six months after transplantation (187 ± 1 days) animals were euthanized and brains fixed, as described above. Vibratome sections through the caudate nucleus were cut in a sagittal plane and stained for tyrosine hydroxylase (TH)-ir, in order to make stereological estimates of grafted TH-ir cells and grafted TH-ir fiber density. Every fourth section was double-labeled for GDNF- and TH-immunoreactivity, using goat anti-GDNF (BAF-212 at 1:250, R&D Systems, Minneapolis, MN) and mouse anti-TH (MAB318 at 1:1000, Chemicon, Temecula, CA). GDNF-immunoreactivity was visualized using biotinylated horse anti-goat IgG (BA-9500 at 1:250, Vector Laboratories, Burlingame, CA), and the ABC technique with DAB as chromagen. TH-immunoreactivity was visualized using biotinylated horse anti-mouse IgG (BA-2000 at 1:200, Vector Laboratories), and the ABC technique with nickel-intensified DAB as chromogen. Quantification comprised unbiased stereological counts of TH-ir in the caudate nucleus. These measures were made using StereoInvestigator 7 software (MicroBrightField Inc.).
Results
Time-course of GDNF expression following AAV2-GDNF injection
The number of cells expressing GDNF in the caudate nucleus did not change significantly between 1 and 6 months after injection of AAV2-GDNF (Fig. 2). The mean number of cells (± S.E.) expressing GDNF in response to the one injection of AAV2-GDNF was 27919 ± 6158. In addition, the volume of the caudate nucleus occupied by diffuse staining for GDNF-ir did not diminish over the time period examined (Fig. 2). The mean volume occupied by elevated GDNF staining was 50 ± 6 mm3.
Fig. 2.
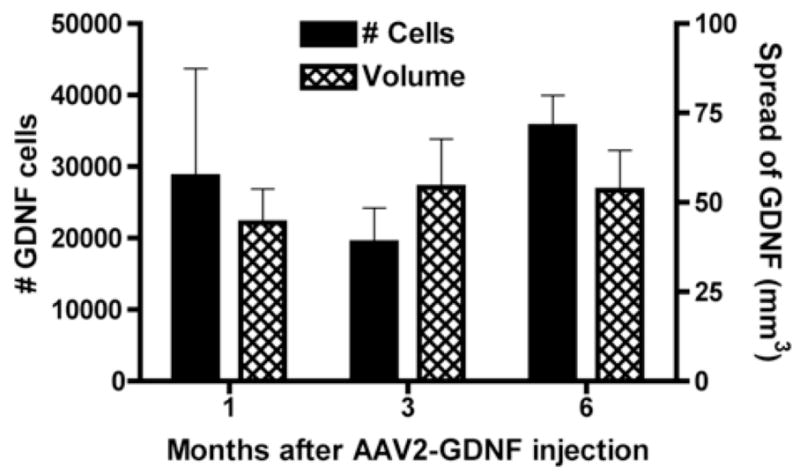
Sustained GDNF expression in caudate nucleus following injection of AAV2-GDNF. No significant change in mean number of GDNF-containing cells or mean volume of GDNF immunoreactivity for up to 6 months in normal monkeys (4 monkeys at 1 month, 3 monkeys each at 3 and 6 months). Error bars show standard error of the mean.
Effect of AAV2-GDNF injection on survival and outgrowth of grafted fetal dopamine neurons
The number of surviving grafted TH-ir cells in each caudate nucleus was counted in monkeys that received half of the donor VM in each caudate nucleus and AAV-2-GDNF in only one caudate nucleus. The side of the brain with AAV2-GDNF consistently contained more surviving TH+ cells. The increase ranged from 2- to 10-fold; a mean 4-fold greater number TH-ir neurons were in the graft on the side of the brain with AAV2-GDNF compared with the contralateral side (Figs. 3 and 4).
Fig. 3.
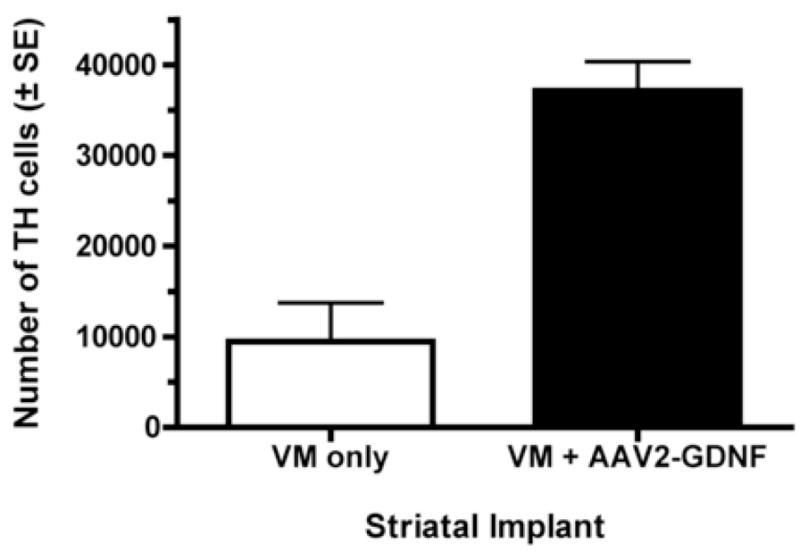
AAV2-GDNF increases grafted TH+ cell survival. The 2 halves of a fetal VM were implanted on opposite sides of the brain in the caudate nucleus of 3 MPTP-treated monkeys. One caudate nucleus also was injected with AAV2-GDNF anterior to the tissue graft. The effect of AAV2-GDNF on the survival of grafted TH+ neurons was statistically significant (paired t-test; t(2)=7.2, p<0.02). Bars represent mean ± standard error of the mean.
Fig. 4.
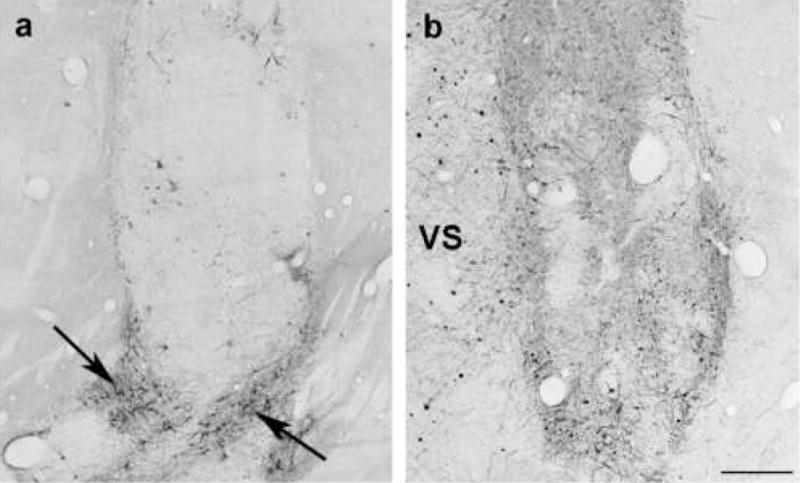
Greater number of grafted TH-stained cells is seen in the side of the brain injected with AAV2-GDNF. Each caudate nucleus received one of the halves of a fetal VM, while only one side was treated with AAV2-GDNF. The panels show the 2 caudate nuclei of the same brain (a, graft only; b, graft plus AAV2-GDNF). Some clusters of grafted TH-stained neurons survived in the absence of AAV2-GDNF (arrows). However, a more extensive pattern of TH-positive fiber outgrowth was seen on the side that received the vector (VS), and the neuropil of this graft showed a substantially higher TH fiber density. Scale bar is 500 μm.
The effect of GDNF on outgrowth of grafted fetal DA neurons was measured by comparing TH-ir fiber densities on the side of the graft facing the AAV2-GDNF (or vehicle) implant with the TH-ir fiber density on the non-implanted side of the graft. This estimate revealed that the TH-ir fiber density was 2–3 fold higher adjacent to the AAV2-GDNF implant (Fig. 5 and 6). The preferential outgrowth of TH+ toward the AAV2-GDNF injection site also was observed in the monkey that received a unilateral graft of half a fetal VM and an AAV2-GDNF injection. TH staining intensity in this additional monkey was 2.7 times higher on the side facing the vector (rostral to the graft), compared with the side opposite the vector implant site (caudal to the graft), consistent with the data obtained in monkeys with bilateral grafts. As expected, inclusion of the unilaterally grafted monkey in the statistical analysis of TH+ outgrowth did not alter the conclusion that AAV2-GDNF enhanced grafted TH+ fiber outgrowth (t (5)=9.0, p<0.0005).
Fig. 5.
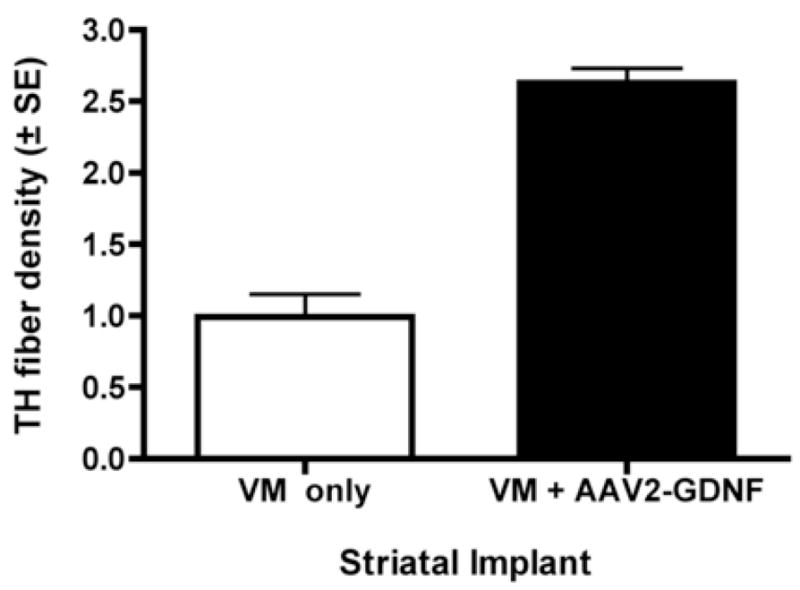
AAV2-GDNF increases grafted TH+ cell outgrowth. The effect of AAV2-GDNF on the outgrowth of grafted TH+ neurons was determined by comparing the ratio of the TH staining intensity on the side facing the AAV2-GDNF injection (rostral) with the side facing away from the vector (caudal). To control for rostral-caudal differences in TH density, the same ratio was derived for the rostral and caudal regions of fetal grafts that received a saline injection instead of the vector. A significantly greater TH staining ratio density was seen on the AAV2-GDNF injected side (paired t-test; t(2)=6.0, p<0.05).
Fig. 6.
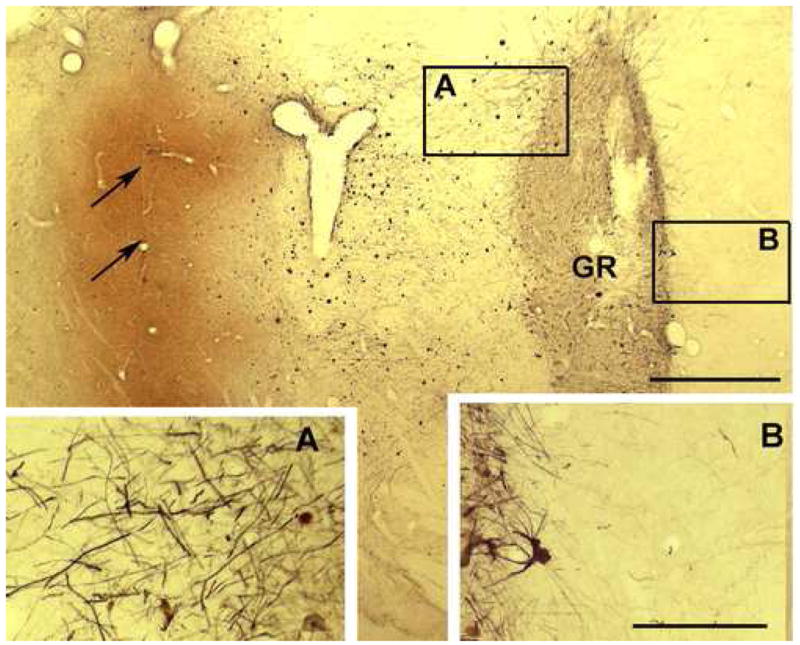
Outgrowth of fetal TH+ fibers (black) from a graft (GR) is preferentially directed (A vs. B) toward the region of GDNF staining (orange) associated with AAV2-GDNF implant (arrows). Scale bars: main figure 500μm, A and B 250μm.
Discussion
Our study of the time-course of GDNF overexpression in normal monkeys revealed that there was no decrease in expression between 1 and 6 months following injection of AAV2-GDNF in the caudate nucleus of monkeys. This outcome was in line with our expectations, based on the absence of toxicity, low immunogenicity and sustained transgene expression of AAV vectors using the chicken β-actin/cytomegalovirus (Tenenbaum et al., 2004). These results showing sustained overexpression of GDNF in normal monkeys striatum justified an investigation of the potential of AAV2-GDNF to enhance the survival and outgrowth of grafted fetal DA neurons in MPTP-treated monkeys in a relatively long-term study. In fact, the volume of striatum occupied by transduced cells in the monkey was larger than predicted by rodent studies, in which rAAV serotype 2 vectors have delivered genes only to a restricted region surrounding the injection needle (Paterna et al., 2004; Taymans et al., 2007). While this apparent difference in distribution of GDNF may be due to species or methodological differences, the observation of substantial spread of GDNF promised adequate exposure of the graft to GDNF. Intrastriatal injection AAV2-GDNF was observed to increase markedly the survival and outgrowth of co-grafted fetal TH-positive cells in the striatum of MPTP-treated monkeys when examined 6 months after implantation. Thus, these data suggest that supplementing intrastriatal fetal VM grafts with in vivo injections of viral vector delivering the GDNF gene may decrease the number of VM grafts necessary to re-supply the host striatum with adequate levels of DA. This would be a significant achievement, as the use of eight donors for one human host (Hauser et al., 1999; Olanow et al., 2003) is an obstacle to neural grafting as a treatment option for Parkinson’s disease.
In addition to striatal cells expressing GDNF, we noticed other apparently non-cellular structures that stained for GDNF. These appeared in the vicinity and beyond the area of diffuse GDNF staining. Their appearance was not dependent on the presence of fetal tissue, as they appeared in brains injected with AAV2-GDNF vector alone, and in brains from our studies injected with other vectors carrying the GDNF gene, specifically AAV5 or equine infectious anemia virus, but not in brains injected with AAV2 vector harboring the gene for green fluorescent protein. In the time course study (Fig. 2) their occurrence was more prevalent at later time points than at one month. While we do not have a definitive identification of these structures, it is possible that they are associated with extracellular matrix complexes (Lapchak et al., 1998; Ruoslahti, 1996; Venstrom and Reichardt, 1993).
There have been indications from rodent experiments that overexpression of GDNF using a recombinant lentiviral vector can lead eventually to down-regulation of TH (see Introduction and Georgievska et al., 2004a). While we do not have a time-course to suggest whether the level of TH expression is altered over the course of 6 months studied, our data showing a robust increase in TH+ cell survival and TH+ fiber outgrowth suggests that a down-regulation did not occur in our study in monkeys using a recombinant AAV. There are several plausible reasons for a more persistent beneficial effect of GDNF on TH in grafted fetal DA neurons in the monkey compared to the rat. As the development of primate brain is extended over a longer period of time than in rodents, it may be that TH in grafted DA neurons will be down-regulated in the monkey, if exposed to elevated GDNF levels for longer than the 6 months studied, possibly when the grafted neurons have reached a stage of maturity when they no longer would normally require high levels of GDNF. Alternatively, since the relationship between GDNF concentration, time of GDNF exposure and TH activity has not been defined, it may be that down-regulation of the enzyme will not occur over time if exposure to GDNF does not exceed a certain threshold. In addition, as TH in primate brain exists in multiple isoforms (Haycock, 2002) which may be differentially regulated (Lehmann et al., 2006), it is feasible that the effect of high GDNF concentrations over time will be different in the primate and rodent brain. Finally, the different parkinsonian models used here and in the rodent study (Georgievska et al., 2004a) may have a bearing on the effects of GDNF on TH. So, while no evidence of TH down-regulation was seen in the current study, further work will be necessary to determine whether, and under what conditions, this may occur in primate brain.
Some parkinsonian patients that received fetal VM grafts in recent clinical trials developed off-medication dyskinesias or graft-induced dyskinesias (GID) (Freed et al., 2001; Hagell et al., 2002; Olanow et al., 2003). The proportion of patients in these 3 studies with GID severe enough to constitute clinical therapeutic problems has been estimated to be between 7 and 15% (Hagell and Cenci, 2005). There is yet no agreement on the cause(s) of this side-effect. Possible explanations have been based on the cellular composition of the graft, the immune response, and the integration of graft and host cells, and damage from the transplantation procedure (Hagell and Cenci, 2005). A leading theory is that GID are due to an incomplete and uneven striatal DA reinnervation by fetal grafts (Carlsson et al., 2006; Hagell and Cenci, 2005; Steece-Collier et al., 2003). If this latter suggestion is correct, then targeted overexpression of GDNF in combination with fetal grafts in the striatum in Parkinson’s disease may lessen the propensity for GID, as this treatment was observed to increase cell survival and outgrowth of donor DA neurons in the MPTP-treated monkey. While a possible differential effect of GDNF on outgrowth of A9 and A10 neurons needs to be investigated in vivo (Borgal et al., 2007), the observed enhanced survival of grafted DA neurons by GDNF indicates that this combination strategy would enable fewer VM donors to be used to re-supply the parkinsonian striatum with adequate DA levels. The significance of reducing the number of donors required for one host brain in the transplantation treatment for Parkinson’s disease is that it would lessen both the trauma of the surgery and the risk of a deleterious immune response (Hagell and Cenci, 2005). Thus, there are several theoretical arguments that favor the use of fetal VM grafts together with a viral vector carrying the GDNF gene to lessen the susceptibility for development of GID in Parkinson’s disease.
Another advantage to the use of viral vector delivered GDNF to support fetal grafts in Parkinson’s disease is that GDNF by itself may provide protection of the ongoing degenerating host DA neurons. In fact, clinical trials have been conducted to examine the effect of brain infusion of GDNF on clinical outcome in patients with Parkinson’s disease. While some encouraging data emerged, side-effects were noted, which may have resulted from the high concentrations of GDNF delivered locally (Eslamboli, 2005; Kirik et al., 2004; Sherer et al., 2006). The use of viral vectors to deliver GDNF in a stable, and potentially a regulated manner, offers a more promising approach to protect remaining DA neurons in Parkinson’s disease than direct infusions of GDNF (Kordower, 2003; McBride and Kordower, 2002). Thus, in patients with Parkinson’s disease who have received fetal VM grafts, viral vector-mediated over-expression of GDNF might be expected to enhance the benefit of the transplant, in addition to retarding the neurodegeneration of the host nigrostriatal DA system.
In summary, the present studies have demonstrated a prolonged overexpression of GDNF in monkey brain following intrastriatal injection of an AAV2-GDNF, and that co-implantation of this viral vector in the vicinity of an intrastriatal fetal VM graft in the MPTP-treated monkey led to the increased survival and outgrowth of TH+ neurons. The use of viral vectors to overexpress GDNF, or other neurotrophic or neurotropic factors, in localized regions of the brain may allow neural grafting to become a viable treatment strategy for Parkinson’s disease. The beneficial effects of GDNF delivered by a viral vector reported here for fetal DA neurons also could be applied to tailor the fate of stem cells transplanted to the parkinsonian brain.
Acknowledgments
Jeremy Bober provided outstanding assistance in the immunohistochemical aspects of the work. We thank the staff of St. Kitts Biomedical Research Facility for their excellent contributions to the in vivo primate work. We are grateful to Xing-Hua Zeng and the Gene Therapy Center for expert assistance. The work was supported by NS44281 and NS40822.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn YH, Bensadoun JC, Aebischer P, Zurn AD, Seiger A, Bjorklund A, Lindvall O, Wahlberg L, Brundin P, Kaminski Schierle GS. Increased fiber outgrowth from xeno-transplanted human embryonic dopaminergic neurons with co-implants of polymer-encapsulated genetically modified cells releasing glial cell line-derived neurotrophic factor. Brain Research Bulletin. 2005;66:135–142. doi: 10.1016/j.brainresbull.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Annett LE. Functional studies of neural grafts in parkinsonian primates. In: Dunnett SB, Bjorklund A, editors. Functional Neural Transplantation. Raven Press; New York: 1994. [Google Scholar]
- Bankiewicz K, Mandel RJ, Sofroniew MV. Trophism, transplantation, and animal models of Parkinson’s disease. Exp Neurol. 1993;124:140–149. doi: 10.1006/exnr.1993.1185. [DOI] [PubMed] [Google Scholar]
- Borgal L, Hong M, Sadi D, Mendez I. Differential effects of glial cell line-derived neurotrophic factor on A9 and A10 dopamine neuron survival in vitro. Neuroscience. 2007;147:712–719. doi: 10.1016/j.neuroscience.2007.03.057. [DOI] [PubMed] [Google Scholar]
- Brundin P, Duan WM, Sauer H. Functional effects of mesencephalic dopamine neurons and adrenal chromaffin cells grafted to the rat striatum. In: Dunnett SB, Bjorklund A, editors. Functional Neural Transplantation. Raven Press: New York; 1994. pp. 9–46. [Google Scholar]
- Brundin P, Karlsson J, Emgard M, Schierle GS, Hansson O, Petersen A, Castilho RF. Improving the survival of grafted dopaminergic neurons: a review over current approaches. Cell Transplant. 2000;9:179–195. doi: 10.1177/096368970000900205. [DOI] [PubMed] [Google Scholar]
- Carlsson T, Winkler C, Lundblad M, Cenci MA, Bjorklund A, Kirik D. Graft placement and uneven pattern of reinnervation in the striatum is important for development of graft-induced dyskinesia. Neurobiol Dis. 2006;21:657–668. doi: 10.1016/j.nbd.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Bohn MC. Ontogeny and distribution of glial cell line-derived neurotrophic factor (GDNF) mRNA in rat. Brain Res Dev Brain Res. 1995;85:80–88. doi: 10.1016/0165-3806(94)00197-8. [DOI] [PubMed] [Google Scholar]
- Doucet G, Brundin P, Descarries L, Bjorklund A. Effect of prior dopamine denervation on survival and fiber outgrowth from intrastriatal fetal mesencephalic grafts. Eur J Neurosci. 1990;2:279–290. doi: 10.1111/j.1460-9568.1990.tb00419.x. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Sladek JR, Jr, Taylor JR, Collier TJ, Redmond DE, Jr, Roth RH. Early gestational mesencephalon grafts, but not later gestational mesencephalon, cerebellum or sham grafts, increase dopamine in caudate nucleus of MPTP-treated monkeys. Neuroscience. 1996;72:477–484. doi: 10.1016/0306-4522(95)00564-1. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Taylor JR, Sladek JR, Jr, Collier TJ, Redmond DE, Jr, Roth RH. Striatal dopaminergic correlates of stable parkinsonism and degree of recovery in old-world primates one year after MPTP treatment. Neuroscience. 2000;95:399–408. doi: 10.1016/s0306-4522(99)00437-6. [DOI] [PubMed] [Google Scholar]
- Eslamboli A. Assessment of GDNF in primate models of Parkinson’s disease: comparison with human studies. Rev Neurosci. 2005;16:303–310. doi: 10.1515/revneuro.2005.16.4.303. [DOI] [PubMed] [Google Scholar]
- Espejo M, Cutillas B, Arenas TE, Ambrosio S. Increased survival of dopaminergic neurons in striatal grafts of fetal ventral mesencephalic cells exposed to neurotrophin-3 or glial cell line-derived neurotrophic factor. Cell Transplant. 2000;9:45–53. doi: 10.1177/096368970000900107. [DOI] [PubMed] [Google Scholar]
- Fine A, Hunt SP, Oertel WH, Nomoto M, Chong PN, Bond A, Waters C, Temlett JA, Annett L, Dunnett S, Jenner P, Marsden CD. Transplantation of embryonic marmoset dopaminergic neurons to the corpus striatum of marmosets rendered parkinsonian by 1-methyl-4-phenyl-1,2-3,6-tetrahydropyridine. Prog Brain Res. 1988;78:479–489. doi: 10.1016/s0079-6123(08)60321-0. [DOI] [PubMed] [Google Scholar]
- Freed CR, Breeze RE, Rosenberg NL, Schneck SA, Kriek E, Qi JX, Lone T, Zhang YB, Snyder JA, Wells TH, et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson’s disease. N Engl J Med. 1992;327:1549–1555. doi: 10.1056/NEJM199211263272202. [DOI] [PubMed] [Google Scholar]
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Georgievska B, Carlsson T, Lacar B, Winkler C, Kirik D. Dissociation between short-term increased graft survival and long-term functional improvements in Parkinsonian rats overexpressing glial cell line-derived neurotrophic factor. Eur J Neurosci. 2004a;20:3121–3130. doi: 10.1111/j.1460-9568.2004.03770.x. [DOI] [PubMed] [Google Scholar]
- Georgievska B, Kirik D, Bjorklund A. Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induces time- and dose-dependent downregulation of tyrosine hydroxylase in the intact nigrostriatal dopamine system. J Neurosci. 2004b;24:6437–6445. doi: 10.1523/JNEUROSCI.1122-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- Grieger JC, Samulski RJ. Adeno-associated virus as a gene therapy vector: vector development, production and clinical applications. Adv Biochem Eng Biotechnol. 2005;99:119–145. [PubMed] [Google Scholar]
- Hagell P, Cenci MA. Dyskinesias and dopamine cell replacement in Parkinson’s disease: a clinical perspective. Brain Res Bull. 2005;68:4–15. doi: 10.1016/j.brainresbull.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Hagell P, Piccini P, Bjorklund A, Brundin P, Rehncrona S, Widner H, Crabb L, Pavese N, Oertel WH, Quinn N, Brooks DJ, Lindvall O. Dyskinesias following neural transplantation in Parkinson’s disease. Nat Neurosci. 2002;5:627–628. doi: 10.1038/nn863. [DOI] [PubMed] [Google Scholar]
- Hauser RA, Freeman TB, Snow BJ, Nauert M, Gauger L, Kordower JH, Olanow CW. Long-term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch Neurol. 1999;56:179–187. doi: 10.1001/archneur.56.2.179. [DOI] [PubMed] [Google Scholar]
- Haycock JW. Species differences in the expression of multiple tyrosine hydroxylase protein isoforms. J Neurochem. 2002;81:947–953. doi: 10.1046/j.1471-4159.2002.00881.x. [DOI] [PubMed] [Google Scholar]
- Kirik D, Georgievska B, Bjorklund A. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci. 2004;7:105–110. doi: 10.1038/nn1175. [DOI] [PubMed] [Google Scholar]
- Kordower JH. In vivo gene delivery of glial cell line--derived neurotrophic factor for Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S120–132. doi: 10.1002/ana.10485. discussion S132–124. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Hilt DC, Jiao S, Collin F, Miyoshi Y, Yi A, Zhang Z, Gash DM. Topographical distribution of [125I]-glial cell line-derived neurotrophic factor in unlesioned and MPTP-lesioned rhesus monkey brain following a bolus intraventricular injection. Brain Res. 1998;789:9–22. doi: 10.1016/s0006-8993(97)01495-9. [DOI] [PubMed] [Google Scholar]
- Lehmann IT, Bobrovskaya L, Gordon SL, Dunkley PR, Dickson PW. Differential regulation of the human tyrosine hydroxylase isoforms via hierarchical phosphorylation. J Biol Chem. 2006;281:17644–17651. doi: 10.1074/jbc.M512194200. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Hagell P. Cell therapy and transplantation in Parkinson’s disease. Clin Chem Lab Med. 2001;39:356–361. doi: 10.1515/CCLM.2001.056. [DOI] [PubMed] [Google Scholar]
- McBride JL, Kordower JH. Neuroprotection for Parkinson’s disease using viral vector-mediated delivery of GDNF. Prog Brain Res. 2002;138:421–432. doi: 10.1016/S0079-6123(02)38091-9. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Kordower JH, Freeman TB. Fetal nigral transplantation as a therapy for Parkinson’s disease. Trends Neurosci. 1996;19:102–109. doi: 10.1016/s0166-2236(96)80038-5. [DOI] [PubMed] [Google Scholar]
- Paterna JC, Feldon J, Bueler H. Transduction profiles of recombinant adeno-associated virus vectors derived from serotypes 2 and 5 in the nigrostriatal system of rats. J Virol. 2004;78:6808–6817. doi: 10.1128/JVI.78.13.6808-6817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond DE., Jr Cellular replacement therapy for Parkinson’s disease--where we are today? Neuroscientist. 2002;8:457–488. doi: 10.1177/107385802237703. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Jr, Sladek JR, Jr, Roth RH, Elsworth JD, Collier TJ, Leranth C, Taylor JR, Sortwell CE, Bloch J, Aebischer P. Restoring normal nigrostriatal anatomy in dopamine-depleted monkeys. Exp Neurol. 2002;175:419. [Google Scholar]
- Rosenblad C, Martinez-Serrano A, Bjorklund A. Glial cell line-derived neurotrophic factor increases survival, growth and function of intrastriatal fetal nigral dopaminergic grafts. Neuroscience. 1996;75:979–985. doi: 10.1016/0306-4522(96)00343-0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Brain extracellular matrix. Glycobiology. 1996;6:489–492. doi: 10.1093/glycob/6.5.489. [DOI] [PubMed] [Google Scholar]
- Sautter J, Tseng JL, Braguglia D, Aebischer P, Spenger C, Seiler RW, Widmer HR, Zurn AD. Implants of polymer-encapsulated genetically modified cells releasing glial cell line-derived neurotrophic factor improve survival, growth, and function of fetal dopaminergic grafts. Exp Neurol. 1998;149:230–236. doi: 10.1006/exnr.1997.6718. [DOI] [PubMed] [Google Scholar]
- Schaar DG, Sieber BA, Dreyfus CF, Black IB. Regional and cell-specific expression of GDNF in rat brain. Exp Neurol. 1993;124:368–371. doi: 10.1006/exnr.1993.1207. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Fiske BK, Svendsen CN, Lang AE, Langston JW. Crossroads in GDNF therapy for Parkinson’s disease. Mov Disorder. 2006;21:136–141. doi: 10.1002/mds.20861. [DOI] [PubMed] [Google Scholar]
- Sinclair SR, Svendsen CN, Torres EM, Martin D, Fawcett JW, Dunnett SB. GDNF enhances dopaminergic cell survival and fibre outgrowth in embryonic nigral grafts. Neuroreport. 1996;7:2547–2552. doi: 10.1097/00001756-199611040-00029. [DOI] [PubMed] [Google Scholar]
- Sladek JR, Jr , Elsworth JD, Taylor JR, Roth RH, Redmond DE., Jr . Techniques for neural transplantation in non-human primates. In: Ricordi C, Landes RG, editors. Techniques for Neural Transplantation. CRC Press: Austin; 1995. pp. 391–408. [Google Scholar]
- Sondhi D, Peterson DA, Giannaris EL, Sanders CT, Mendez BS, De B, Rostkowski AB, Blanchard B, Bjugstad K, Sladek JR, Jr, Redmond DE, Jr, Leopold PL, Kaminsky SM, Hackett NR, Crystal RG. AAV2-mediated CLN2 gene transfer to rodent and non-human primate brain results in long-term TPP-I expression compatible with therapy for LINCL. Gene Therapy. 2005;12:1618–1632. doi: 10.1038/sj.gt.3302549. [DOI] [PubMed] [Google Scholar]
- Spencer DD, Robbins RJ, Naftolin F, Marek KL, Vollmer T, Leranth C, Roth RH, Price LH, Gjedde A, Bunney BS, et al. Unilateral transplantation of human fetal mesencephalic tissue into the caudate nucleus of patients with Parkinson’s disease. N Engl J Med. 1992;327:1541–1548. doi: 10.1056/NEJM199211263272201. [DOI] [PubMed] [Google Scholar]
- Steece-Collier K, Collier TJ, Danielson PD, Kurlan R, Yurek DM, Sladek JR., Jr Embryonic mesencephalic grafts increase levodopa-induced forelimb hyperkinesia in parkinsonian rats. Mov Disorder. 2003;18:1442–1454. doi: 10.1002/mds.10588. [DOI] [PubMed] [Google Scholar]
- Stromberg I, Bjorklund L, Johansson M, Tomac A, Collins F, Olson L, Hoffer B, Humpel C. Glial cell line-derived neurotrophic factor is expressed in the developing but not adult striatum and stimulates developing dopamine neurons in vivo. Exp Neurol. 1993;124:401–412. doi: 10.1006/exnr.1993.1214. [DOI] [PubMed] [Google Scholar]
- Sullivan AM, Pohl J, Blunt SB. Growth/differentiation factor 5 and glial cell line-derived neurotrophic factor enhance survival and function of dopaminergic grafts in a rat model of Parkinson’s disease. Eur J Neurosci. 1998;10:3681–3688. doi: 10.1046/j.1460-9568.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Roth RH, Sladek JR, Jr, Collier TJ, Redmond DE., Jr Grafting of fetal substantia nigra to striatum reverses behavioral deficits induced by MPTP in primates: a comparison with other types of grafts as controls. Exp Brain Res. 1991;85:335–348. doi: 10.1007/BF00229411. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Roth RH, Sladek JR, Jr, Redmond DE., Jr . Behavioral effects of MPTP administration in the vervet monkey: a primate model of Parkinson’s disease. In: Woodruff ML, Nonneman AJ, editors. Toxin-Induced Models of Neurological Disorders. Plenum Press: New York; 1994. pp. 139–174. [Google Scholar]
- Taylor JR, Elsworth JD, Roth RH, Sladek JR, Jr, Redmond DE., Jr Severe long-term 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in the vervet monkey (Cercopithecus aethiops sabaeus) Neuroscience. 1997;81:745–755. doi: 10.1016/s0306-4522(97)00214-5. [DOI] [PubMed] [Google Scholar]
- Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L, Wilson JM, Debyser Z, Baekelandt V. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum Gene Ther. 2007;18:195–206. doi: 10.1089/hum.2006.178. [DOI] [PubMed] [Google Scholar]
- Tenenbaum L, Chtarto A, Lehtonen E, Velu T, Brotchi J, Levivier M. Recombinant AAV-mediated gene delivery to the central nervous system. J Gene Med. 2004;6(Suppl 1):S212–222. doi: 10.1002/jgm.506. [DOI] [PubMed] [Google Scholar]
- Venstrom KA, Reichardt LF. Extracellular matrix. 2: Role of extracellular matrix molecules and their receptors in the nervous system. Faseb J. 1993;7:996–1003. doi: 10.1096/fasebj.7.11.8370483. [DOI] [PubMed] [Google Scholar]
- Widner H, Tetrud J, Rehncrona S, Snow B, Brundin P, Gustavii B, Bjorklund A, Lindvall O, Langston JW. Bilateral fetal mesencephalic grafting in two patients with parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) N Engl J Med. 1992;327:1556–1563. doi: 10.1056/NEJM199211263272203. [DOI] [PubMed] [Google Scholar]
- Wilby MJ, Sinclair SR, Muir EM, Zietlow R, Adcock KH, Horellou P, Rogers JH, Dunnett SB, Fawcett JW. A glial cell line-derived neurotrophic factor-secreting clone of the Schwann cell line SCTM41 enhances survival and fiber outgrowth from embryonic nigral neurons grafted to the striatum and to the lesioned substantia nigra. J Neurosci. 1999;19:2301–2312. doi: 10.1523/JNEUROSCI.19-06-02301.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurek DM. Glial cell line-derived neurotrophic factor improves survival of dopaminergic neurons in transplants of fetal ventral mesencephalic tissue. Exp Neurol. 1998;153:195–202. doi: 10.1006/exnr.1998.6884. [DOI] [PubMed] [Google Scholar]


