Abstract
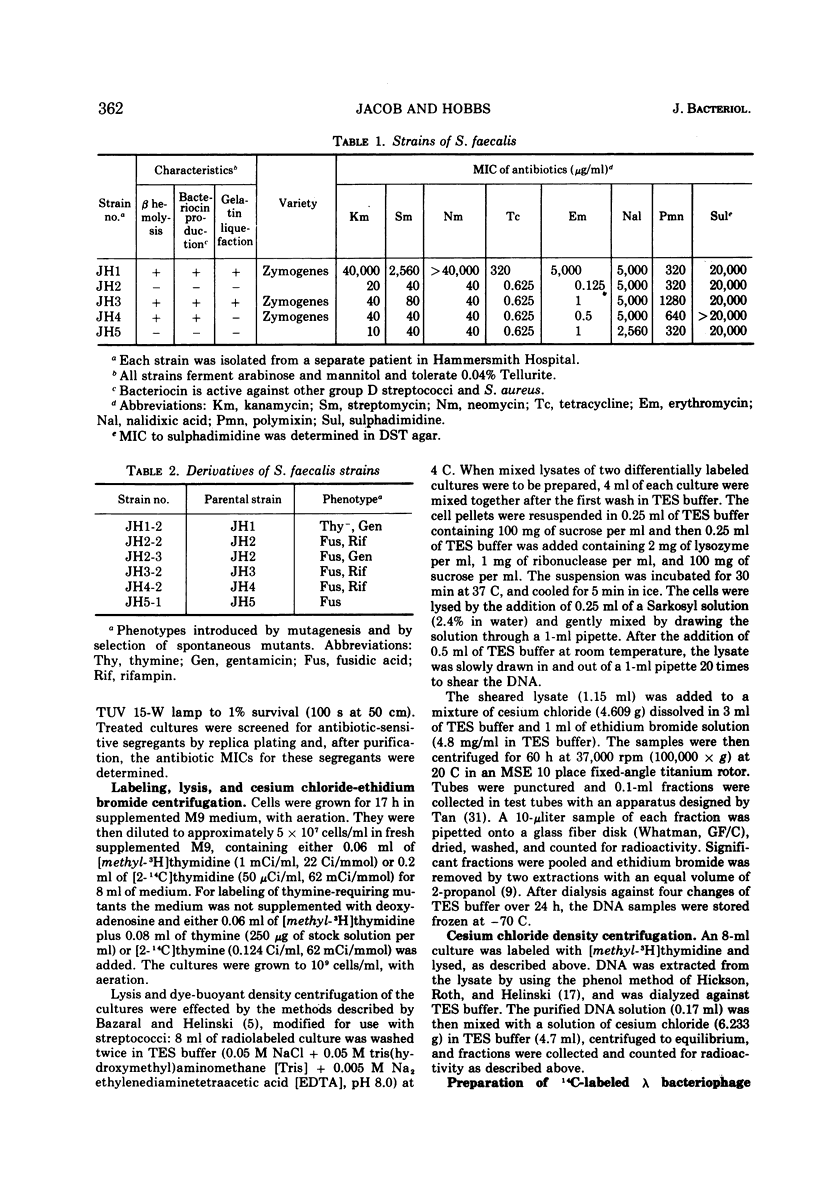
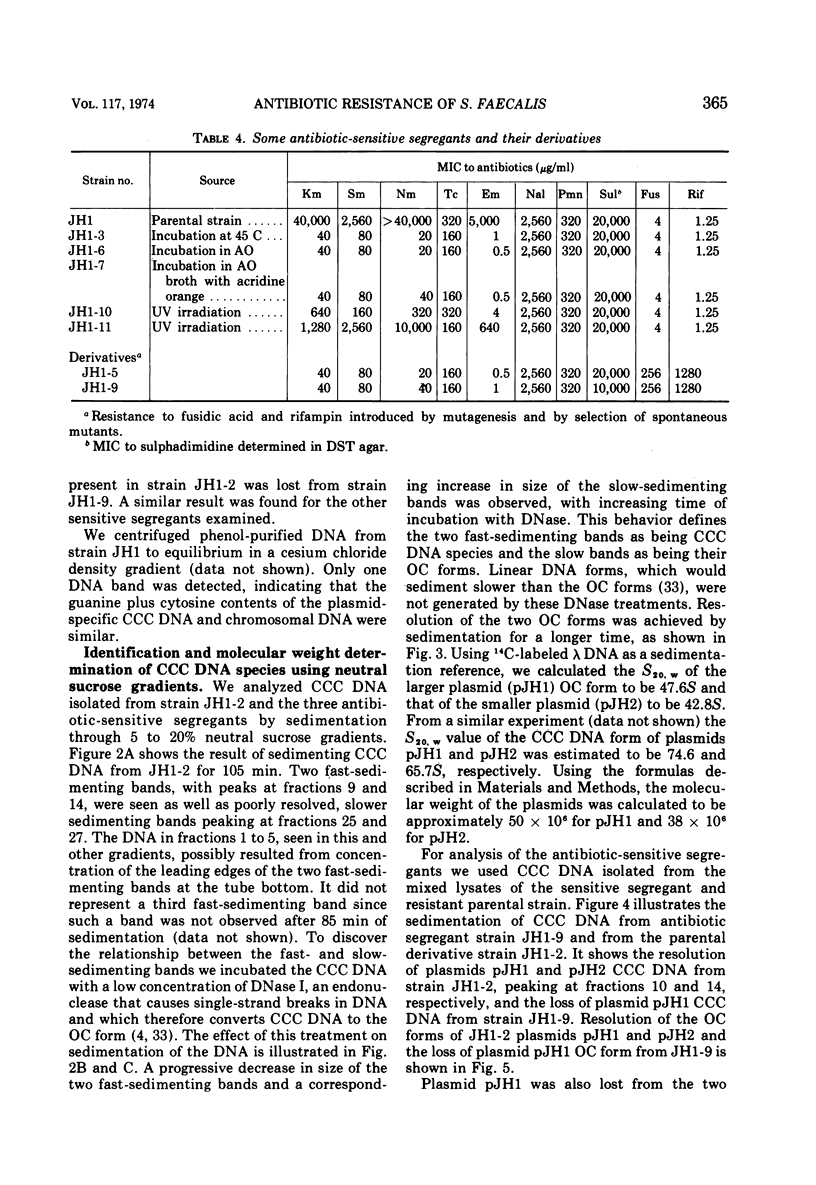
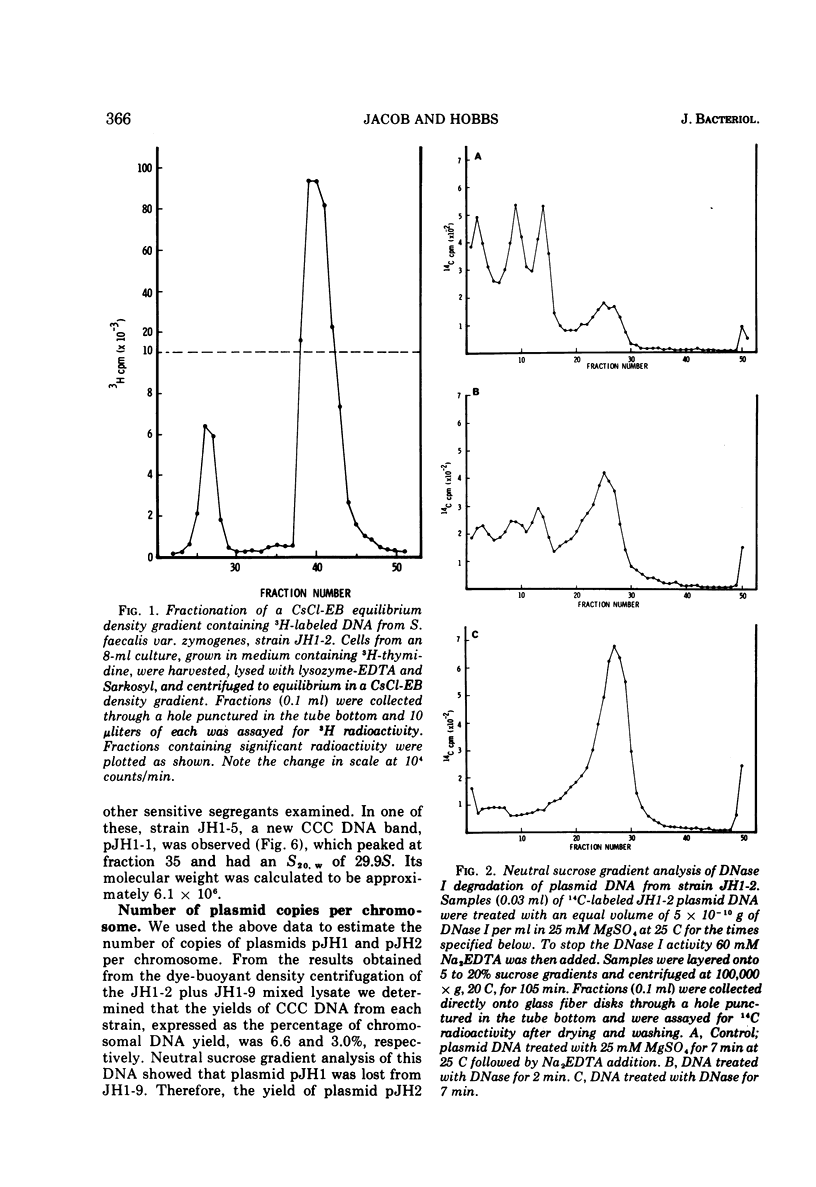
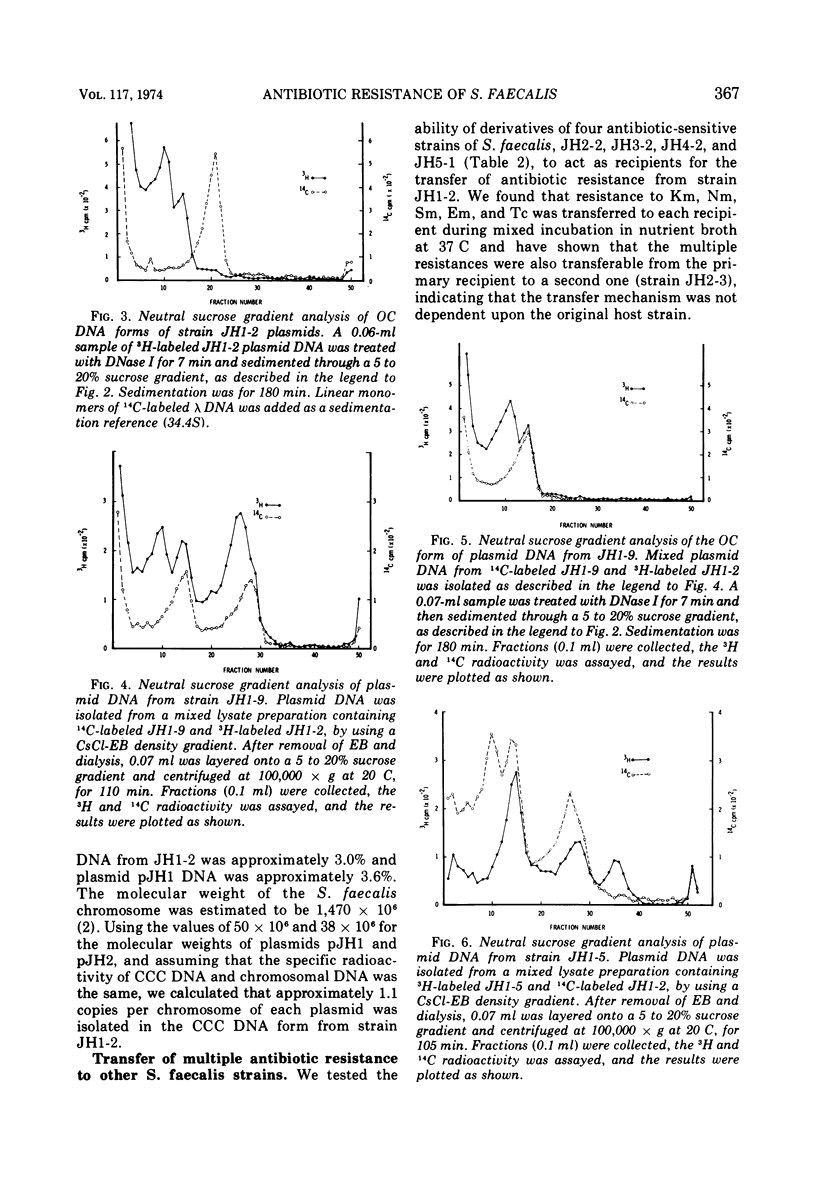
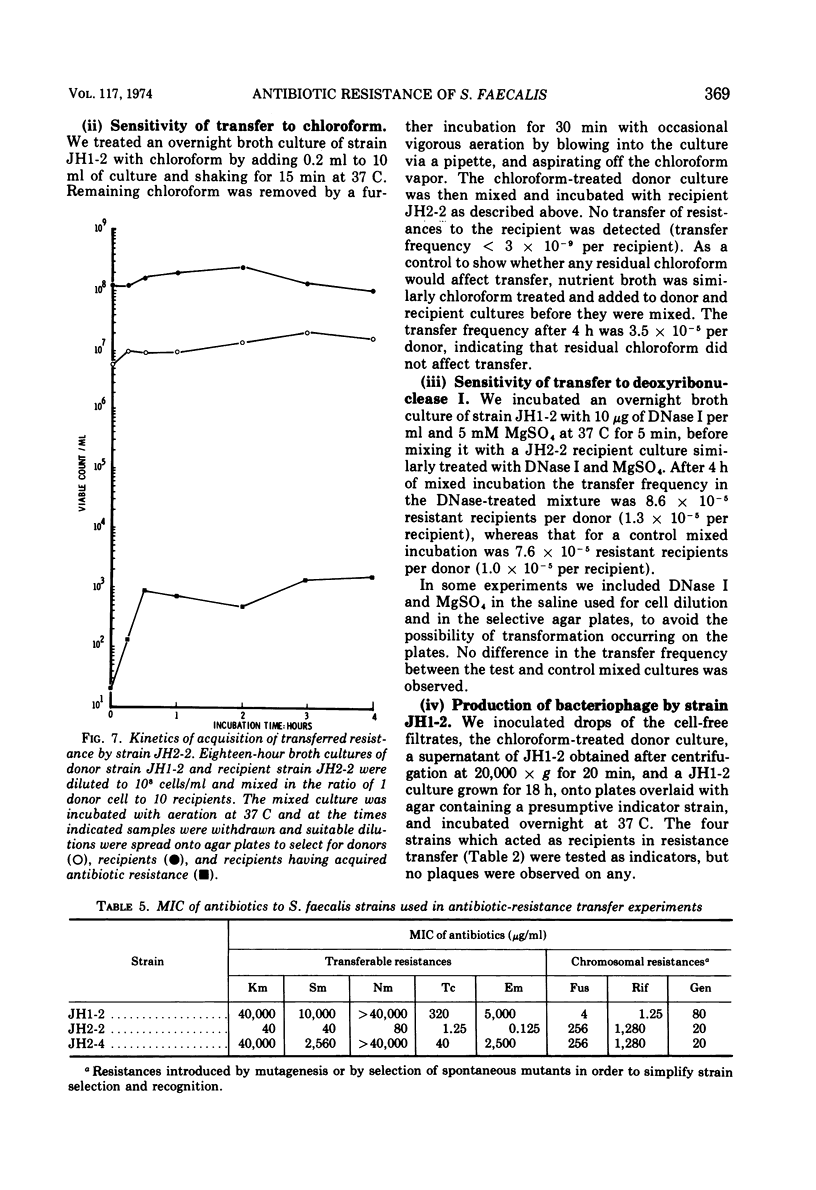
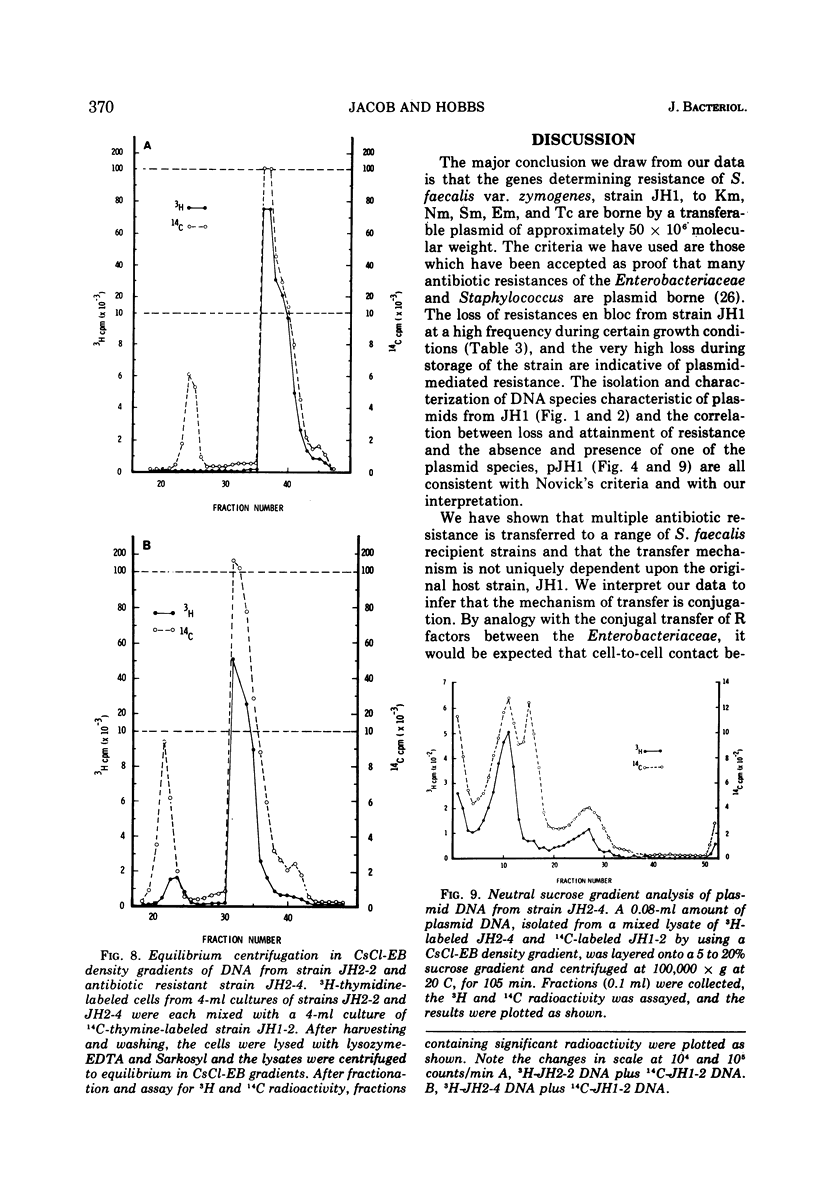
A strain of Streptococcus faecalis var. zymogenes, designated JH1, had high-level resistance to the antibiotics streptomycin, kanamycin, neomycin, erythromycin, and tetracycline. These resistances were lost en bloc from approximately 0.1% of cells grown in nutrient broth at 45 C. The frequency of resistance loss was not increased by growth in the presence of the “curing” agents acriflavine or acridine orange, but after prolonged storage in nutrient agar 17% of cells became antibiotic sensitive. Covalently closed circular deoxyribonucleic acid (DNA) molecules were isolated from the parental strain and from antibiotic-sensitive segregants by using cesium chloride-ethidium bromide gradients. DNA molecular species were identified by using neutral sucrose gradients. Strain JH1 contained two covalently closed circular DNA species of molecular weights 50 × 106 and 38 × 106. An antibiotic-sensitive segregant, strain JH1-9, had lost the larger molecular species. A second sensitive segregant, strain JH1-5, had also lost the larger molecular species but a new molecular species of approximate molecular weight 6 × 106 was present. The antibiotic resistances that were curable from the parental strain were transferred to antibiotic-sensitive strains of S. faecalis and to strain JH1-9, during mixed incubation in nutrient broth at 37 C. Data to be described are interpreted to suggest that the transfer is by a conjugal mechanism. Analysis of the plasmid species in recipient clones showed that all had received the plasmid of molecular weight 50 × 106. Strain JH1-5 was not a good recipient. Analysis of one successful recipient clone of JH1-5 revealed that it had gained the 50 × 106 molecular weight plasmid but lost the 6 × 106 molecular weight species. These data are interpreted to mean that the multiple antibiotic resistance is borne by a transferable plasmid of 50 × 106 molecular weight, and that in clone JH1-5 this plasmid suffered a large deletion leaving only a 6 × 106 remnant which was incompatible with the complete replicon.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew M. H. Use of trimethoprim to obtain thymine-requiring mutants of Streptococcus faecalis. J Gen Microbiol. 1973 Jan;74(1):195–199. doi: 10.1099/00221287-74-1-195. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak A. L., Christiansen C., Stenderup A. Bacterial genome sizes determined by DNA renaturation studies. J Gen Microbiol. 1970 Dec;64(3):377–380. doi: 10.1099/00221287-64-3-377. [DOI] [PubMed] [Google Scholar]
- Baxter-Gabbard K. L. A simple method for the large-scale preparation of sucrose gradients. FEBS Lett. 1972 Jan 15;20(1):117–119. doi: 10.1016/0014-5793(72)80031-0. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Characterization of multiple circular DNA forms of colicinogenic factor E-1 from Proteus mirabilis. Biochemistry. 1968 Oct;7(10):3513–3520. doi: 10.1021/bi00850a028. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Chabbert Y. A. Plasmid-linked tetracycline and erythromycin resistance in group D "streptococcus". Ann Inst Pasteur (Paris) 1972 Dec;123(6):755–759. [PubMed] [Google Scholar]
- Cuzin F., Vogt M., Dieckmann M., Berg P. Induction of virus multiplication in 3T3 cells transformed by a thermosensitive mutant of polyoma virus. II. Formation of oligometric polyoma DNA molecules. J Mol Biol. 1970 Feb 14;47(3):317–333. doi: 10.1016/0022-2836(70)90305-0. [DOI] [PubMed] [Google Scholar]
- Datta N., Lawn A. M., Meynell E. The relationship of F type piliation and F phage sensitivity to drug resistance transfer in R+F- Escherichia coli K 12. J Gen Microbiol. 1966 Nov;45(2):365–376. doi: 10.1099/00221287-45-2-365. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Helinski D. R., Clewell D. B. Circular DNA. Annu Rev Biochem. 1971;40:899–942. doi: 10.1146/annurev.bi.40.070171.004343. [DOI] [PubMed] [Google Scholar]
- Hershey A. D., Burgi E., Ingraham L. COHESION OF DNA MOLECULES ISOLATED FROM PHAGE LAMBDA. Proc Natl Acad Sci U S A. 1963 May;49(5):748–755. doi: 10.1073/pnas.49.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson F. T., Roth T. F., Helinski D. R. Circular DNA forms of a bacterial sex factor. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1731–1738. doi: 10.1073/pnas.58.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B., Clayton D. A., Vinograd J. Complex mitochondrial DNA. Cold Spring Harb Symp Quant Biol. 1968;33:435–442. doi: 10.1101/sqb.1968.033.01.050. [DOI] [PubMed] [Google Scholar]
- KAISER A. D. The production of phage chromosome fragments and their capacity for genetic transfer. J Mol Biol. 1962 Apr;4:275–287. doi: 10.1016/s0022-2836(62)80005-9. [DOI] [PubMed] [Google Scholar]
- KORN D., WEISSBACH A. Thymineless induction in Escherichia coli K12 (lambda). Biochim Biophys Acta. 1962 Nov 26;61:775–790. doi: 10.1016/0926-6550(62)90060-9. [DOI] [PubMed] [Google Scholar]
- Kornberg A., Zimmerman S. B., Kornberg S. R., Josse J. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. INFLUENCE OF BACTERIOPHAGE T2 ON THE SYNTHETIC PATHWAY IN HOST CELLS. Proc Natl Acad Sci U S A. 1959 Jun;45(6):772–785. doi: 10.1073/pnas.45.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W. Transfer of tetracycline-resistance between strains of Staphylococcus aureus in mixed cultures. J Gen Microbiol. 1971 Dec;69(2):229–237. doi: 10.1099/00221287-69-2-229. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAYCROFT R. E., ZIMMERMAN L. N. NEW MODE OF GENETIC TRANSFER IN STREPTOCOCCUS FAECALIS VAR. LIQUEFACIENS. J Bacteriol. 1964 Apr;87:799–801. doi: 10.1128/jb.87.4.799-801.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Tan K. B. A simple device for fractionating density gradients. Anal Biochem. 1972 Jan;45(1):306–308. doi: 10.1016/0003-2697(72)90031-0. [DOI] [PubMed] [Google Scholar]
- Toala P., McDonald A., Wilcox C., Finland M. Susceptibility of group D streptococcus (enterococcus) to 21 antibiotics in vitro, with special reference to species differences. Am J Med Sci. 1969 Dec;258(6):416–430. doi: 10.1097/00000441-196912000-00006. [DOI] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963 Mar;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. T., 2nd, Sinsheimer R. L. Vegetative bacteriophage lambda-DNA. I. Infectivity in a spheroplast assay. J Mol Biol. 1967 Nov 28;30(1):147–164. doi: 10.1016/0022-2836(67)90250-1. [DOI] [PubMed] [Google Scholar]



