Abstract
γ-Aminobutyric acid type A (GABAA) receptors are an important target for general anesthetics in the central nervous system. Site-directed mutagenesis techniques have identified amino acid residues that are important for the positive modulation of GABAA receptors by general anesthetics. In the present study, we investigate the role of an amino acid residue in transmembrane (TM) domain 3 of the GABAA receptor β2 subunit for modulation by the general anesthetic 2,6-diisopropylphenol (propofol). Mutation of methionine 286 to tryptophan (M286W) in the β2 subunit abolished potentiation of GABA responses by propofol but did not affect direct receptor activation by propofol in the absence of GABA. In contrast, substitution of methionine 286 by alanine, cysteine, glutamate, lysine, phenylalanine, serine, or tyrosine was permissive for potentiation of GABA responses and direct activation by propofol. Using propofol analogs of varying molecular size, we show that the β2(M286W) mutation resulted in a decrease in the ‘cut-off’ volume for propofol analog molecules to enhance GABA responses at GABAAα1β2γ2s receptors. This suggests that mutation of M286 in the GABAA β2 subunit alters the dimensions of a ‘binding pocket’ for propofol and related alkylphenol general anesthetics.
1. Introduction
2,6-Diisopropylphenol (propofol) has proven to be a useful intravenous general anesthetic since its introduction into clinical practice in the 1980s. There have been a number of efforts to understand the molecular mechanism of action of this clinically useful drug (Trapani et al., 2000). One hypothesis, supported by substantial experimental evidence, cites the ability of propofol to positively modulate the function of γ-aminobutyric acid type A (GABAA) receptors, a property shared with other general anesthetics (Franks and Lieb, 1994; Belelli et al., 1999; Krasowski and Harrison, 1999; Trapani et al., 2000). Propofol has been shown in electrophysiological assays to allosterically enhance (potentiate) the actions of GABA at the GABAA receptor (Hales and Lambert, 1991). In addition, propofol prolongs inhibitory postsynaptic currents mediated by GABAA receptors (Bai et al., 2001) and alters receptor deactivation and desensitization (Bai et al., 1999). Propofol can also open the GABAA receptor ion channel in the absence of GABA (termed ‘direct activation’), although this usually occurs at higher concentrations of propofol than necessary to potentiate responses to GABA (Hales and Lambert, 1991; Hara et al., 1993).
Molecular pharmacology studies have defined individual amino acid residues within GABAA receptor molecules that are critical for the allosteric effects of general anesthetics (Belelli et al., 1999; Krasowski and Harrison, 1999). Progress in this effort followed from the observation that receptors related to the GABAA receptor, such as the strychnine-sensitive glycine receptor (Mascia et al., 1996) and the GABAC ρ receptor (Mihic and Harris, 1996) differ in their sensitivity to general anesthetics. Amino acid residues in transmembrane (TM) domains 2 and 3 of GABAA receptor α and β subunits are particularly important for the modulatory actions of ether, alkane, and alcohol anesthetics (Mihic et al., 1997; Krasowski et al., 1998a,b; Koltchine et al., 1999; Ueno et al. 1999, 2000; Krasowski and Harrison, 2000; Jenkins et al., 2001) (Fig. 1), as well as for certain intravenous anesthetics such as propofol, etomidate, and the barbiturates (Amin, 1999; Belelli et al., 1999). In the case of propofol, mutation of methionine 286 to tryptophan (M286W) in TM3 of the GABAAβ1 receptor subunit abolishes potentiation of GABA responses (Krasowski et al., 1998b).
Fig. 1.
Amino acid sequence alignment of TM2 and TM3 from human glycine α1 (Grenningloh et al., 1987), GABAA α1 (Schofield et al., 1989), α2 (Hadingham et al., 1993a), β1 (Schofield et al., 1989), rat or human GABAA β2 (Ymer et al., 1989; Hadingham et al., 1993b), and human GABAA ρ1 receptor subunits (Cutting et al., 1991). Residue positions in bold type within TM2 and TM3 of glycine α1 (S267 and A288), GABAA α1 and α2 (S270 and A291), GABAA β1 (S265 and M286), and GABAA β2 (N265 and M286) receptor subunits are critical for potentiation of agonist responses by alcohol, alkane, and ether anesthetics (Mihic et al., 1997; Ye et al., 1998; Krasowski et al., 1998a; Koltchine et al., 1999; Ueno et al. 1999, 2000; Yamakura et al., 1999; Krasowski and Harrison, 2000; Jenkins et al., 2001). GABAA β1(M286) is necessary for potentiation of GABA responses by propofol (Krasowski et al., 1998b). GABAA β2(M286) is the main residue position of interest for the current study. GABAA α1(S270), α1(A291), and β2(N265) are also considered in this paper.
In the present study we further define the importance of this TM3 residue of the β subunit for the modulatory effects of propofol. We also test the effect of tryptophan substitution within TM2 of the β2 subunit and within TM2 and TM3 of the α1 subunit. The final objective was to utilize propofol analogs of different molecular size (Krasowski et al., 2001) to test whether amino acid substitutions for GABAA β2(M286) can alter the ‘cut-off’ for propofol analogs in potentiating GABA responses at GABAAα1β2γ2s receptors. The cut-off for a homologous series of molecules at a given receptor is believed to reflect a binding pocket of limited dimensions, such that molecules beyond a certain size cannot fit into the binding pocket (Franks and Lieb, 1994; Krasowski and Harrison, 1999; Dwyer and Bradley, 2000). The classic homologous series to show cut-off is the n-alcohols, which exhibit cut-offs specific for different ligand-gated ion channels (Li et al., 1994; Peoples and Weight, 1995; Mascia et al., 1996; Mihic and Harris, 1996). This has been taken to suggest that these receptors contain cavities of discrete size for the alcohols, which would be consistent with the finding that alcohol cut-off for modulation of glycine α1 and GABAC ρ receptors can be manipulated by mutagenesis within TM2 and TM3 (Wick et al., 1998). The idea in the present study was to investigate whether amino acid substitutions at GABAAβ2(M286) could produce a similar effect for analogs to propofol and thereby test the hypothesis thatβ2(M286) influences the dimensions of a propofol ‘binding pocket’.
2. Methods
2.1. Site-directed mutagenesis
The GABAAα1 (Schofield et al., 1989) and γ2s (Pritchett et al., 1989) receptor subunit cDNAs are of human origin. The GABAAβ2 receptor subunit cDNA is from the rat (Ymer et al., 1989). The human GABAAα1 and γ2s receptor subunit cDNAs were generously provided by the late Dr Dolan Pritchett (University of Pennsylvania, Philadelphia, PA, USA). The rat GABAA β2 subunit cDNA was provided by Dr Dennis Grayson (University of Illinois at Chicago, Chicago, IL, USA).
The β2(N265W), β2(M286F), and β2(M286Y) mutations were introduced by the unique site elimination method using the USE kit (Pharmacia Biotech, Piscataway, NJ, USA) as previously described (Krasowski et al., 1998a,b). For the α1(S270W,A291W) double mutation, the S270W and A291W mutations were initially introduced separately. The vector containing the S270W mutation was digested with Bsu36I and NotI (New England Biolabs) to create a 1 kb fragment, which then replaced the same fragment in the similarly digested vector containing the A291W mutation.
The β2(M286A), β2(M286C), β2(M286E), β2(M286K), β2(M286S), and β2(M286W) mutations in the GABAA β2 subunit were introduced with the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA, USA) as previously described (Krasowski et al., 1998a,b). Positive clones were confirmed by double-stranded sequencing of the entire cDNA insert.
2.2. Cell culture and transfection of receptor cDNAs
GABAA receptor cDNAs were expressed via the vector pCIS2 which contains one copy of the strong promoter from cytomegalovirus and a polyadenylation sequence from SV40. Human embryonic kidney (HEK) 293 cells (American Type Culture Collection, Rockville, MD, USA) were maintained in culture and passaged weekly by trypsin treatment for a maximum of 20 times before being discarded and replaced with early passage cells. HEK 293 cells were maintained in Eagle’s minimum essential medium (Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA), l-glutamine (0.292 μg/ml; Gibco BRL, Grand Island, NY, USA), penicillin G sulfate (100 units/ml; Gibco BRL), and streptomycin sulfate (100 μg/ml; Gibco BRL).
For electrophysiological experiments, cells were plated on glass coverslips coated with poly-D-lysine (Sigma). Each coverslip of cells was individually transfected with the calcium phosphate precipitation technique as previously described (Krasowski et al., 1998a). Each transfection used 1–5 μg of each cDNA; the cDNA was in contact with the cells for 24 h under an atmosphere containing 3% CO2 before being removed and replaced with fresh culture medium in an atmosphere of 5% CO2.
2.3. Electrophysiology and design of pharmacology experiments
Stock solutions of GABA (Sigma) and the propofol analogs were diluted into extracellular solution daily before use. The propofol analogs were all prepared as stock solutions in dimethyl sulfoxide before being dissolved in the extracellular medium. The maximum final concentration of dimethyl sulfoxide was 0.2% (v/v), which was determined during carrier control experiments to have no significant effect on GABA-induced currents in the GABAA receptors analyzed in this study.
Electrophysiological recordings were performed at room temperature using the whole-cell patch clamp technique as previously described (Krasowski et al., 1998a,b). The coverslips were transferred 48–96 h after removal of the cDNA to a large chamber that was continuously perfused (2–3 ml/min) with extracellular medium containing (in mM): 145 NaCl, 3 KCl, 1.5 CaCl2, 1 MgCl2, 5.5 D-glucose, and 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.4, osmolarity 320–330 mOsmol. The electrode solution contained (in mM): 145 N-methyl-D-glucamine hydrochloride, 5 K2ATP, 5 HEPES/KOH, 2 MgCl2, 0.1 CaCl2, and 1.1 ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), pH 7.2, osmolarity 315 mOsmol. Pipette-to-bath resistance was 4–6 MΩ. Cells were voltage-clamped at _60 mV. Since the intracellular and extracellular solutions contained symmetrical chloride concentrations, the chloride equilibrium potential was approximately 0 mV.
All drugs were applied to the cell by local perfusion (Krasowski et al., 1998a,b) using a motor-driven solution exchange device (Bio Logic Rapid Solution Changer RSC-100; Molecular Kinetics, Pullman, WA, USA). Laminar flow was maintained by applying all solutions at identical flow rates via a multi-channel infusion pump (Stoelting, Wood Dale, IL, USA). The solution changer was driven by protocols in the acquisition program pCLAMP5 (Axon Instruments, Foster City, CA, USA). Responses were low-pass-filtered at 5 kHz and digitized (TL-1-125 interface; Axon Instruments) using pCLAMP5 and stored for off-line analysis.
Potentiation of GABA responses by propofol and other drugs was always assessed by co-application of the drug with an EC20 GABA concentration (i.e. the concentration of GABA that produces 20% of the maximal response to GABA). Anesthetics were always preapplied prior to co-application with GABA to ensure that the anesthetic had reached equilibrium with the receptor. At the end of each potentiation experiment, a maximal GABA response was elicited by an appropriately high concentration of GABA to verify that the average control GABA response fell between EC10 and EC30. This experimental design permits comparison not only of anesthetic EC50 between various receptor combinations but also of the maximal magnitude (relative efficacy) of agonist potentiation between anesthetics (Krasowski and Harrison, 2000). Anesthetic direct activation currents were expressed as a fraction of the maximal current response to GABA.
2.4. Data analysis
Drug-induced potentiation of a GABA-induced current was defined as the percentage increase of the control GABA response (defined as the average of the pre-drug and post-drug GABA-induced currents). Concentration response data were fitted (KaleidaGraph; Reading, PA, USA) with the equation: I/Imax=100[drug]nH/([drug]nH+(EC50)nH), where I/Imax is the percentage of the maximum obtainable response, EC50 the concentration producing a half-maximal response, and nH is the Hill coefficient. Pooled data are presented throughout as mean±SE. Statistical significance was determined by two-way analysis of variance with Dunnet’s post-hoc test, unless otherwise specified.
2.5. Molecular volume of the propofol analogs and amino acid residues
Molecular volumes for the propofol analogs were calculated using the cerius2 qsar software version 3.0 (Molecular Simulations, San Diego, CA, USA). The molecular volumes of the propofol analogs are listed in Fig. 7. The molecular volumes of the amino acid residue side chains considered in this study are (Harpaz et al., 1994): alanine (A; 0.026 nm3), asparagine (N; 0.064 nm3), cysteine (C; 0.04 nm3), glutamic acid (E; 0.077 nm3), glycine (G; 0 nm3), lysine (K; 0.106 nm3), methionine (M; 0.104 nm3), phenylalanine (F; 0.13 nm3), serine (S; 0.03 nm3), tryptophan (W; 0.168 nm3), tyrosine (Y; 0.133 nm3), and valine (V; 0.075 nm3).
Fig. 7.
Summary of the effects of six propofol analogs on the wild-type GABAA α1β2γ2s receptor and the GABAA α1β2(M286A)γ2s, α1β2(M286S)γ2s, and α1β2(M286W)γ2s receptors. The wild-type GABAA α1β2γ2s receptor supports potentiation of GABA responses by 2,6-dimethylphenol, 2-isopropylphenol, 2,6-diethylphenol, 2,6-diethylphenyl isothiocyanate, and propofol, but not the bulkier analog 2,6-di-tert-butylphenol. The mutation of methionine 286 to the smaller alanine or serine residues does not change this profile. On the other hand, the β2(M286W) mutation results in a receptor now insensitive to 2,6- diethylphenol, 2,6-diethylphenyl isothiocyanate, and propofol, as well as 2,6-di-tert-butylphenol. Thus, the molecular volume cut-off for propofol potentiation of GABA responses is reduced from 0.193 nm3 in the GABAA α1β2γ2, α1β2(M286A)γ2s, and α1β2(M286S)γ2s receptors (‘Wildtype cut-off’, single arrow) to less than 0.16 nm3 in the GABAA α1β2(M286W)γ2s receptor (‘Altered cut-off’, double arrow).
2.6. Sources of drugs
The sources of the propofol analogs and other anesthetics were as follows: 2,6-dimethylphenol, 2-isopropylphenol (Aldrich Chemical Co., Milwaukee, WI, USA), 2,6-diethylphenyl isothiocyanate (Lancaster Synthesis, Windham, NH, USA), and methohexital sodium (Brevital sodium; Eli Lilly and Co., Indianapolis, IN, USA). Each analog was of the highest purity grade available commercially. Propofol, 2,6-diethylphenol, and 2,6-di-tert-butylphenol were generously provided by Drs. J.B. Glen and Roger James of Zeneca Pharmaceuticals (Macclesfield, Cheshire, UK; see James and Glen, 1980). Research-grade etomidate was a generous gift from Professor Alfred Doenicke (Institute of Anesthesiology, Ludwig Maximillians University of Munchen, Germany).
3. Results
3.1. Receptor characteristics and GABA concentration–response relationships for wild-type and mutated GABAAα1β2γ2s receptors
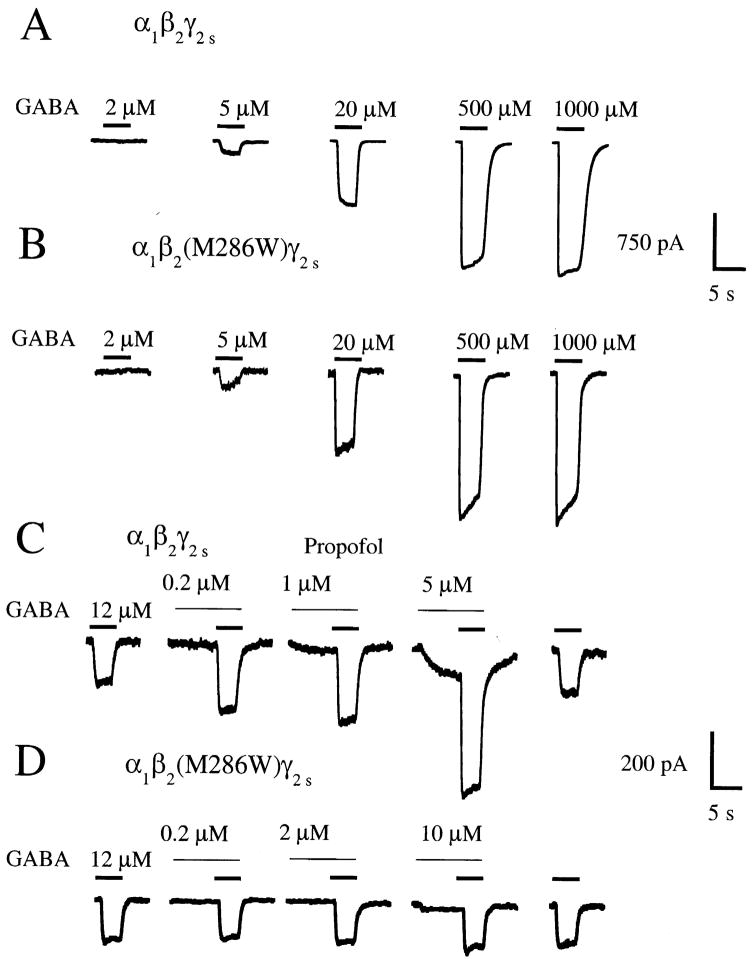
The wild-type GABAA receptor and all of the receptors containing point mutations in the α1 or β2 subunits responded to GABA in a concentration-dependent manner. Fig. 2(A) and (B) shows responses to GABA for the wild-type GABAAα1β2γ2s receptor and for the GABAAα1β2(M286W)γ2s receptor. Fig. 3 displays GABA concentration–response curves for the wild-type GABAAα1β2γ2s receptor and for the series of eightα1β2(M286X)γ2s mutated receptors.
Fig. 2.
GABAA α1β2γ2s and α1β2(M286W)γ2s receptors have similar sensitivity to GABA, but the α1β2(M286W)γ2s receptor is insensitive to the modulatory effects of propofol. (A) and (B) Traces from individual HEK 293 cells expressing either GABAA α1β2γ2s or GABAA α1β2(M286W)γ2s receptors in response to application of 2, 5, 20, 500, and 1000 μM GABA. Time of application is indicated by the bars above the traces. (C) Submaximal (EC20) GABA current responses at the wild-type GABAA α1β2γ2s receptor are enhanced by co-application of 0.2, 1, or 5 μM propofol. Note also the direct activation by 1 and 5 μM propofol, evident during the pre-application of propofol. (D) In contrast, propofol does not potentiate submaximal GABA currents at the GABAA α1β2(M286W)γ2s receptor, but does produce direct receptor activation at a concentration of 10 μM. Traces shown in (A)–(D) are recordings from individual HEK 293 cells transfected with cDNAs encoding the indicated receptor subunit combinations.
Fig. 3.
Concentration–response curves for GABA at the wild-type GABAA α1β2γ2s receptor and the α1β2(M286X)γ2s series of mutant receptors (see Table 1 for parameters derived by curve fitting to the pooled data).
The EC50 values for GABA for the α1β2(M286X)γ2s series of receptors varied over approximately one order of magnitude, with the greatest absolute difference found in the α1β2(M286C)γ2s receptor, which had a 4.5-fold higher EC50 to GABA than the α1β2γ2s receptor. GABA EC50, Hill slope, and maximal response data for all receptors are summarized in Table 1. In contrast to the relatively modest effects on GABA sensitivity produced by the series of β2(M286X) mutant subunits, the GABAA α1(S270W,A291W)β2γ2s receptor had an EC50 value for GABA 25 times lower than that for the wild-type GABAAα1β2γ2s receptor. This is consistent with the previously described effects of theα2(S270W) andα2(A291W) mutations, which shift the GABA concentration–response relationship for the GABAAα2β1γ2s receptor to the left by 7.1- and 3.4-fold, respectively (Krasowski et al., 1998b; Koltchine et al., 1999).
Table 1.
Summary data of concentration-response relationships for GABA activation and for potentiation of GABA responses and direct activation by propofol at wild-type and mutant GABAA receptors expressed in HEK 293 cellsa
| GABA activation | Propofol potentiation of GABA responses | Propofol direct activation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GABAA receptor combination | EC50 (μM) | Hill slope | ncells | Maximal GABA response (pA) | ncells | EC50 (μM) | Hill slope | Emax | ncells | EC50 (μM) | Hill slope | Emax | ncells |
| α1β2γ2s | 29.5±2.4 | 1.4±0.1 | 7 | 631±54 | 300 | 1.5±0.2 | 1.8±0.5 | 241±16 | 16 | 10.4±0.8 | 1.4±0.1 | 59.8±1.6 | 10–16 |
| α1β2(N265W)γ2s | 9.1±1.1*** | 1.4±0.2 | 8 | 468±172 | 8 | 2.3±1.1 | 1.2±0.5 | 205±39 | 5 | 12.4±1.8 | 1.3±0.2 | 32.9±1.5*** | 5 |
| α1β2(M286A)γ2s | 10.6±0.8*** | 1.2±0.1 | 7 | 447±172 | 25 | 2.3±0.4 | 2.0±0.5 | 149±10** | 6 | 11.7±1.0 | 2.7±0.6 | 51.2±3.9 | 4 |
| α1β2(M286C)γ2s | 132±12*** | 1.1±0.1 | 6 | 346±51 | 19 | 1.5±0.1 | 3.7±0.7 | 126±5*** | 6 | 41.8±10.8* | 2.2±0.9 | 41.0±5.9 | 6 |
| α1β2(M286E)γ2s | 13.6±1.1*** | 1.1±0.1 | 9 | 519±95 | 22 | 4.2±0.7* | 2.4±0.8 | 77±7.7*** | 6 | 49.3±2.2*** | 1.8±0.1 | 49.3±3.8 | 6 |
| α1β2(M286F)γ2s | 11.9±0.6*** | 1.7±0.1 | 9 | 315±58* | 9 | 2.6±0.2* | 1.7±0.2 | 285±9.0 | 7 | 27.7±1.8*** | 1.4±0.1 | 53.5±1.3 | 5 |
| α1β2(M286K)γ2s | 98.9±3.8*** | 1.6±0.1 | 7 | 371±36* | 17 | 2.6±0.6 | 2.8±1.3 | 98±10*** | 6 | 51.7±4.9*** | 3.0±0.8 | 68.0±4.7 | 5 |
| α1β2(M286S)γ2s | 24.7±2.4 | 1.0±0.1 | 7 | 570±79 | 20 | 6.8±0.6*** | 2.3±0.4 | 239±16 | 6 | 9.0±1.4 | 2.0±0.6 | 50.6±3.0 | 5 |
| α1β2(M286W)γ2s | 24.8±2.6 | 1.3±0.2 | 7 | 508±118 | 41 | none*** | N/A | N/A | 7 | 24.1±3.5* | 2.7±0.9 | 51.5±5.5 | 11 |
| α1β2(M286Y)γ2s | 60.0±4.8*** | 1.5±0.1 | 10 | 441±62 | 10 | 3.9±0.5*** | 2.4±0.5 | 102±7*** | 9 | 14.8±2.2 | 1.7±0.3 | 49.3±2.2* | 5 |
| α1(S270I,A291W)β2γ2s | 1.2±0.1*** | 1.1±0.1 | 5 | 250±82** | 5 | 2.0±0.2*** | 2.3±0.4 | 141±5*** | 5 | N/A | N/A | N/A | N/A |
The EC50, Hill slope, and maximal effect values are presented as mean±SE. ncells indicates number of cells contributing to mean values.
P<0.05,
P<0.01,
P<0.0001 compared with response in wild-type GABAA α1β2γ2s receptor combination.
3.2. Propofol pharmacology of wild-type and mutant GABAAα1β2γ2s receptors
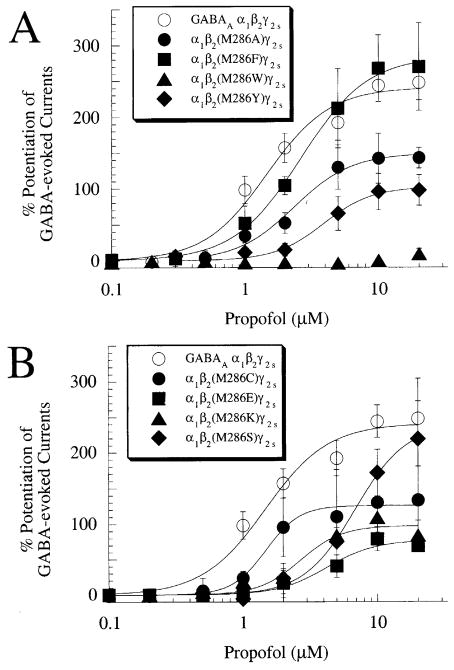
Propofol potentiated submaximal (EC20) GABA responses at the wild-type GABAA α1β2γ2s receptor (Figs. 2(C) and 4) but failed to enhance submaximal GABA responses at the GABAA α1β2(M286W)γ2s receptor (Figs. 2(D) and 4(A); Table 1). In contrast to the lack of potentiation of GABA responses, propofol directly activated the GABAA α1β2(M286W)γ2s receptor, although with a 2.3-fold higher EC50 value than at the wild-type GABAA α1β2γ2s receptor (Figs. 2(D) and 5(A); Table 1). Complete concentration–response curves for potentiation of GABA responses and direct activation by propofol for the wild-type GABAA α1β2γ2s receptor and for the series of α1β2(M286X)γ2s mutated receptors are illustrated in Figs. 4 and 5, with summary of data for all receptors presented in Table 1.
Fig. 4.
Concentration–response relationships for potentiation of GABA responses by propofol at the wild-type GABAA α1β2γ2s receptor and the α1β2(M286X)γ2s series of mutant receptors. (A) Propofol potentiates GABA responses at GABAA α1β2γ2s, α1β2(M286A)γ2s, α1β2(M286F)γ2s, and α1β2(M286Y)γ2s receptors but fails to enhance GABA responses at GABAA α1β2(M286W)γ2s receptors. (B) Propofol potentiates GABA responses at GABAA α1βγ2s, α1β2(M286C)γ2s, α1β2(M286E)γ2s, α1β2(M286K)γ2s, and α1β2(M286S)γ2s receptors (see Table 1 for parameters derived by curve fitting to the pooled data).
Fig. 5.
Concentration–response relationships for direct activation by propofol at the wild-type GABAA α1β2γ2s receptor and the α1β2(M286X)γ2s series of mutant receptors (see Table 1 for parameters derived by curve fitting to the pooled data).
To test the importance of the α subunit for the effects of propofol, potentiation of GABA responses by propofol was studied at the GABAA α1(S270W,A291W)β2γ2s receptor. This receptor showed potentiation of GABA responses by propofol similar to that at the wild-type GABAA α1β2γ2s receptor (Table 1).
3.3. Correlation analysis of the properties of the amino acid residue side-chain at position 286 of the GABAA β2 subunit with the GABA and propofol pharmacology
We next investigated the relationship between the physical properties of the amino acid residue at position 286 of the GABAAβ2 subunit and the responses to GABA and propofol. The amino acid properties chosen were molecular volume (Harpaz et al., 1994), polarity (Zimmerman et al., 1968), hydropathy (Kyte and Doolittle, 1982), and hydrophilicity (Hopp and Woods, 1981). There was no significant correlation between any of these amino acid properties and the GABA EC50, GABA maximal current, EC50 for potentiation of GABA responses by propofol, potentiation of control GABA response by 2, 5, 10, or 20 μM propofol, EC50 for propofol direct activation, or maximal direct activation by propofol (Pearson’s correlation r2=0.002–0.56, P<0.05 for each). In addition, the GABA EC50 value did not correlate with the EC50 values for direct activation or potentiation of GABA responses by propofol (r2=0.07 and 0.13, respectively, P<0.05 for each); similarly, the EC50 value for potentiation of GABA responses by propofol also did not correlate with the EC50 value for propofol direct activation (r2=0.01, P<0.05).
3.4. Effects of methohexital and etomidate at wild-type and mutant GABAA α1β2γ2s receptors
We next determined the effects of methohexital (a barbiturate) and etomidate on submaximal GABA responses at the GABAA α1β2γ2s and α1β2(M286W)γ2s receptors, to test whether this mutant receptor was capable of being modulated by anesthetics from different chemical classes than propofol. Methohexital (10 μM) potentiated EC20 GABA responses at both receptors: 199±39% potentiation at the GABAA α1β2γ2s receptor (n=5) and 118±18% at the GABAA α1β2(M286W)γ2s receptor (n=6) (P<0.05 compared with response at the wild-type receptor). Etomidate (10 μM) also potentiated EC20 GABA responses at both receptors: 237±45% potentiation at the GABAA α1β2γ2s receptor (n=6) and 162±11% at the GABAA α1β2(M286W)γ2s receptor (n=5) (P<0.05 compared with response at the wild-type receptor).
3.5. Alteration of the cut-off for propofol analogs by mutations in the GABAA β2 subunit
Two approaches were adopted to test the possibility that substitutions at the β2(M286) position might alter the size of propofol analog that could potentiate GABA responses. One approach was to mutate β2(M286) to a smaller amino acid residue to see if this would allow an analog bulkier than propofol to gain access to the propofol binding site by creating a larger binding cavity (an increase in cut-off). The analog chosen was 2,6-di-tertbutylphenol, shown in a separate study to be completely inactive at wild- type GABAA α1β2γ2s receptors (Krasowski et al., 2001). The second approach investigated whether submaximal GABA responses at the GABAA α1β2(M286W)γ2s receptor could still be potentiated by analogs smaller than propofol (a decrease in cut-off), even though propofol itself is inactive at this mutant receptor.
In the first set of experiments, the effects of 2,6-ditert-butylphenol were tested at GABAA α1β2(M286A)γ2s and GABAA α1β2(M286S)γ2s receptors. The M286A and M286S mutations result in 0.078 and 0.074 nm3 decreases in amino acid molecular volume at the 286 position, respectively, while 2,6-di-tert-butylphenol is 0.034 nm3 greater in molecular volume than propofol. 2,6-Di-tert-butylphenol did not, however, potentiate GABA responses at the GABAA α1β2(M286A)γ2s and GABAA α1β2(M286S)γ2s receptors (Table 2). 2,6-Ditert-butylphenol also produced no direct activation at either receptor at concentrations up to 500 μM (n=5). In contrast, four analogs smaller than propofol (molecular volume=0.193 nm3), 2,6-dimethylphenol (0.126 nm3), 2-isopropylphenol (0.143 nm3), 2,6-diethylphenol (0.160 nm3), and 2,6-diethylphenyl isothiocyanate (0.187 nm3), each potentiated GABA responses at the GABAA α1β2(M286A)γ2s and α1β2(M286S)γ2s receptors (Table 2). Thus, the GABAA α1β2(M286A)γ2s and GABAA α1β2(M286S)γ2s receptors have a cut-off for propofol analogs similar to that for the wild-type GABAA α1β2γ2s receptor, at least for the six propofol analogs tested (Fig. 7).
Table 2.
Molecular volume of propofol and related alkylphenol analogs and their effects on wild-type and mutated GABAA α1β2γ2s receptors a
| Propofol analog | Molecular volume (nm3) | Concentration (μM) | GABAA α1β2γ2s | GABAA α1β2(M286A)γ2s | GABAA α1β2(M286S)γ2s | GABAA α1β2(M286W)γ2s |
|---|---|---|---|---|---|---|
| 2,6-Dimethylphenol | 0.447 | 200 | 131±12 | 78±13 | 90±15 | 49±11** |
| 500 | 199±49 | 170±29 | 166±33 | 47±10 | ||
| 2-Isopropylphenol | 0.495 | 100 | 53±13 | 49±11 | 32±10 | 80±21 |
| 200 | 78±26 | 80±16 | 67±19 | 45±22 | ||
| 2,6-Diethylphenol | 0.548 | 20 | 175±56 | 168±47 | 133±21 | −3±6b** |
| 50 | 210±58 | 222±51 | 201±29 | 2±5b** | ||
| 2,6-Diethylphenyl isothiocyanate | 0.638 | 50 | 172±41 | 129±25 | 157±35 | −2±4b** |
| 100 | 184±27 | 190±30 | 200±31 | 2±5b** | ||
| Propofol | 0.641 | 2 | 133±28 | 52±14 | 75±34 | −3±3b** |
| 5 | 179±31 | 123±31 | 172±33 | −4±2b** | ||
| 2,6-Di-tert- butylphenol | 0.711 | 100 | −2±3b | 0±3b | 2±4b | −2±2b |
| 200 | 1±4b | −3±3b | 0±2b | −1±5b |
Molecular volume is a calculated parameter for each of the analogs (see Section 2). The other values indicate mean±SE for percent potentiation of an EC20 test concentration of GABA by the compound, with data from 5 to 12 cells contributing to each mean value.
Compound did not produce potentiation of GABA responses at the indicated concentration (i.e. effect did not differ significantly from no alteration of control GABA response).
P<0.05,
P<0.001 compared with response in wild-type GABAA α1β2γ2s receptor combination.
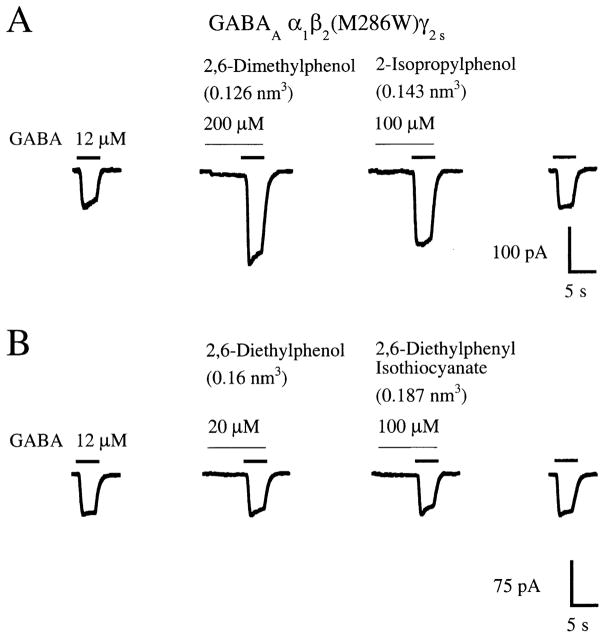
In the second set of experiments, the actions of propofol and five analogs were tested at the GABAA α1β2(M286W)γ2s receptor. The M286W mutation results in a 0.064 nm3 increase in molecular volume at position 286. The two analogs with the smallest molecular volumes, 2,6-dimethylphenol (0.126 nm3) and 2-isopropylphenol (0.143 nm3), enhanced submaximal GABA currents at the GABAA α1β2(M286W)γ2s receptor (Figs. 6 and 7; Table 2). In contrast, 2,6-diethylphenol (0.16 nm3), 2,6- diethylphenyl isothiocyanate (0.187 nm3), propofol (0.193 nm3), and 2,6-di-tertbutylphenol (0.227 nm3) all failed to potentiate submaximal GABA currents at the GABAA α1β2(M286W)γ2s receptor (Figs. 2(D), 6 and 7; Table 2). Thus, the GABAA β2(M286W) mutation reduces the propofol analog cut-off for potentiation of GABA responses from 0.193 nm3 (propofol) to below 0.16 nm3 (2,6-diethylphenol) (Fig. 7).
Fig. 6.
The GABAA β2(M286W) mutation alters the cut-off for potentiation of GABA responses by propofol analogs at the GABAA α1β2γ2s receptor. (A) The ‘small’ propofol analogs 2,6-dimethylphenol and 2-isopropylphenol (molecular volumes=0.126 and 0.143 nm3, respectively) potentiate submaximal (EC20) GABA responses at the GABAA α1β2(M286W)γ2s receptor. (B) In contrast, the larger propofol analogs 2,6-diethylphenol (0.16 nm3) and 2,6-diethylphenyl isothiocyanate (0.187 nm3) fail to enhance submaximal GABA responses at the GABAA α1β2(M286W)γ2s receptor. Traces shown in (A) and (B) are recordings from individual HEK 293 cells transfected with cDNAs encoding the GABAA α1, β2(M286W), and γ2s receptor subunits.
4. Discussion
We explain these experimental data by proposing that mutation of M286 in the GABAA receptor β2 subunit alters the dimensions of a binding cavity for the general anesthetic propofol. We will first discuss our results in relation to those of other studies employing site-directed mutagenesis to probe sites of anesthetic action on GABAA receptor subunits and then turn to discussion of the putative propofol binding cavity.
4.1. Contrasting effects of mutations within GABAA α or β subunit isoforms
The effects of the GABAA β2 mutations described in this study contrast with previously described effects of mutations within the GABAA α2 subunit or the glycineα1 subunits. The GABAA β2(M286W) mutation, which abolishes potentiation of GABA responses by propofol, has only small effects on the potency of GABA or for direct activation by propofol at the GABAA α1β2γ2s receptor (1.2 to 2.6-fold shifts of the respective concentration–response curves; see Table 1). In contrast, site-directed substitutions of GABAA α2(S270) or α2(A291), or of glycine α1(S267) or α1(A288) (see Fig. 1), markedly affect both the ability of general anesthetics to potentiate receptor function and receptor gating by agonists or anesthetics (Ye et al., 1998; Krasowski et al., 1998a,b; Koltchine et al., 1999; Ueno et al. 1999, 2000; Yamakura et al., 1999).
More specifically, the molecular volume of the amino acid side chain at the GABAA α2(S270), glycine α1(S267), or glycine α1(A288) positions correlates negatively with agonist EC50 and with anesthetic potentiation of agonist responses (Ye et al., 1998; Koltchine et al., 1999; Yamakura et al., 1999). In contrast, the present study and others show that site-directed substitutions of the 265 or 286 positions of GABAA β1 or β2 subunits do not produce any consistent alterations in agonist EC50 (Krasowski et al., 1998a,b; Amin, 1999; Ueno et al., 1999). This may relate to the dominant role of GABAA α subunits in determining agonist potency and efficacy (Levitan et al., 1988; Sigel et al., 1990; Ebert et al., 1994; O’Shea and Harrison, 2000).
4.2. Interpretation of the effects of site-directed mutations
There are a number of ways in which amino acid substitutions at β2(M286) could potentially disrupt the modulatory effects of propofol. First, the mutations could result in receptors that are already ‘maximally sensitive’ to GABA, such that propofol cannot shift the concentration–response relationship to GABA further to the left. This mechanism, which may apply to GABAA receptors containing the β2(L259S) mutation (Thompson et al., 1999), is improbable in the GABAA α1β2(M286W)γ2s receptor due to the minimal alterations in EC50 for GABA and the observation that etomidate and methohexital potentiate submaximal responses at this receptor (albeit with responses significantly smaller than at the wild-type GABAA α1β2γ2s receptor).
A second possibility is that the mutated receptor is more easily gated by propofol, which might obscure the measurement of potentiation of GABA-evoked currents. This problem has been encountered in other studies of anesthetic effects on mutated GABAA α2 and β2 receptor subunits (Krasowski et al., 1998b; Thompson et al., 1999). However, the GABAA α1β2(M286W)γ2s receptor analyzed in this study actually shows less direct activation by propofol concentrations below 20 μM than the wild-type GABAA α1β2γ2s receptor, discounting the possibility that direct activation obscures potentiation of GABA responses by propofol at this mutated receptor.
The remaining possibilities to explain the effects of the β2 mutations are that the mutations disrupt the binding site for propofol or, alternatively, disrupt conformational change(s) of the GABAA receptor that occur following the binding of propofol to the receptor. Distinguishing between these two possibilities is difficult because fine-resolution, three-dimensional structural data for the ligand-gated ion channels are not yet available. We therefore pursued an alternate approach, namely that of considering whether site-directed mutagenesis ofβ2(M286) altered the characteristics of a putative propofol binding cavity.
4.3. Probable characteristics of a propofol binding cavity in the GABAA receptor
A number of investigators have suggested that general anesthetic molecules may exert their effects by binding fortuitously to cavities naturally present on proteins, thereby stabilizing certain conformational states such as the open and closed states of the GABAA receptor ion channel (LaBella, 1981; Franks and Lieb, 1985; Williams and Akabas, 1999). Although GABAA receptors and other ligand-gated ion channels have so far resisted attempts to determine high-resolution structures, there have been a number of studies which demonstrate general anesthetic binding to cavities in soluble proteins that serve as models for true anesthetic targets. The high resolution structures of firefly luciferase complexed with bromoform (Franks et al., 1998) and human serum albumin complexed with halothane and propofol (Bhattacharya et al., 2000) demonstrate striking examples of anesthetic binding to pre-existing amphiphilic protein cavities.
In addition, alkylphenol binding cavities have been demonstrated on a variety of proteins, including the enzymes phenol hydroxylase (Neujahr, 1990; Enroth et al., 1998), vanillyl-alcohol oxidase (Mattevi et al., 1997; van den Heuvel et al., 1998), and p-cresol methylhydroxylase (McIntire et al., 1985; Cunane et al., 2000). In these three enzymes, structural determination by X-ray crystallography has revealed hydrophobic cavities that selectively bind alkylphenols of specific shapes and sizes. For instance, the 0.2 nm3 catalytic cavity of vanillyl-alcohol oxidase is completely filled by the competitive inhibitor p-(-1-heptenyl)phenol, which explains why alkylphenols bearing aliphatic substituents longer than seven carbon atoms at the para position do not bind to the enzyme (Mattevi et al., 1997; van den Heuvel et al., 1998). The substrate binding cavity of phenol hydroxylase has a shape and size that can accommodate 4-tert-butylphenol but not 2-isopropylphenol (Enroth et al., 1998; Neujahr, 1990).
Much like the structure–affinity relationships for alkylphenols at the three enzymes described above, the structure–activity relationships for propofol analogs for modulating GABAA receptors or producing loss of righting reflex in Xenopus laevis tadpoles exhibit distinct ligand requirements, for which steric factors seem to play a major role (Krasowski et al., 2001). For example, propofol (molecular volume=0.193 nm3) and 2,6-diethylphenyl isothiocyanate (0.187 nm3) are ‘active’, whereas 2,6-dicyclopentylphenol (0.24 nm3) and 2,6-diisopropylphenyl isocyanate (0.211 nm3) are completely inactive. These structure–activity relationships are consistent with the hypothesis that propofol analogs interact with a binding cavity of limited dimensions on the GABAA receptor, similar perhaps to the alkylphenol binding cavities on phenol hydroxylase, vanillyl-alcohol oxidase, or p-cresol methylhydroxylase.
4.4. Alteration of the cut-off for propofol analogs by mutation of the GABAA β2 subunit
If β2(M286) is indeed part of a binding cavity for propofol, then mutagenesis of this residue might alter the specificity of propofol analogs that can modulate GABAA receptor function. This is precisely what happened, with the propofol analog cut-off for potentiation of GABA responses reduced from 0.193 nm3 (propofol) in the wild-type GABAA α1β2γ2s receptor to below 0.16 nm3 (2,6-diethylphenol) in the GABAA α1β2(M286W)γ2s receptor (Fig. 6). In this context, it is of interest to consider studies of the catalytic pockets of alcohol dehydrogenase (Weinhold and Benner, 1995) and L-hydroxyisocaproate dehydrogenase (Feil et al., 1997). In both of these enzymes, it is possible to alter substrate specificity by site-directed mutagenesis of amino acid residues lining the substrate binding pocket.
Future studies will continue to elucidate the role of the amino acid residues involved in the actions of anesthetics. Even more exciting, perhaps, is the use of targeted gene manipulations in mice to test the relevance of certain ligand-gated ion channels in mediating the behavioral actions of general anesthetics (Belelli et al., 1999; Krasowski and Harrison, 1999). The GABAA receptor β2(M286W) mutation described in this study may be an ideal candidate for the creation of knock-in mice, in which the endogenous β2 subunit is replaced by the mutated, anesthetic-insensitive subunit.
Acknowledgments
Funding for this study was provided by the C.V. Starr Foundation (New York City, NY, USA) and the Rice Foundation (Chicago, IL, USA) to N.L.H., by National Institutes of Health grants P01-GM62195 and GM56850 to N.L.H., and by National Institute of Mental Health training fellowship MH11504 to M.D.K. K.N. was supported by a research fellowship from the Uehara Memorial Foundation (Tokyo, Japan). The authors are grateful to Amiinah Kung, Irene Paraskevakis, and Steve Lopez for invaluable technical support. The authors also thank Drs J.B. Glen and Roger James of Zeneca Pharma ceuticals for providing propofol analogs and Professor Alfred Doenicke for supplying etomidate.
References
- Amin J. A single hydrophobic residue confers barbiturate sensitivity to γ-aminobutyric acid type C receptor. Molecular Pharmacology. 1999;55:411–423. [PubMed] [Google Scholar]
- Bai D, Pennefather PS, MacDonald JF, Orser BA. The general anesthetic propofol slows deactivation and desensitization of GABAA receptors. Journal of Neuroscience. 1999;19:10635–10646. doi: 10.1523/JNEUROSCI.19-24-10635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather PS, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Molecular Pharmacology. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Belelli D, Pistis M, Peters JA, Lambert JJ. General anaesthetic action at transmitter-gated inhibitory amino acid receptors. Trends in Pharmacological Sciences. 1999;20:496–502. doi: 10.1016/s0165-6147(99)01405-4. [DOI] [PubMed] [Google Scholar]
- Bhattacharya AA, Curry S, Franks NP. Binding of the general anesthetics propofol and halothane to human serum albumin: high-resolution crystal structures. Journal of Biological Chemistry. 2000;275:38731–38738. doi: 10.1074/jbc.M005460200. [DOI] [PubMed] [Google Scholar]
- Cunane LM, Chen ZW, Shamala N, Mathews FS, Cronin CN, McIntire WS. Structures of the flavocytochrome p-cresol methylhydroxylase and its enzyme substrate complex: gated substrate entry and proton relays support the proposed catalytic mechanism. Journal of Molecular Biology. 2000;295:357–374. doi: 10.1006/jmbi.1999.3290. [DOI] [PubMed] [Google Scholar]
- Cutting GR, Lu L, O’Hara BF, Kasch LM, Montrose-Rafizadeh C, Donovan DM, Shimada S, Antonarakis SE, Guggino WB, Uhl GR, Kazazian HH. Cloning of the γ-aminobutyric acid (GABA) ρ1 cDNA: a GABA receptor subunit highly expressed in the retina. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DS, Bradley RJ. Chemical properties of alcohols and their protein binding sites. Cellular and Molecular Life Sciences. 2000;57:265–275. doi: 10.1007/PL00000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B, Wafford KA, Whiting PJ, Krogsgaard-Larsen P, Kemp JA. Molecular pharmacology of γ-aminobutyric acid type A receptor agonists and partial agonists in oocytes injected with different α, β, and γ receptor subunit combinations. Molecular Pharmacology. 1994;46:957–963. [PubMed] [Google Scholar]
- Enroth C, Neujahr H, Schneider G, Lindqvist Y. The crystal structure of phenol hydroxylase in complex with FAD and phenol provides evidence for a concerted conformational change in the enzyme and its cofactor during catalysis. Structure. 1998;6:605–617. doi: 10.1016/s0969-2126(98)00062-8. [DOI] [PubMed] [Google Scholar]
- Feil IK, Hendle J, Schomburg D. Modified substrate specificity of L-hydroxyisocaproate dehydrogenase derived from structure-based protein engineering. Protein Engineering. 1997;10:255–262. doi: 10.1093/protein/10.3.255. [DOI] [PubMed] [Google Scholar]
- Franks NP, Jenkins A, Conti E, Lieb WR, Brick P. Structural basis for the inhibition of firefly luciferase by a general anesthetic. Biophysical Journal. 1998;75:2205–2211. doi: 10.1016/S0006-3495(98)77664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. The firefly throws light on anesthesia. Chemistry in Britain. 1985;21:919–921. [Google Scholar]
- Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Grenningloh G, Rienitz A, Schmitt B, Methfessel C, Zensen M, Beyreuther K, Gundelfinger ED, Betz H. The strychnine binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987;328:215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whiting PJ. Cloning of cDNA sequences encoding human α2 and α3 γ-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant α1-, α2-, α3-, and α5-containing human γ-aminobutyric acidA receptors. Molecular Pharmacology. 1993a;43:970–975. [PubMed] [Google Scholar]
- Hadingham KL, Wingrove PB, Wafford KA, Bain C, Kemp JA, Palmer KJ, Wilson AW, Wilcox AS, Sikela JM, Ragan CI, Whiting PJ. Role of the β subunit in determining the pharmacology of human γ-aminobutyric acid type A receptors. Molecular Pharmacology. 1993b;44:1211–1218. [PubMed] [Google Scholar]
- Hales TG, Lambert JJ. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. British Journal of Pharmacology. 1991;104:619–628. doi: 10.1111/j.1476-5381.1991.tb12479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Kai Y, Ikemoto Y. Propofol activates GABAA receptor-chloride ionophore complex in dissociated hippocampal pyramidal neurons of the rat. Anesthesiology. 1993;79:781–788. doi: 10.1097/00000542-199310000-00021. [DOI] [PubMed] [Google Scholar]
- Harpaz Y, Gerstein M, Chothia C. Volume changes on protein folding. Structure. 1994;2:641–649. doi: 10.1016/s0969-2126(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Hopp TP, Woods KR. Prediction of protein antigenic determinants from amino acid sequences. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R, Glen JB. Synthesis, biological evaluation, and preliminary structure-activity considerations of a series of alkylphenols as intravenous anesthetic agents. Journal of Medicinal Chemistry. 1980;23:1350–1357. doi: 10.1021/jm00186a013. [DOI] [PubMed] [Google Scholar]
- Jenkins A, Greenblatt EP, Faulkner HJ, Bertaccini E, Light A, Lin A, Andreasen A, Viner A, Trudell JR, Harrison NL. Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. Journal of Neuroscience. 2001;21 (RC-136):1–4. doi: 10.1523/JNEUROSCI.21-06-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltchine VV, Finn SE, Jenkins A, Nikolaeva N, Lin A, Harrison NL. Agonist gating and isoflurane potentiation in the human γ-aminobutyric acid type A receptor determined by the volume of a TM2 residue. Molecular Pharmacology. 1999;56:1087–1093. doi: 10.1124/mol.56.5.1087. [DOI] [PubMed] [Google Scholar]
- Krasowski MD, Finn SE, Ye Q, Harrison NL. Trichloroethanol modulation of recombinant GABAA, glycine, and GABA ρ1 receptors. Journal of Pharmacology and Experimental Therapeutics. 1998a;284:934–942. [PubMed] [Google Scholar]
- Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cellular and Molecular Life Sciences. 1999;55:1278–1303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasowski MD, Harrison NL. The actions of ether, alcohol, and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations. British Journal of Pharmacology. 2000;129:731–743. doi: 10.1038/sj.bjp.0703087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasowski MD, Jenkins A, Flood P, Kung AY, Hopfinger AJ, Harrison NL. The general anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of GABA current at the GABAA receptor but not with lipid solubility. Journal of Pharmacology and Experimental Therapeutics. 2001;297:338–351. [PubMed] [Google Scholar]
- Krasowski MD, Koltchine VV, Rick CE, Ye Q, Finn SE, Harrison NL. Propofol and other intravenous anesthetics have sites of action on the γ-aminobutyric acidA receptor distinct from that for isoflurane. Molecular Pharmacology. 1998b;53:530–538. doi: 10.1124/mol.53.3.530. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. Journal of Molecular Biology. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- LaBella FS. Is there a general anesthesia receptor? Canadian Journal of Physiology and Pharmacology. 1981;59:432–442. doi: 10.1139/y81-067. [DOI] [PubMed] [Google Scholar]
- Levitan ES, Blair LA, Dionne VE, Barnard EA. Biophysical and pharmacological properties of cloned GABAA receptor subunits expressed in Xenopus oocytes. Neuron. 1988;1:773–781. doi: 10.1016/0896-6273(88)90125-0. [DOI] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. Alcohol action on a neuronal membrane receptor: evidence for a direct interaction with the receptor protein. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:8200–8204. doi: 10.1073/pnas.91.17.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia MP, Machu TK, Harris RA. Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. British Journal of Pharmacology. 1996;119:1331–1336. doi: 10.1111/j.1476-5381.1996.tb16042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattevi A, Fraaije MW, Mozzarelli A, Olivi L, Coda A, van Berkel WJ. Crystal structures and inhibitor binding in the octameric flavoenzyme vanillyl-alcohol oxidase: the shape of the active-site cavity controls substrate specificity. Structure. 1997;5:907–920. doi: 10.1016/s0969-2126(97)00245-1. [DOI] [PubMed] [Google Scholar]
- McIntire W, Hopper DJ, Singer TP. p-Cresol methylhydroxylase: assay and general properties. Biochemical Journal. 1985;228:325–335. doi: 10.1042/bj2280325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ, Harris RA. Inhibition of ρ1 receptor GABAergic currents by alcohols and volatile anesthetics. Journal of Pharmacology and Experimental Therapeutics. 1996;277:411–416. [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Molecular sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Neujahr HY. Phenol hydroxylase. In: Nuller F, editor. Chemistry and Biochemistry of Flavoenzymes. 2. CRC Press; Boca Raton, FL, USA: 1990. pp. 65–85. [Google Scholar]
- O’Shea SM, Harrison NL. Arg 274 and Leu 277 of the GABAA receptor α2 subunit define agonist efficacy and potency. Journal of Biological Chemistry. 2000;275:22764–22768. doi: 10.1074/jbc.M001299200. [DOI] [PubMed] [Google Scholar]
- Peoples RW, Weight FF. Cutoff in potency implicates alcohol inhibition of N-methyl-D-aspartate receptors in alcohol intoxication. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:2825–2829. doi: 10.1073/pnas.92.7.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Schofield PR, Pritchett DB, Sontheimer H, Kettenmann H, Seeburg PH. Sequence and expression of human GABAA α1 and β1 subunits. FEBS Letters. 1989;244:361–364. doi: 10.1016/0014-5793(89)80563-0. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Trube G, Mohler H, Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990;5:703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Smith MZ, Wingrove PB, Whiting PJ, Wafford KA. Mutation at the putative GABAA ion-channel gate reveals changes in allosteric modulation. British Journal of Pharmacology. 1999;127:1349–1358. doi: 10.1038/sj.bjp.0702687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani G, Altomare C, Sanna E, Biggio G, Liso G. Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Current Medicinal Chemistry. 2000;7:249–271. doi: 10.2174/0929867003375335. [DOI] [PubMed] [Google Scholar]
- Ueno S, Lin A, Nikolaeva N, Trudell JR, Mihic SJ, Harris RA, Harrison NL. Tryptophan scanning mutagenesis in TM2 of the GABAA receptor α subunit: effects on channel gating and regulation by ethanol. British Journal of Pharmacology. 2000;131:296–302. doi: 10.1038/sj.bjp.0703504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Wick MJ, Ye Q, Harrison NL, Harris RA. Subunit mutations affect ethanol actions on GABAA receptors expressed in Xenopus oocytes. British Journal of Pharmacology. 1999;127:377–382. doi: 10.1038/sj.bjp.0702563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel RHH, Fraaije MW, Laane C, van Berkel WJH. Regio- and stereospecific conversion of 4-alkylphenols by the covalent flavoprotein vanillyl-alcohol oxidase. Journal of Bacteriology. 1998;180:5646–5651. doi: 10.1128/jb.180.21.5646-5651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhold EG, Benner SA. Engineering yeast alcohol dehydrogenase. Replacing Trp54 by Leu broadens substrate specificity. Protein Engineering. 1995;8:457–461. doi: 10.1093/protein/8.5.457. [DOI] [PubMed] [Google Scholar]
- Wick MJ, Mihic SJ, Ueno S, Mascia MP, Trudell JR, Brozowski SJ, Ye Q, Harrison NL, Harris RA. Mutations of γ-aminobutyric acid and glycine receptors change alcohol cutoff: evidence for an alcohol receptor? Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6504–6509. doi: 10.1073/pnas.95.11.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DB, Akabas MH. γ-aminobutyric acid increases the water accessibility of M3 membrane-spanning segment residues in γ-aminobutyric acid type A receptor. Biophysical Journal. 1999;77:2563–2574. doi: 10.1016/s0006-3495(99)77091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura T, Mihic SJ, Harris RA. Amino acid volume and hydropathy of a transmembrane site determine glycine and anesthetic sensitivity of glycine receptors. Journal of Biological Chemistry. 1999;274:23006–23012. doi: 10.1074/jbc.274.33.23006. [DOI] [PubMed] [Google Scholar]
- Ye Q, Koltchine VV, Mihic SJ, Mascia MP, Wick M, Finn SE, Harrison NL, Harris RA. Enhancement of glycine receptor function by ethanol is inversely correlated with molecular volume at position α267. Journal of Biological Chemistry. 1998;273:3314–3319. doi: 10.1074/jbc.273.6.3314. [DOI] [PubMed] [Google Scholar]
- Ymer S, Schofield PR, Draguhn A, Werner P, Kohler M, Seeburg PH. GABAA receptor β subunit heterogeneity: functional expression of cloned cDNAs. EMBO Journal. 1989;8:1665–1670. doi: 10.1002/j.1460-2075.1989.tb03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, Eliezer N, Simha R. The characterization of amino acid sequences in proteins by statistical methods. Journal of Theoretical Biology. 1968;21:170–201. doi: 10.1016/0022-5193(68)90069-6. [DOI] [PubMed] [Google Scholar]