Abstract
Obestatin, derived from the same gene as the hunger hormone ghrelin, may reduce food intake in animals. The role of obestatin in human physiology is unclear. We evaluated cross-sectional associations between participant characteristics and fasting levels of obestatin as well two other hormones associated with energy balance, ghrelin and leptin. Data are from the baseline visit of the Omni-Heart Trial that enrolled adults with elevated blood pressure (systolic 120 to 159 mm Hg or a diastolic of 80 to 99 mm Hg) but who were otherwise healthy. Partial spearman correlations and linear regression models estimated the association between age, gender, BMI, physical activity, and smoking with fasting hormones. Obestatin was directly associated with ghrelin (r=0.45, P<0.05). On average, overweight (BMI 25 to 30) and obese (BMI>30) individuals had obestatin concentrations that were 12.6 (SD 8.8) and 25.4 (SD 8.4) pg/ml lower compared to normal weight (BMI <25) individuals, respectively (P for trend=0.002). Overweight (BMI 25 to 30) and obese (BMI>30) individuals had ghrelin concentrations that were 161.7 (SD 69.6) and 284.7 (SD 66.5) pg/ml lower compared to normal weight (BMI <25) individuals, respectively (P for trend < 0.0001). A 5 unit increase in BMI was associated with 41.3% (SD 4.3%) (P<0.0001) higher leptin. Obestatin and ghrelin are directly correlated and share the same patterns of association with participant characteristics. Modifiable risk factors for chronic diseases, including BMI and physical activity, are associated with fasting levels of leptin, obestatin, and ghrelin.
Keywords: appetite, ghrelin, leptin, body mass index
Introduction
Obestatin was recently identified in one study as a hormone that reduces food intake in animals (1), but others have not replicated these findings (2). Obestatin is derived from the same gene as ghrelin, a hormone that promotes meal initiation in both humans and animals (3). A recent review, predominantly from animal studies, summarized potential roles for obestatin including slowing gastrointestinal motility, inhibiting thirst, inducing sleep, and/or improving memory (4). Research in humans is needed to clarify whether obestatin has opposite effects to ghrelin with respect to food intake, has other effects on human physiology, or has no relevance in humans.
Measuring associations between obestatin and characteristics related to energy balance (i.e. BMI, physical activity) helps inform whether obestatin regulates food intake. Data from these small studies (n < 40) have shown both positive (5) and negative (2, 6) correlations between obestatin and body mass index.
If obestatin and ghrelin have opposing effects on energy balance, one might expect the two hormones to have opposite associations with modifiable factors related to energy balance, such as BMI and physical activity. Ghrelin has an inverse relationship with adiposity, as ghrelin decreases with positive energy balance (7).
Leptin, a hormone associated with long-term energy balance, has been extensively studied since it was identified in 1994 (8). As a signal of long-term energy stores, it increases with increasing BMI (9) and decreases with greater physical activity (10).
We investigated relationships between selected fasting hormones and modifiable risk factors for chronic disease among a larger, more representative sample than previously studied to resolve discrepant findings from previous reports of obestatin, This analysis evaluated associations between obestatin and participant characteristics and compares these associations to those of concurrently measured ghrelin and leptin.
Methods
Optimal Macronutrient Intake Trial to Prevent Heart Disease (OMNI-Heart) Design
Data were collected from the baseline exam of the OMNI-Heart Trial. The primary objective of the OMNI-Heart Trial was to compare the effects of three diets, each rich in a different macronutrient, on cardiovascular disease risk factors (11, 12). The research protocol was approved by the Western Institutional Review Board and all participants provided written informed consent to participate. Briefly, baseline data were collected over three screening visits, except for medical history and physical activity, which were collected during the one week run-in period.
Participants were recruited through mailings and media placements in newspapers. Study participants were adults at least 30 years old with elevated blood pressure (systolic 120 to 159 mm Hg or a diastolic of 80 to 99 mm Hg) but were otherwise healthy. Exclusion criteria included cardiovascular disease, recent cancer, diabetes, LDL cholesterol greater than 220 mg/dL, fasting triacylglycerol greater than 750 mg/dL, weight greater than 350 lb, taking medications that affect blood pressure or blood lipids, unwillingness to suspend vitamin and mineral supplementation, and alcohol consumption greater than 14 drinks per week.
Measures
Age, gender, and race were all self-reported. Smoking status (current, former, or never) and leisure-time physical activity (frequency of moderate and vigorous activities in the past month) were collected by questionnaires administered during the six-day run-in period. Height was measured by trained staff using a stadiometer at the first screening visit, and weight was measured using a calibrated balance beam scale during the last screening visit.
During the third screening visit, blood was drawn after an overnight fast. Blood samples were centrifuged, processed, and stored at −70°C within 2 hours of collection. Samples were analyzed by radioimmunoassay for obestatin (Phoenix Pharmaceutical, Belmont, CA, USA), total ghrelin (Linco, St Charles, MO, USA), and leptin (Linco, St Charles, MO, USA),. Intra-assay coefficients of variation were 8.9% for obestatin, 3.9% for ghrelin, and were 5.5% for leptin.
Statistical Analysis
Analyses included all randomized participants who had stored blood samples from the baseline visit (n = 163). Obestatin was measured in 156 of 163 participants, as there was insufficient specimen volume to assay all three hormones in 7 participants. Outcomes were obestatin, ghrelin, and leptin. Descriptive statistics and histograms were used to check for outliers and evaluate the normality of the distribution of variables. Leptin was loge-transformed to improve normality. Back-transformation from the loge-scale for interpretation is equivalent to the percent change in leptin per unit change in exposure variable. Scatterplots were used to assess bivariate relationships between variables.
Linear regression was used to estimate the association between fasting hormones and exposures controlling for potential confounders. Participant characteristics of interest were age, gender, BMI, smoking, and physical activity. Continuous variables were centered at the mean and scaled to approximately one standard deviation. BMI was modeled continuously (per 5kg/m2) as a linear term, with a spline at BMI of 30, with a squared term to allow for non-linearity in the association, and categorically (per NHLBI Obesity Classification). The approach that best summarized the data, considering scatterplots, interpretability of the coefficients, and goodness of fit statistics, is presented. Smoking was categorized as current, former, and never. Physical activity in the past month was categorized as sedentary (moderate and vigorous activity less than twice per week), moderate (moderate activity at least twice per week), or vigorous (vigorous activity at least twice per week).
Spearman correlations were used to assess the association between fasting hormones. In addition to crude associations, partial correlations were estimated adjusting for age, gender, BMI, smoking, and physical activity.
P<0.05 was considered statistically significant, and no adjustments for multiple comparisons were made. Statistical analyses were conducted using SAS for WINDOWS (version 9.1; SAS Institute, Cary, NC).
Results
Participant Characteristics
The study population was diverse, having approximately equal numbers of obese and non-obese (BMI ≥30 vs.<30), gender, and race (black/white) (Table 1). Almost two-thirds (61%) reported never smoking, and about a quarter (23%) engaged in vigorous physical activity at least twice per week over the past month.
Table 1.
Baseline Characteristics of OMNI-Heart Participants.
| Mean ± SD or N (%) | |
|---|---|
| Participant Characteristics (n = 1631) | |
| Age, years | 53.5 ± 10.8 |
| Women | 73 (45) |
| Race | |
| African American | 90 (55) |
| Non-Hispanic white | 65 (40) |
| Other | 8 (6) |
| Body mass index (BMI), kg/m2 | 30.3 ± 6.1 |
| Obesity status | |
| Not overweight or obese, BMI<25 | 34 (21) |
| Overweight, BMI 25-29.9 | 54 (33) |
| Obese, BMI ≥ 30 | 75 (46) |
| Smoking | |
| Never | 100 (61) |
| Former | 45 (28) |
| Current | 18 (11) |
| Physical Activity2 | |
| Sedentary | 62 (38) |
| Moderate | 63 (39) |
| Vigorous | 38 (23) |
| Fasting Hormones (n=163) | |
| Obestatin, pg/ml3 | 119.9 ± 41.8 |
| Ghrelin, pg/ml | 817.0 ± 355.5 |
| Leptin, ng/ml4 | 11.2 (5.9, 26.2) |
163 out of 164 participants with blood analyzed for hormone concentrations
Moderate=moderate physical activity at least 2 times per week; Vigorous=vigorous physical activity at least 2 times per week; Referent is sedentary=any physical activity less than twice per week.
n=156
Median (25th percentile, 75th percentile)
Associations Between Participant Characteristics and Fasting Hormones
Obestatin
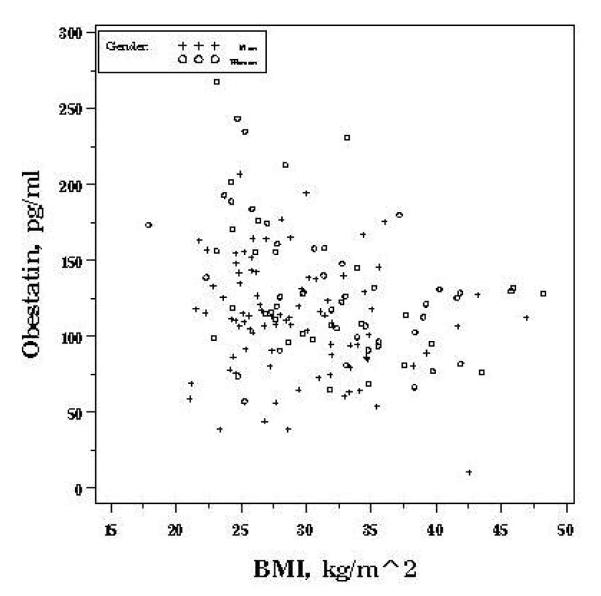
Mean (±SD) fasting obestatin (pg/ml) was higher in women (130.9 ± 45.1) compared to men (111.0 ± 36.9), P=0.003. Gender remained independently associated with obestatin in multivariate analyses. On average, obestatin was 25.6 (SD 6.5) pg/ml higher in women compared to men after adjustment for confounders (P<0.0001) (Table 2). Race was not associated with obestatin concentration. In comparison to non-smokers, obestatin levels were higher in former smokers by an average of 14.9 pg/ml (P=0.04) and in current smokers by an average of 19.2 pg/ml (P=0.08)). On average, overweight (BMI 25 to 30) and obese (BMI>30) individuals had obestatin concentrations that were 12.6 (SD 8.8) and 25.4 (SD 8.4) pg/ml lower compared to normal weight (BMI <25) individuals, respectively (P for trend=0.002). As displayed in Figure 1a, obestatin was rather uniform across BMI’s greater than 30. Models allowing for non-linearity in the association better fit the data; hence, BMI is included as a categorical term. In multivariate analyses, BMI and sedentary lifestyle were inversely associated with obestatin (Table 2). Age was not independently associated with obestatin.
Table 2.
Estimated Associations Between Hormone Concentrations Per Unit Difference in Participant Characteristics, OMNI-Heart.
| Crude1 | Adjusted2 | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SE | P | P-trend | Mean | SE | P | |
| Obestatin, mean difference, pg/ml (n=156) 3 | |||||||
| Age, per 5 y | 3.6 | 1.6 | 0.02 | 1.8 | 1.5 | 0.25 | |
| Women vs. men | 19.8 | 6.6 | 0.003 | 25.6 | 6.5 | <0.0001 | |
| Black vs. non-black | −5.4 | 7.1 | 0.45 | ||||
| BMI4 | |||||||
| Overweight | −12.5 | 9.2 | −12.6 | 8.8 | |||
| Obese | −26.2 | 8.7 | 0.002 | −25.4 | 8.4 | 0.002 | |
| Smoking | |||||||
| Former Smoker | 11.8 | 7.7 | 0.12 | 14.9 | 7.2 | 0.04 | |
| Current Smoker | 23.7 | 11.8 | 0.05 | 19.2 | 11.0 | 0.08 | |
| Physical Activity5 | |||||||
| Moderate | 14.7 | 7.5 | 10.3 | 7.2 | |||
| Vigorous | 18.3 | 8.7 | 0.03 | 17.3 | 8.4 | 0.03 | |
|
| |||||||
| Ghrelin, mean difference, pg/ml (n=163) 3 | |||||||
| Age, per 5 y | 18.2 | 12.9 | 0.16 | 4.4 | 12.0 | 0.72 | |
| Women vs. men | 117.2 | 55.4 | 0.04 | 174.1 | 51.9 | 0.001 | |
| Black vs. non-black | −81.6 | 57.9 | 0.16 | ||||
| BMI4 | |||||||
| Overweight | −180.1 | 73.3 | −161.7 | 69.6 | |||
| Obese | −324.7 | 69.2 | <0.0001 | −284.7 | 66.5 | <0.0001 | |
| Smoking | |||||||
| Former Smoker | −7.2 | 63.5 | 0.91 | 6.6 | 58.1 | 0.91 | |
| Current Smoker | 275.4 | 93.4 | 0.004 | 254.2 | 84.8 | 0.003 | |
| Physical Activity5 | |||||||
| Moderate | 180.6 | 61.4 | 143.1 | 57.7 | |||
| Vigorous | 234.5 | 70.7 | 0.001 | 210.8 | 66.6 | 0.001 | |
|
| |||||||
| Leptin, mean percent difference, (n=163) 3 | |||||||
| Age, per 5 y | 5.1% | 3.8% | 0.18 | 5.6% | 2.4% | 0.02 | |
| Women vs. men | 131.5% | 12.9% | <0.0001 | 103.0% | 10.2% | <0.0001 | |
| Black vs. non-black | 45.6% | 16.9% | 0.008 | ||||
| BMI, per 5 kg/m2 | 51.5% | 5.4% | <0.0001 | 41.3% | 4.3% | <0.0001 | |
| Smoking | |||||||
| Former Smoker | −4.2% | 19.1% | 0.82 | 10.0% | 11.4% | 0.38 | |
| Current Smoker | −50.4% | 28.0% | 0.07 | −23.7% | 16.6% | 0.16 | |
| Physical Activity5 | |||||||
| Moderate | −54.5% | 17.6% | 0.002 | −25.3% | 11.3% | ||
| Vigorous | −96.3% | 20.3% | <0.0001 | −40.4% | 13.2% | 0.002 | |
Crude association from linear regression model.
Adjusted estimates from linear regression model accounting for age, gender, BMI, smoking, and physical activity.
Leptin estimates are percent change due to back-transformation from the loge scale. For obestatin and ghrelin, values are mean pg/ml change per unit change in exposure.
Referent is normal (BMI <25 kg/m2); overweight=BMI 25 to 30 kg/m2; obese=BMI ≥30 kg/m2). Reported p-value is p for trend.
Referent is sedentary=Moderate PA <2 times/week); Moderate=Moderate PA ≥ 2 times/wk; Vigorous=Vigorous PA ≥ 2 times/wk. Reported p-value is p for trend
Figure 1a.
Gender-specific associations between fasting obestatin and BMI, OMNI-Heart.
Ghrelin
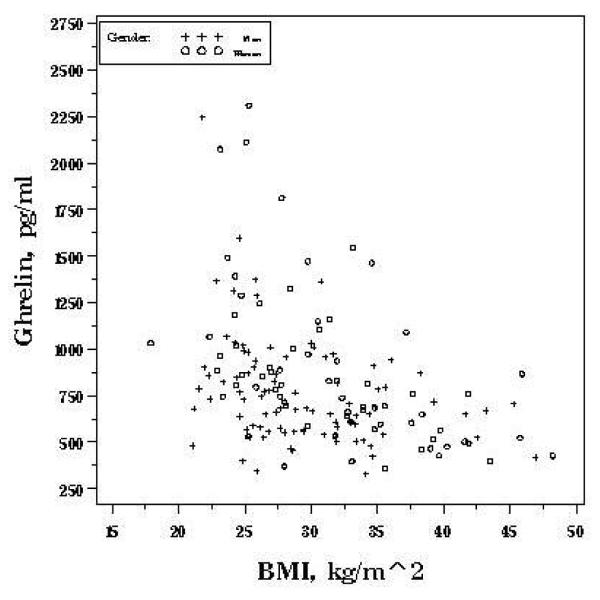
As with obestatin, mean ghrelin (pg/ml) was also higher in women (881.7 (SD 413.5)) compared to men (764.5 (SD 292.5)), P=0.04. Higher BMI was independently associated with lower ghrelin concentrations (P<0.0001) (Table 2). Overweight (BMI 25 to 30) and obese (BMI>30) individuals had ghrelin concentrations that were 161.7 (SD 69.6) and 284.7 (SD 66.5) pg/ml lower compared to normal weight (BMI <25) individuals, respectively (P for trend < 0.0001). Similar to obestatin, ghrelin was relatively uniform across BMI’s greater than 30 (Figure 1b), suggesting a threshold relationship., Models allowing for non-linearity in the association better fit the data, so BMI is included as a categorical term. Current smoking and engaging in physical activity were both associated with significantly higher ghrelin. Age was not significantly associated with ghrelin.
Figure 1b.
Gender-specific associations between fasting ghrelin and BMI, OMNI-Heart.
Leptin
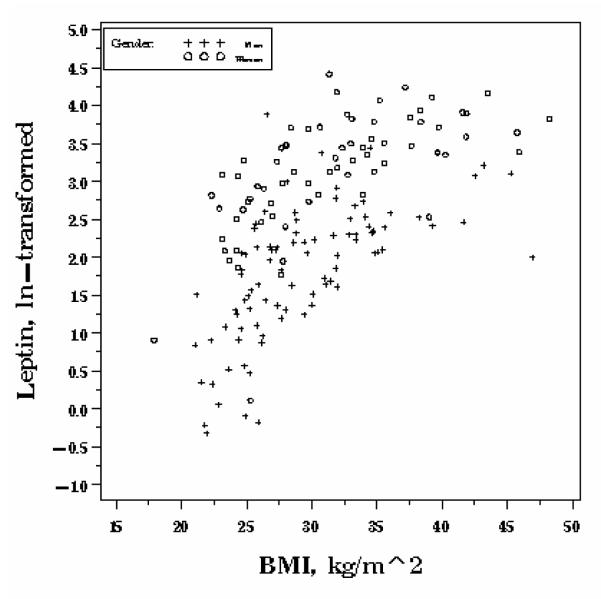
Median fasting leptin (ng/ml) was higher in women (26.8 (IQR 15.5, 40.4)) than men (7.6 (IQR 3.7, 11.0)), P <0.0001. After multivariate adjustment, leptin was 103.0% higher in women than men (SD 10.2%,P <0.0001) (Table 2). Unlike obestatin and ghrelin, leptin increased exponentially across the entire range of BMI (Figure 1c). Models allowing for non-linearity in the association between BMI and fasting leptin improved goodness of fit. Each 5 unit increase in BMI was independently associated with a 41.3% (SD 4.3%) increase in leptin (P<0.0001). Engaging in physical activity at least twice per week was associated with decreased leptin concentrations, by 25.3% (SD=11.3%) for moderate and 40.4% (SD=13.2%) for vigorous intensity (P for trend=0.002). On average, those who were 5 years older had fasting leptin concentrations that were 5.6% higher (P=0.02) after adjusting for covariates. Smoking was not associated with leptin.
Figure 1c.
Gender-specific associations between fasting leptin and BMI, OMNI-Heart.
Associations Between Fasting Hormones
Although we postulated obestatin and ghrelin would be inversely correlated, the hormones were directly related with a high correlation (crude r=0.55, P<0.05) (Table 3). This relationship was only slightly attenuated after adjustment for age, gender, BMI, smoking, and physical activity (r=0.45, P<0.05). Leptin was not associated with obestatin or ghrelin after adjustment for confounders.
Table 3.
Spearman Correlations Between Fasting Hormones, OMNI-Heart.
| Crude | Adjusted1 | |||
|---|---|---|---|---|
| Obestatin | Ghrelin | Obestatin | Ghrelin | |
| Leptin | −0.05 | −0.22 | −0.08 | −0.05 |
| Obestatin | 0.552 | 0.452 | ||
Partial Spearman correlations adjusted for age (continuous), gender, BMI, (continuous), smoking (current, former, never), and physical activity (sedentary, moderate, vigorous). N=163 except for comparisons including obestatin (n=156).
P<0.05
Discussion
Assuming fasting concentrations are a suitable proxy for obestatin activity, these data do not support hypotheses that obestatin has opposing effects compared to ghrelin. Obestatin and ghrelin were directly associated, and both hormones were higher in women, among smokers, and among those who consistently engage in intense physical activity. If obestatin and ghrelin had opposing effects on energy balance, they would be inversely associated; instead, obestatin and ghrelin were directly correlated.
Cross-sectional, correlational analyses such as this one are limited in their ability to explain mechanism. For example, one alternative explanation for the observed results is that more obestatin is secreted in response to elevated ghrelin levels. This hypothesis is supported by data from an animal study suggesting obestatin modulates ghrelin’s action (13). In order to test this hypothesis, well-controlled human studies collecting multiple measures of ghrelin and obestatin before and after a meal are needed.
There were strong associations between hormone levels and participant characteristics related to energy balance. Both obestatin and ghrelin were inversely associated with BMI. Data from other studies on the association between BMI and obestatin are limited, but two small studies (n<=30) comparing obese to lean participants also reported significantly lower levels among obese individuals (2, 6) while another study (n<40) reported statistically significant increases in obestatin among overweight compared to normal weight individuals (5). Similar to our findings, recent data measuring ghrelin and obestatin in obese and anorexic individuals observed a positive correlation between ghrelin and obestatin (14). Our data, contributing a sample size more than four times that of previous studies, indicate that obestatin levels are lower with increased adiposity.
Previous research supports an inverse association between ghrelin and BMI (15, 16). Ghrelin also decreases with short-term positive energy balance (7). Similar to obestatin, ghrelin levels were lower among overweight and obese individuals compared to normal weight individuals, but levels were fairly homogenous among obese individuals. Leptin, which is released from adipose tissue, was directly associated with BMI as reported by others (17, 18).
Even with a crude measure of physical activity, each of the hormones was significantly associated after controlling for confounders. Studies using more accurate and repeated measures of physical activity, as have been reported for leptin and ghrelin (19), could help to inform the relationship between hormones related to appetite regulation and physical activity.
Some limitations should be considered in interpreting these analyses. The mechanism by which obestatin may reduce food intake has not been identified, as obestatin is likely not the ligand for the G protein receptor GPR39 as previously reported (4). It may be important to measure hormone concentrations after a meal in addition to fasting levels. Furthermore, in cross-sectional studies one cannot assess temporality of the associations, so data from longitudinal studies could inform whether changing modifiable characteristics (i.e. BMI, physical activity) alters hormone concentrations.
Among the strengths of this study was its large and diverse sample size compared to previous research in this area. This study is one of the largest to report associations between both non-modifiable and modifiable risk factors for obesity-associated chronic diseases and fasting hormones leptin, obestatin, and ghrelin. These data provide more precise estimates of the association between gender, obesity, physical activity, and smoking with fasting appetite hormone levels among a study population with approximately equal numbers by gender and obese versus non-obese individuals.
In conclusion, our data suggest fasting levels of obestatin and ghrelin have similar patterns of association with characteristics associated with energy balance (i.e. BMI, physical activity). A better understanding of the biological mechanism of action of obestatin, i.e. through identification of its receptor, could help to explain these findings. Studies with repeated measures of self-reported appetite and obestatin could also inform whether obestatin has a role in energy balance independent of leptin and ghrelin.
Acknowledgements
This research was funded by the Department of Health and Human Services, NIH NEI Training grant Number EY 07127 Clinical Trials Training Program in Vision Research, Jean Hankin Nutrition Epidemiology Grant, and OMNI-Heart Trial (HL67098, DK63214, HL68712, and RR02635).
Footnotes
Authors have no conflicts of interest to disclose.
No supplementary materials have been submitted.
Literature Cited
- 1.Zhang JV, Ren PG, Avsian-Kretchmer O, et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science. 2005 Nov 11;310(5750):996–9. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- 2.Guo ZF, Zheng X, Qin YW, Hu JQ, Chen SP, Zhang Z. Circulating preprandial ghrelin to obestatin ratio is increased in human obesity. J Clin Endocrinol Metab. 2007 Feb 13; doi: 10.1210/jc.2006-2306. [DOI] [PubMed] [Google Scholar]
- 3.Cummings DE, Foster-Schubert KE, Overduin J. Ghrelin and energy balance: Focus on current controversies. Curr Drug Targets. 2005 Mar;6(2):153–69. doi: 10.2174/1389450053174569. [DOI] [PubMed] [Google Scholar]
- 4.Tang SQ, Jiang QY, Zhang YL, et al. Obestatin: Its physicochemical characteristics and physiological functions. Peptides. 2008 Apr;29(4):639–45. doi: 10.1016/j.peptides.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Vicennati V, Genghini S, De Iasio R, Pasqui F, Pagotto U, Pasquali R. Circulating obestatin levels and the ghrelin/obestatin ratio in obese women. Eur J Endocrinol. 2007 Sep;157(3):295–301. doi: 10.1530/EJE-07-0059. [DOI] [PubMed] [Google Scholar]
- 6.Huda MS, Durham BH, Wong SP, Deepak D, Kerrigan D, McCulloch P, Ranganath L, et al. Plasma obestatin levels are lower in obese and post-gastrectomy subjects, but do not change in response to a meal. Int J Obes (Lond) 2007 Jul 31; doi: 10.1038/sj.ijo.0803694. [DOI] [PubMed] [Google Scholar]
- 7.Marzullo P, Verti B, Savia G, et al. The relationship between active ghrelin levels and human obesity involves alterations in resting energy expenditure. J Clin Endocrinol Metab. 2004 Feb;89(2):936–9. doi: 10.1210/jc.2003-031328. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994 Dec 1;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 9.Monti V, Carlson JJ, Hunt SC, Adams TD. Relationship of ghrelin and leptin hormones with body mass index and waist circumference in a random sample of adults. J Am Diet Assoc. 2006 Jun;106(6):822, 8. doi: 10.1016/j.jada.2006.03.015. quiz 829-30. [DOI] [PubMed] [Google Scholar]
- 10.Koutsari C, Karpe F, Humphreys SM, Frayn KN, Hardman AE. Plasma leptin is influenced by diet composition and exercise. Int J Obes Relat Metab Disord. 2003 Aug;27(8):901–6. doi: 10.1038/sj.ijo.0802322. [DOI] [PubMed] [Google Scholar]
- 11.Carey VJ, Bishop L, Charleston J, et al. Rationale and design of the optimal macro-nutrient intake heart trial to prevent heart disease (OMNI-heart) Clin Trials. 2005;2(6):529–37. doi: 10.1191/1740774505cn123oa. [DOI] [PubMed] [Google Scholar]
- 12.Appel LJ, Sacks FM, Carey VJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: Results of the OmniHeart randomized trial. JAMA. 2005 Nov 16;294(19):2455–64. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 13.Zizzari P, Longchamps R, Epelbaum J, Bluet-Pajot MT. Obestatin partially affects ghrelin stimulation of food intake and growth hormone secretion in rodents. Endocrinology. 2007 Apr;148(4):1648–53. doi: 10.1210/en.2006-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakahara T, Harada T, Yasuhara D, et al. Plasma obestatin concentrations are negatively correlated with body mass index, insulin resistance index, and plasma leptin concentrations in obesity and anorexia nervosa. Biol Psychiatry. 2008 Aug 1;64(3):252–5. doi: 10.1016/j.biopsych.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Shiiya T, Nakazato M, Mizuta M, et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002 Jan;87(1):240–4. doi: 10.1210/jcem.87.1.8129. [DOI] [PubMed] [Google Scholar]
- 16.Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001 Apr;50(4):707–9. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 17.Chu NF, Stampfer MJ, Spiegelman D, Rifai N, Hotamisligil GS, Rimm EB. Dietary and lifestyle factors in relation to plasma leptin concentrations among normal weight and overweight men. Int J Obes Relat Metab Disord. 2001 Jan;25(1):106–14. doi: 10.1038/sj.ijo.0801468. [DOI] [PubMed] [Google Scholar]
- 18.Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: Effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab. 1996 Dec;81(12):4406–13. doi: 10.1210/jcem.81.12.8954050. [DOI] [PubMed] [Google Scholar]
- 19.Littman AJ, Vitiello MV, Foster-Schubert K, et al. Sleep, ghrelin, leptin and changes in body weight during a 1-year moderate-intensity physical activity intervention. Int J Obes (Lond) 2007 Mar;31(3):466–75. doi: 10.1038/sj.ijo.0803438. [DOI] [PubMed] [Google Scholar]