Introduction
Pancreatic cancer, the 4th leading cause of cancer deaths in men and women in the United States,1 disproportionately affects the elderly. The overall annual incidence of pancreatic cancer is approximately 11 cases per 100,000 population and over 80% of pancreatic cancer patients are over the age of 60. The incidence increases sharply with age. Patients 20-29 years old have an annual incidence of 0.1 cases of pancreatic cancer per 100,000 population, while patients 80 and older have an annual incidence of 87.2 cases per 100,000 population.2 The same is true for other periampullary adenocarcinomas including distal bile duct cancer, ampullary cancer and duodenal cancer.
People aged 65 and older represent the fastest growing subset of the United States population. By the year 2030 people over 65 are expected to account for one fifth of the population. By 2050, people over 85 years of age are expected to account for 24% of the elderly population and 5% of the overall population.3 The growing elderly population is expected to have a significant effect on the surgical workforce, with a 28% increase in General Surgery volume expected between 2001 and 2020.4
Currently, pancreatic resection is the only potentially curative option for patients with pancreatic and other periampullary cancers. The 5-year survival following pancreaticoduodenectomy for pancreatic cancer is approximately 15-20%.5 5-year survival is higher for patients with distal bile duct cancer (20-25%), ampullary cancer (30-40%), and duodenal cancer (50-60%).5 In the 1970s and early 1980s the high morbidity and mortality rates following pancreatic surgery led many surgeons to recommend palliative care only for patients with periampullary cancers.6, 7 As pancreatic resection became safer and was accepted as the standard of care the indications remained narrow and it was not commonly performed in patients over 70 and rarely, if ever, in patients over 80.
In the last three decades the mortality rates following pancreatic resection for pancreatic cancer, periampullary cancer, and other benign and malignant diseases have dropped to less than 2% at experienced centers.8-12 Given the low mortality rates the indications for pancreatic resection continue to expand. However, pancreatic resection still carries significant risk with reported morbidity rates exceeding 30%.13-15 Starting in the 1990s many individual centers began reporting their results following pancreatic resection in the elderly.16-25 The age cutoffs for “elderly” vary significantly among the different studies varying from 65 to 80 years. The majority of studies report statistically higher morbidity rates in the group they define as “older” patients when compared to younger patients.17, 19, 22, 24, 25 Some studies show no difference in mortality,20-22, 24, 25 while other demonstrate higher mortality in the “older” patients.17-19 Likewise, some studies show that survival is the same in the elderly population following pancreatic resection,17, 22 while others demonstrate lower survival rates in elderly patients.20 Recent population-based studies suggest increased morbidity and worse short-term outcomes in elderly patients undergoing pancreatic resection.26, 27
Currently, age alone is not a contraindication to pancreatic resection. However, the complex nature of pancreatic surgery and the relatively low survival rates following pancreatic resection for pancreatic cancer make decisions regarding surgery in the elderly population difficult. This chapter will review the literature to understand the independent effect of age on the receipt of surgical resection for early stage pancreatic cancer as well as the effect of age on both long- and short-term outcomes following pancreatic resection. The information provided will help guide surgeons, oncologists, and primary care physicians in counseling elderly patients regarding the risks and benefits of surgical resection.
Age and Surgical Resection Rates
At the national level, surgical resection is underutilized for patients with pancreatic cancer with only 27-35% of patients with locoregional disease being resected.28-30 An analysis of the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked data demonstrated that in patients with locoregional pancreatic cancer, the likelihood of being evaluated by a surgeon decreased 8% with each increasing year of age (Odds Ratio (OR) for surgical evaluation = 0.92, 95% Confidence Interval (CI) 0.90 – 0.93) in a multivariate logistic regression analysis.31 This model controlled for year of diagnosis, sex, race, marital status, socioeconomic status, SEER region, nodal status, and patient comorbidities.
In addition, when evaluating the factors that influenced the receipt of surgical resection, the study demonstrated patients were 9% less likely to undergo surgical resection with each increasing year of age (OR = 0.91, 95% CI 0.90 – 0.92). A decrease in surgical resection rates was noted regardless of patient comorbidities. Even in patients with no comorbidities, resection rates decreased from 40% of patients 66-70, to 37% of patients 70-74, to 32% of patients 75-79, to 21% of patients 80-84, to only 7% of patients 85 and older.32
Age and Short-Term Outcomes Following Pancreatic Resection
Single-Institution Studies
In the past fifteen years there have been multiple single-institution retrospective studies evaluating outcomes following pancreatic resection in elderly patients. These studies differ in their definition of “elderly”. In addition, some series include both periampullary cancer and non-cancer patients while others are restricted to cancer patients. Table 1 summarizes the age group definitions, number of patients, overall morbidity rates by age group, mortality rates by age group, and lengths of stay for the largest series.
Table 1. Previous Studies Evaluating Short-term Outcomes in Elderly Patients Undergoing Pancreatic Resection.
| Year | Age Group | N | Mortality | Overall Morbidity | Length of Stay (median in days) | |
|---|---|---|---|---|---|---|
| Single-institution | ||||||
| Fong et al.20 | 1995 | <70 | 350 | 4.0% | 39% | 20 |
| >=70 | 138 | 5.8% | 45% | 20 | ||
| Richter et al.23 | 1996 | <=70 | 293 | 1.9%* | 22%* | NR |
| >70 | 45 | 4.3%* | 39%* | NR | ||
| Sohn et al.24 | 1998 | <80 | 681 | 1.6% | 41%* | 13 |
| >=80 | 46 | 4.3% | 57%* | 15* | ||
| al-Sharaf et al.16 | 1999 | <70 | 74 (total) | 4.0% | NR | NR |
| >=70 | 74 (total) | 7.0% | NR | NR | ||
| 3.2% (head) | 16%* (head) | |||||
| Bottger et al.18 | 1999 | <=70 | 300 (total) | 1.7%* (distal) | 10%* (distal) | NR |
| 2.3% (head) | 28%* (head) | |||||
| >70 | 300 (total) | 14%* (distal) | 29%* (distal) | NR | ||
| Bathe et al.17 | 2000 | 60-74 | 54 | 3.7% | 31%* | 18 (mean)a |
| >=75 | 16 | 25.0% | 63%* | 25 (mean) | ||
| Hodul et al.21 | 2000 | <70 | 74 | 1.4% | 65% | NR |
| >=70 | 48 | 0.0% | 60% | NR | ||
| Brozetti et al.19 | 2006 | <70 | 109 | 3.7%* | 46%* | 16.3 (mean) |
| >=70 | 57 | 10.5%* | 49%* | 16 (mean) | ||
| Makary et al.22 | 2006 | <80 | 2,491 | 1.7% | 42% | 10 |
| 80-89 | 197 | 4.1%** | 53%** | 11 | ||
| >=90 | 10 | 0.0% | 50% | 12 | ||
| Scurtu et al.25 | 2006 | 70-75 | 38 | 0.0% | 37% | 17 (mean) |
| >=75 | 32 | 6.2% | 50% | 19 (mean) | ||
| Population-based | ||||||
| Finlayson et al.26 | 2007 | 65-69 | 7,125 (weighted)b | 6.7%* | NR | 17.4* (mean) |
| 70-79 | 13,478 (weighted) | 9.3%* | NR | 18.2* (mean) | ||
| >=80 | 2,915 (weighted) | 15.5%* | NR | 20.4* (mean) | ||
| Riall et al.27 | 2008 | <60 | 1,780 | 2.4%* | NR | 11* |
| 60-69 | 887 | 5.8%* | NR | 13* | ||
| 70-79 | 855 | 7.4%* | NR | 14* | ||
| >=80 | 214 | 11.4%* | NR | 15* |
P<0.05 younger vs. older group or overall chi-square P-value if multiple groups
P<0.05 compared to patients < 80
Mean is given and not median when noted
Number is weighted since it comes from a random sample
Mortality
Seventy years of age is the most common cutoff for “elderly” used in these studies.16, 18-21, 23 The two overlapping series from Johns Hopkins22, 24 compare octogenarians to younger patients while the later series reports specifically on ten patients older than 90.22
In the vast majority of studies, the reported mortality rates were higher in the group defined as elderly (Table 1).16, 17, 19, 20, 22-25 Given small numbers of patients, this difference often did not achieve statistical significance. In the large series from Johns Hopkins (n=2,698) the mortality rate was 4.1% in patients 80 and older and 1.7% in patients <80 (P<0.05). It should be noted that, while the mortality rates were higher in older patients they were less than 5% in several series18, 21-24 with the exception of a few outliers. It is important to remember that these series were generated at high-volume centers specializing in pancreatic surgery and the differences in mortality rates between age groups are more striking in the population-based studies discussed below.
Overall Morbidity
Most studies demonstrate increased morbidity following surgical resection in the age group defined as elderly. As you can see from looking at Table 2 the overall postoperative morbidity rates varied widely from institution to institution ranging from as low as 16% to as high as 65%. Despite this the morbidity rates are consistently higher in the older age group. Richter and colleagues23 in Germany reported a 21.5% morbidity rate in patients ≤70 (n=293) and a 39.1% morbidty rate in patients >70 years (n=45, P<0.05). Likewise, Bathe and colleagues in Miami reported a 31% morbidity rate in patients 60-74 years of age (n=54) and a 63% morbidity rate in patients ≥75, (n=16, P=0.03).17 The largest single institution series was from Johns Hopkins, comparing 2,491 patients <80, 197 patients 80-89, and 10 patients ≥90 years old.22 This study reported morbidity rates of 41.6% in the youngest group, 52.8% in the 80-89 year group (P<0.05 compared to patients <80), and 50.0% in patients ≥90 (P=NS when compared to patients <80).
Table 2. Texas Discharge Data: Demographic Characteristics, Diagnoses, and Procedure Type by Age Group.
| <60 years (n=1,780) | 60-69 years (N=887) | 70-79 years (N=855) | 80+ years (N=214) | P-value | |
|---|---|---|---|---|---|
| Female | 53.9% | 49.0% | 49.4% | 54.7% | 0.04 |
| Caucasian | 56.6% | 66.1% | 70.2% | 74.3% | <0.0001 |
| Uninsured | 9.9% | 5.8% | 1.5% | 2.3% | <0.0001 |
| Periampullary Cancer | 44.1% | 68.3% | 71.6% | 76.6% | <0.0001 |
| Resected at HV hospital | 62.3% | 61.9% | 57.4% | 53.7% | 0.01 |
| Elective Procedures | 69.0% | 75.7% | 75.7% | 71.4% | 0.0002 |
| Procedure | <0.0001 | ||||
| Head resection | 59.9% | 76.0% | 75.9% | 72.4% | |
| Distal resection | 31.9% | 17.8% | 19.0% | 22.4% | |
| Pancreatic resection, unspecified | 8.2% | 6.2% | 5.1% | 5.2% |
With permission from Riall et al. Ann Surg 2008;248: p. 462.27
Several studies did not show a statistical difference in overall morbidty rates between the older and younger groups, but all demonstrated a trend in this direction. The first was a French study comparing 38 patients aged 70-74 years to 32 patients ≥75 years.25 While this study did not demonstrate a statistical difference, the morbidity rates were 36.8% and 50.0% in the younger and older groups respectively, suggesting a type II error due to small numbers of patients. An Italian study of 109 patients <70 and 57 patients ≥70 showed morbidity rates of 45.8% and 49.1%, respectively.19 The last study by Hodul and colleagues compared 74 patients <70 years to 48 patients ≥70 years.21 They reported a 65% morbidity rate in the younger group and 60% morbidity rate in the older group. This lack of difference may reflect that fact that the mortality reported in this series exceeds that reported in all other series.
Specific Morbidity
While it did not show a statistically significant difference in overall morbidity rates, the French study by Scurtu and colleagues25 did demonstrate a statistically higher incidence of delayed gastric emptying in the older group (12.5% vs. 0%, P=0.04). This is consistent with the findings in other studies with the early Hopkins study showing a 33% incidence of delayed gastric emptying in the patients ≥80 and an 18.6% rate of delayed gastric emptying in patients <80 (P=0.03). Other studies showed a similar trend in the occurrence of delayed gastric emptying,21 but did not achieve statistical significance. Many studies did not report this directly.
In the study by Hodul et al.21 there was a 9.4% rate of neurologic complications in the older group and none in the younger group. This difference approached but did not achieve significance (P=0.09). No other studies reported this specific complication.
In the studies that reported cardiac complications, they were higher in the older group. Makary et al.22 reported a 12.5% rate of cardiac complications in patients ≥90, an 8.3% rate in patients 80-89, and a 3.6% rate in patients <80 (P<0.05 when comparing the <80 and 80-89 age groups). Bathe et al.17 reported a 12.5% incidence of cardiac complications in patients ≥75 years and a 2.4% incidence in patients 60-74 years (P=NS) and Hodul et al.21 reported a 17% rate in patients ≥70 and a 5% rate in patients <70 years (P=NS). The incidence of pneumonia was not commonly reported but was increased with increasing age in the Hopkins series. 10.0% of patients ≥90, 5.6% of patients 80-89, and 1.1% of patients <80 developed pneumonia in the postoperative period (P<0.05 for both older age groups compared to patients <80).
The incidence of urinary tract infections did not achieve statistical significance in any studies, but was consistently higher in the groups defined as elderly. As might be expected, the incidence of other specific surgical complications including pancreatic fistula, intraabdominal abscess, bile leak, sepsis, and cholangitis did not differ between the age groups in any studies.
Length of Stay
For the groups reporting length of stay, there was not a significant difference between age groups despite the higher observed complication rates in elderly patients. The lengths of stay varied significantly between different series and can be seen in Table 1. Scurtu and colleagues25 reported readmission rates in the 70-74 year and ≥75 year age groups and reported no differences.
Population-Based Studies
There is some concern that the results from the previously described series might lead to unrealistic expectations of outcomes following pancreatic surgery in the elderly population given that these are all single-institution studies performed at high-volume centers. It is well documented that the surgical outcomes following pancreatic surgery are better at high-volume, experienced centers.33-42 Two recent population-based studies evaluate short-term outcomes following pancreatic resection. Since both studies use administrative data sources they report primarily mortality rates, lengths of stay, and discharge disposition, but do not report overall morbidity rates or rates of specific complications.
Mortality
A recent study used the Nationwide Inpatient Sample (NIS) to examine the operative mortality and discharge disposition among over 200,000 patients aged 65 and older undergoing lung, esophageal, and pancreatic surgery.26 For patients undergoing pancreatic surgery the operative mortality increased from 6.7% in patients aged 65-69, to 9.3% in patients aged 70-79, to 15.5% in patients aged 80 and older (P<0.0001). Likewise, a study using the Texas Hospital Inpatient Discharge Public Use Data File reported on 3,736 patients undergoing pancreatic resection for all causes in Texas.27 1,780 patients were <60, 887 were 60-69, 855 were 70-79, and 214 were 80 or older. The unadjusted mortality rates increased from 2.4%, to 5.8%, to 7.4%, to 11.4% with each increasing age group respectively (P<0.0001).
In the Texas study, with increasing age group patients were more likely to be female and Caucasian. They were also more likely to have a diagnosis of periampullary cancer, to have insurance, to be more severely ill, to have a higher risk of mortality, and to be resected at a low-volume hospital (<11 pancreatic resections per year) as the age group increased (Table 2). In a multivariate logistic regression analysis, age was an independent predictor of operative mortality (Table 3). This model controlled for gender, race, year of surgery, diagnosis, admission status (emergent vs. elective), risk of mortality, severity of illness, hospital volume, and type of procedure (left or right pancreatectomy). When compared to patients <60 years of age, patients aged 60-69 were 2.53 times more likely to die, patients aged 70-79 were 1.81 times more likely to die, and patients aged 80 and older were 4.45 times more likely to die (Table 3).27
Table 3. Multivariate Logistic Regression Analysis: Effect of Age on Mortality and Discharge Status.
| In-hospital Mortality | Discharge other than home* | |||
|---|---|---|---|---|
| Factor | Odds Ratio | 95% CI | OR | 95% CI |
| Age Group | ||||
| <60 | 1.00 | ∼ | 1.00 | ∼ |
| 60 – 69 | 2.53 | 1.53 – 4.17 | 1.91 | 1.27 – 2.87 |
| 70 – 79 | 1.81 | 1.12 – 2.93 | 5.61 | 3.95 – 7.96 |
| 80+ | 4.45 | 2.31 – 8.57 | 16.35 | 10.41 – 25.67 |
| Gender | ||||
| Male | 1.00 | ∼ | 1.00 | ∼ |
| Female | 0.77 | 0.54 – 1.11 | 1.16 | 0.90 – 1.50 |
| Race | ||||
| White | 1.00 | ∼ | 1.00 | ∼ |
| Black | 1.57 | 0.91 – 2.67 | 1.50 | 1.00 – 2.26 |
| Hispanic | 0.95 | 0.59 – 1.54 | 0.98 | 0.69 – 1.39 |
| Other | 1.48 | 0.75 – 2.91 | 1.35 | 0.82 – 2.52 |
| Hospital Volume | ||||
| Low-volume (≤10/year) | 1.00 | ∼ | 1.00 | ∼ |
| High-volume (>10/year) | 0.50 | 0.34 – 0.73 | 0.46 | 0.35 – 0.60 |
| Year of Operation | 0.89 | 0.81 – 0.97 | 1.07 | 1.00 – 1.15 |
| Diagnosis | ||||
| Periampullary cancer | 1.00 | ∼ | 1.00 | ∼ |
| Other | 1.47 | 0.97 – 2.21 | 1.27 | 0.93 – 1.72 |
| Risk of Mortality | ||||
| 1 | 1.00 | ∼ | 1.00 | ∼ |
| 2 | 1.87 | 0.70 – 4.96 | 1.49 | 0.98 – 2.28 |
| 3 | 6.23 | 2.55 – 15.22 | 3.20 | 2.11 – 4.86 |
| 4 | 60.92 | 25.2 – 147.3 | 4.81 | 2.95 – 7.85 |
| Illness Severity | ||||
| 1 | 1.00 | ∼ | 1.00 | ∼ |
| 2 | 1.19 | 0.14 – 10.49 | 3.94 | 0.50 – 30.78 |
| 3 | 0.57 | 0.07 – 4.97 | 9.63 | 1.27 – 72.97 |
| 4 | 1.03 | 0.12 – 8.97 | 15.00 | 1.96 – 115.12 |
| Admission Status | ||||
| Elective | 1.00 | ∼ | 1.00 | ∼ |
| Urgent/Emergent | 1.45 | 0.98 – 2.14 | 1.30 | 0.98 – 1.72 |
| Procedure | ||||
| Head resection | 1.00 | ∼ | 1.00 | ∼ |
| Distal resection | 0.46 | 0.25 – 0.85 | 0.66 | 0.44 – 0.99 |
| Pancreatectomy, not specified | 0.47 | 0.20 – 1.09 | 1.11 | 0.62 – 1.97 |
Includes skilled nursing facilities, intermediate nursing facilities, other inpatient acute care hospitals, and Medicare- and Medicaid-certified long-term care hospitals or nursing facilities. Excludes hospital mortalities.
With permission from Riall et al. Ann Surg 2008;248:p. 464.27
In the Texas study, low-volume hospitals (<11 resections per year) had higher mortality rates than high-volume hospitals (7.3% vs. 3.2%, P<0.0001). Importantly, the difference in mortality between high- and low-volume hospitals was accentuated with each increasing age group. For patients <60 years, mortality was 2.0% at high-volume hospitals and 3.0% at low-volume hospitals. This increased to 3.5% at high-volume hospitals and 9.5% at low-volume hospitals for patients 60-69 years. In the 70-79year age group, the mortality rate increased to 4.5% at high-volume hospitals and 11.4% at low-volume hospitals. Finally, in the patients 80 and older, mortality was 8.7% at high-volume hospitals and 14.5% at low-volume hospitals (Table 4). These results imply that the effect of hospital volume is even greater in elderly patients and these patients are best treated at high-volume centers. In addition, this study also documents that elderly patients are less likely to receive care at high-volume hospitals (Table 2). 63% of patients <60, 62% of patients 60-69, 57% of patients 70-79, and only 54% of patients 80 and older were resected at high volume hospitals (P=0.01).27
Table 4. Texas Discharge Data – Mortality by Age Group at High- and Low-Volume Hospitals*.
| Unadjusted Mortality | ||
|---|---|---|
| Age Group | High-Volume (>10 cases/year) | Low-Volume (<=10 cases/year) |
| <60 | 2.0% | 3.0% |
| 60-69 | 3.5% | 9.5% |
| 70-79 | 4.5% | 11.4% |
| >=80 | 8.7% | 14.5% |
Table created from existing data.27
Length of Stay and Disposition at Discharge
In both the Texas study and the NIS study,26, 27 lengths of stay were significantly higher with increasing age. While these studies do not directly measure overall or specific morbidity rates, the length of stay is a good surrogate with longer lengths of stay most likely reflecting a complicated postoperative course. In the NIS study, the mean length of stay increased from 17.4 days in the 65-69 age group, to 18.2 days in the 70-79 age group, to 20.4 days in the 80 and older age group (P<0.0001).26 A similar pattern was found in the Texas data with mean length of stay increasing from 16.0 days (median = 11 days) in patients < 60 to 16.5 days (median = 13 days) in patients 60-69, to 17.6 days (median = 14 days) in patients 70-79, to 17.9 days (median = 15 days) in patients 80 and older (P=0.02).27
In addition, both studies found the number of patients requiring ongoing inpatient nursing care at discharge increased significantly with age. In the NIS study, 10.6% of patients 65-59 were unable to be discharged home, 19.2% of patients aged 70-79 were unable to be discharged home, and 36.7% of patients 80 and older were unable to be discharged home (P<0.0001).26 Similarly, in the Texas study, 3.5% of patients <60, 6.2% of patients 60-69, 20.2% of patients 70-79, and 38.2% of patients 80 and older were unable to be discharged home (P<0.0001).27 This information is not provided in the single-institution studies and is important in counseling elderly patients regarding the expected postoperative course.
In a multivariate logistic regression analysis, age was an independent predictor of discharge status (Table 3). When compared to patients <60, patients aged 60-69 were 1.9 times as likely (95% CI 1.3 – 2.9), patients aged 70-79 were 5.6 times as likely (95% CI 4.0 – 7.9), and patients 80 and older were 16.4 times as likely to require inpatient nursing care at discharge (95% CI 10.4 – 25.7).
Age and Long-Term Survival Following Pancreatic Resection for Periampullary Cancer
Several single-institution studies and two population based studies have evaluated long-term survival in elderly patients undergoing pancreatic resection for periampullary cancer. It is critical to remember that elderly patients have increased operative mortality as well as increased competing mortality risks. As a result, the survival statistics reported following pancreatic resection for periampullary cancer may not be generalizable to the elderly population. This can make it difficult for elderly patients and their physicians to realistically assess the risks and benefits of surgery for periampullary cancer and may contribute to the nihilistic attitude and underutilization of surgical resection in elderly patients.
Single-Institution Studies
Several single-institution studies describing the short-term outcomes following pancreatic surgery in the elderly also reported on long-term survival. The reported 5-year survival rates are shown in Table 5. As you can see in Table 5, most series reported a lower 5-year survival rate in the elderly group, but this rarely achieved statistical significance due to small sample sizes. The Memorial Sloan Kettering group did show a statistical difference in 5-year survival between patients 70 and older (21%, median = 18 months) and patients <70 (29%, median = 24 months, P=0.03, Figure 1).20 All of these patients had periampullary cancers including pancreatic cancer, distal bile duct cancer, ampullary cancer, and duodenal cancer. The largest single-institution series from Johns Hopkins reported on 1,022 patients <80 and 102 patients 80-89 with pancreatic cancer only. They demonstrated a 2-year survival rate of 37.7% in the younger group (median = 18 months) and 33.0% in the older group (median = 11 months, P=0.0002, Figure 2).22
Table 5. Previous Studies Evaluating Long-term Survival in Elderly Patients Undergoing Pancreatic Resection for Pancreatic or Periampullary Cancer.
| Year | Age Group | Diagnosis | 5-year Survival | Median Survival | P-value | |
|---|---|---|---|---|---|---|
| Single-institution | ||||||
| Fong et al.20 | 1995 | <70 | Periampullary Cancer | 29% | 24 months | 0.03 |
| >=70 | Periampullary Cancer | 21% | 18 months | |||
| Sohn et al.24 | 1998 | <80 | Periampullary Cancer | 27% | 20 months | NS |
| >=80 | Periampullary Cancer | 19% | 19 months | |||
| Bathe et al. 17 | 2000 | 60-74 | Periampullary Cancer | 23% | 24 months | NS |
| >=75 | Periampullary Cancer | 31% | 9 months | |||
| Bathe et al.44 | 2001 | <65 | Periampullary Cancer | NR | 12 months | 0.02 |
| 65-74 | Periampullary Cancer | NR | 25 months | |||
| >=75 | Periampullary Cancer | NR | 11 months | |||
| Makary et al.22 | 2006 | <80 | Periampullary Cancer | 19% | 18 months | 0.002 |
| >=80 | Periampullary Cancer | 12% | 11 months | |||
| Scurtu et al.25 | 2006 | 70-75 | Pancreatic Cancer | 33% (3-year) | 12 months | NS |
| >=75 | Pancreatic Cancer | 28% (3-year) | 13 months | |||
| Population-based | ||||||
| Finlayson et al.26 | 2007 | 65-69 | Pancreatic Cancer | 16.4% | NR | 0.28 |
| 70-79 | Pancreatic Cancer | 15.6% | NR | |||
| >=80 | Pancreatic Cancer | 11.3% | NR |
Figure 1.
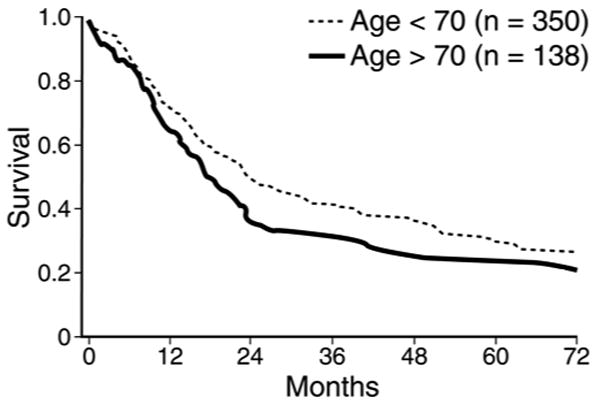
Survival after pancreatic resection for patients age 70 years or older (n=138) compared with that of patients younger than 70 years of age (n=350, p = 0.03).
Figure 2.
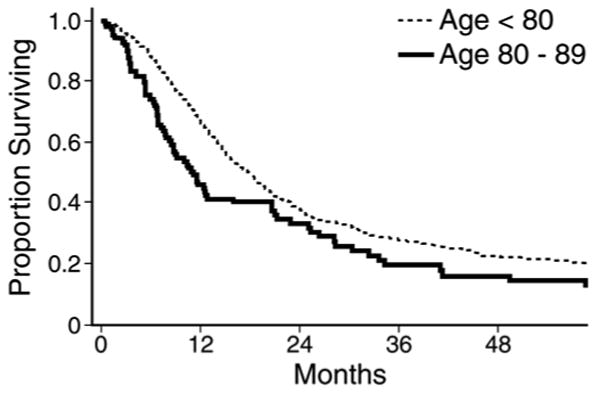
Actuarial survival curves comparing all patients with pancreatic cancer under 80 years of age (n=1022, median survival 18 months, 2-year survival 37.7%) to patients aged 80-89 (n=102, median survival 11 months, 2-year survival 33.0%, P=0.002).
The same series also reported long-term survival in 80-89 year old patients who survived 25 months after surgery. Those individuals who were alive 25 months after surgery had survival curves similar to those of age-matched controls (82 year olds in the general population).22
Population-Based Studies
Finlayson and colleagues used the SEER-Medicare linked data to evaluate 5-year survival following surgery for pancreatic cancer. 5-year survival decreased from 16.4% in patients 65-69, to 15.6% in patients 70-79, to 11.3% in patients 80 and older, but this difference did not achieve statistical significance. (P=0.28). Moreover, patients with more than two comorbidities undergoing pancreatectomy for pancreatic cancer had a 5-year survival rate of 10% compared to 14% for those with less than two comorbidities (P=NS).
In another analysis of the SEER-Medicare database, long-term survival was evaluated in 1,229 patients undergoing pancreatic resection for locoregional pancreatic cancer. In a Cox proportional hazards model, surgical resection improved disease-specific survival in all age groups, but this improvement was most striking in patients 80 and older (Table 6). For patients 66-79 undergoing surgical resection, the hazard ratio (HR) was 0.43 (95% confidence interval (CI) 0.36 – 0.52). For patients 70-79 years and patients 80 and older the benefit of surgical resection remained clear. The 70-79 year old group had a HR of 0.47 (95% CI 0.41 – 0.53) and the 80 and older group had a HR of 0.36 (95% CI, 0.28 – 0.45).32 This finding suggests that, despite the increased morbidity and mortality after pancreatic resection in the elderly, surgical resection remains the only hope for cure and the benefit of surgical resection does not diminish with age.
Table 6. Cox Proportional Hazards Model.
| Covariate | HR | 95% CI |
|---|---|---|
| Resected and < 70 years | 0.43 | 0.36, 0.52 |
| Resected and 70-79 years | 0.47 | 0.41, 0.53 |
| Resected and 80 years or older | 0.36 | 0.28, 0.45 |
In both studies, the survival analysis is limited by a relatively small sample size with fewer than 200 patients over the age of 80. In addition, the younger cohort starts at age 65 since Medicare data is used, perhaps underestimating the disparity in long-term survival between the octogenarians and younger patients. The use of administrative data also limits the studies ability to risk-adjust clinical outcomes and adequately account for case-mix differences among the different age groups.43
While elderly patients have increased morbidity, mortality, and a longer convalescence following pancreatic surgery, the survival benefit of surgical resection for pancreatic cancer is not diminished with age. However, comorbidities and other competing risks in this age group make overall survival lower in older patients. Careful selection of elderly patients is critical. The information in these studies will provide essential information needed for providers and patients to make informed decisions.
Conclusions
Both morbidity and mortality rates following pancreatic resection increase with advanced age. The reported mortality rates following pancreatic surgery are underestimated in single-institution studies. There is a significant publication bias where only centers with good results report their outcomes. Therefore, the population-based data are critical to provide a more realistic view of mortality rates following pancreatic resection. It is essential in counseling elderly patients that they understand that mortality rates are increased, morbidity rates are increased, and the effect of complications often leads to a prolonged convalescence. They will have a longer length of stay and up to a 30-40% chance that they will not be able to go home but will need to recover in an extended care facility following hospital discharge. However, while the morbidity and mortality rates are increased for elderly patients they are well within the acceptable range for major abdominal surgery when performed at experienced centers.
Patients also need to be aware that surgical resection is the only curative option for pancreatic cancer. In reasonable risk elderly patients the benefit of surgical resection does not decrease with age and these patients can experience long-term survival and good quality of life. In fact, once patients over 80 get beyond the two year survival mark without cancer recurrence their survival parallels that of their age-matched counterparts. One should also keep in mind that the reported survival rates are mostly for pancreatic cancer, but patients with other periampullary cancers have improved long-term survival when compared to those with pancreatic cancer.
Elderly patients also need to be aware of the fact that hospital volume and surgeon experience significantly impact outcomes. The mortality rates following surgery in the oldest patients, those over 80, is nearly twice as high at low-volume facilities compared to high-volume facilities. The overall mortality rate and the difference decrease with decreasing age. This likely represents improved processes of care at experienced centers and better ability to manage the complications of pancreatic surgery, which occur more commonly in elderly patients. It is important to educate both physicians and elderly patients about this difference. Currently, elderly patients are less likely to be resected at high-volume centers than younger patients. The reasons for this are unclear but include lack of awareness of the importance of hospital volume and surgeon experience and reluctance of patients in this age group to travel long distances from home for their care.
When reviewing the data, be aware that these studies (both population-based and single-institution) are retrospective and subject to significant selection bias. The elderly patients undergoing resection were clearly carefully chosen. However, there is still nihilism toward aggressive care in these patients, with fewer than 10% of patients over 80 with locoregional disease and no comorbidities being resected, while 40% of patients 66-70 in the same category are resected.
These data provide an excellent foundation to guide informed decision-making in the elderly population with pancreatic and periampullary cancer. Again patients need to know that surgical resection offers the only hope for cure and that the benefit of surgical resection does not diminish with age. The diagnosis (pancreatic vs. other periampullary cancers vs. benign disease/premalignant lesions) needs to be taken into account to fully balance the risks and benefits. Older patients need to be aware of the increased morbidity, mortality, and prolonged convalescence they may experience. They also need to be advised to have their surgery done by experienced surgeons at experienced centers where these complications can be best managed.
Further studies are needed to guide patient selection. The effect of patient comorbidities, cognitive status, preoperative functional status, and frailty need to be more formally assessed in order to best select patients, maximize surgical resection in appropriate candidates, and improve short-term outcomes. Once better characterized, specialized geriatric pathways may optimize surgical resection rates, streamline care, and improve outcomes in this challenging population.26 However, age alone should not be a contraindication to pancreatic resection in the elderly patients with pancreatic cancer.
Acknowledgments
*Work supported by the Dennis W. Jahnigen Career Development Award and National Institutes of Health Grant # 1K07CA130938-01A1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008 Mar-Apr;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Medscape. [September 2, 2008]; http://www.medscape.com/viewarticle/537123_3.
- 3.Sixty-five plus in the United States. [March 14, 2008]; http://www.census.gov/population/socdemo/statbriefs/agebrief.html.
- 4.Etzioni DA, Liu JH, Maggard MA, Ko CY. The aging population and its impact on the surgery workforce. Ann Surg. 2003 Aug;238(2):170–177. doi: 10.1097/01.SLA.0000081085.98792.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riall TS, Cameron JL, Lillemoe KD, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006 Nov;140(5):764–772. doi: 10.1016/j.surg.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro TM. Adenocarcinoma of the pancreas: a statistical analysis of biliary bypass vs Whipple resection in good risk patients. Ann Surg. 1975 Dec;182(6):715–721. doi: 10.1097/00000658-197512000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crile G., Jr The advantages of bypass operations over radical pancreatoduodenectomy in the treatment of pancreatic carcinoma. Surg Gynecol Obstet. 1970 Jun;130(6):1049–1053. [PubMed] [Google Scholar]
- 8.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993 Jan;165(1):68–72. doi: 10.1016/s0002-9610(05)80406-4. discussion 72-63. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy EP, Rosato EL, Sauter PK, et al. Initiation of a critical pathway for pancreaticoduodenectomy at an academic institution--the first step in multidisciplinary team building. J Am Coll Surg. 2007 May;204(5):917–923. doi: 10.1016/j.jamcollsurg.2007.01.057. discussion 923-914. [DOI] [PubMed] [Google Scholar]
- 10.Porter GA, Pisters PW, Mansyur C, et al. Cost and utilization impact of a clinical pathway for patients undergoing pancreaticoduodenectomy. Ann Surg Oncol. 2000 Aug;7(7):484–489. doi: 10.1007/s10434-000-0484-0. [DOI] [PubMed] [Google Scholar]
- 11.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000 Nov-Dec;4(6):567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 12.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg. 1990 Apr;211(4):447–458. doi: 10.1097/00000658-199004000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balcom JHt, Rattner DW, Warshaw AL, Chang Y, Fernandez-del Castillo C. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001 Apr;136(4):391–398. doi: 10.1001/archsurg.136.4.391. [DOI] [PubMed] [Google Scholar]
- 14.Emick DM, Riall TS, Cameron JL, et al. Hospital readmission after pancreaticoduodenectomy. J Gastrointest Surg. 2006 Nov;10(9):1243–1252. doi: 10.1016/j.gassur.2006.08.016. discussion 1252-1243. [DOI] [PubMed] [Google Scholar]
- 15.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006 Nov;10(9):1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210-1191. [DOI] [PubMed] [Google Scholar]
- 16.al-Sharaf K, Andren-Sandberg A, Ihse I. Subtotal pancreatectomy for cancer can be safe in the elderly. Eur J Surg. 1999 Mar;165(3):230–235. doi: 10.1080/110241599750007090. [DOI] [PubMed] [Google Scholar]
- 17.Bathe OF, Levi D, Caldera H, et al. Radical resection of periampullary tumors in the elderly: evaluation of long-term results. World J Surg. 2000 Mar;24(3):353–358. doi: 10.1007/s002689910056. [DOI] [PubMed] [Google Scholar]
- 18.Bottger TC, Engelmann R, Junginger T. Is age a risk factor for major pancreatic surgery? An analysis of 300 resections. Hepatogastroenterology. 1999 Jul-Aug;46(28):2589–2598. [PubMed] [Google Scholar]
- 19.Brozzetti S, Mazzoni G, Miccini M, et al. Surgical treatment of pancreatic head carcinoma in elderly patients. Arch Surg. 2006 Feb;141(2):137–142. doi: 10.1001/archsurg.141.2.137. [DOI] [PubMed] [Google Scholar]
- 20.Fong Y, Blumgart LH, Fortner JG, Brennan MF. Pancreatic or liver resection for malignancy is safe and effective for the elderly. Ann Surg. 1995 Oct;222(4):426–434. doi: 10.1097/00000658-199522240-00002. discussion 434-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodul P, Tansey J, Golts E, Oh D, Pickleman J, Aranha GV. Age is not a contraindication to pancreaticoduodenectomy. Am Surg. 2001 Mar;67(3):270–275. doi: 10.1016/s0016-5085(00)81967-8. discussion 275-276. [DOI] [PubMed] [Google Scholar]
- 22.Makary MA, Winter JM, Cameron JL, et al. Pancreaticoduodenectomy in the very elderly. J Gastrointest Surg. 2006 Mar;10(3):347–356. doi: 10.1016/j.gassur.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Richter A, Schwab M, Lorenz D, Rumstadt B, Trede M. Surgical therapy of pancreatic carcinoma in elderly patients over 70. Langenbecks Arch Chir Suppl Kongressbd. 1996;113:492–494. [PubMed] [Google Scholar]
- 24.Sohn TA, Yeo CJ, Cameron JL, et al. Should pancreaticoduodenectomy be performed in octogenarians? J Gastrointest Surg. 1998 May-Jun;2(3):207–216. doi: 10.1016/s1091-255x(98)80014-0. [DOI] [PubMed] [Google Scholar]
- 25.Scurtu R, Bachellier P, Oussoultzoglou E, Rosso E, Maroni R, Jaeck D. Outcome after pancreaticoduodenectomy for cancer in elderly patients. J Gastrointest Surg. 2006 Jun;10(6):813–822. doi: 10.1016/j.gassur.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Finlayson E, Fan Z, Birkmeyer JD. Outcomes in octogenarians undergoing high-risk cancer operation: a national study. J Am Coll Surg. 2007 Dec;205(6):729–734. doi: 10.1016/j.jamcollsurg.2007.06.307. [DOI] [PubMed] [Google Scholar]
- 27.Riall TS, Reddy DM, Nealon WH, Goodwin JS. The effect of age on short-term outcomes after pancreatic resection: a population-based study. Ann Surg. 2008 Sep;248(3):459–467. doi: 10.1097/SLA.0b013e318185e1b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riall TS, Nealon WH, Goodwin JS, et al. Pancreatic cancer in the general population: Improvements in survival over the last decade. J Gastrointest Surg. 2006 Nov;10(9):1212–1223. doi: 10.1016/j.gassur.2006.08.010. discussion 1223-1214. [DOI] [PubMed] [Google Scholar]
- 29.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007 Aug;246(2):173–180. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cress RD, Yin D, Clarke L, Bold R, Holly EA. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States) Cancer Causes Control. 2006 May;17(4):403–409. doi: 10.1007/s10552-005-0539-4. [DOI] [PubMed] [Google Scholar]
- 31.Riall TS, Townsend CM, Kuo Y, Nealon WH, Freeman JL. Disparities in the evaluation and surgical resection rates of Medicare patients with locoregional pancreatic cancer. Prepared for Surgery. Submitted to the 4th Annual Academic Surgical Congress; August 2008; 2008. [Google Scholar]
- 32.Riall TS, Kuo Y, Townsend CM, Freeman JL, Nealon WH, Goodwin JS. The impact of age on surgical resection rates and long-term survival in patients with locoregional pancreatic cancer. Prepared for Surgery. Submitted to the 4th Annual Academic Surgical Congress; August 2008; 2008. [Google Scholar]
- 33.Gordon TA, Burleyson GP, Tielsch JM, Cameron JL. The effects of regionalization on cost and outcome for one general high-risk surgical procedure. Ann Surg. 1995 Jan;221(1):43–49. doi: 10.1097/00000658-199501000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lieberman MD, Kilburn H, Lindsey M, Brennan MF. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg. 1995 Nov;222(5):638–645. doi: 10.1097/00000658-199511000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho V, Heslin MJ. Effect of hospital volume and experience on in-hospital mortality for pancreaticoduodenectomy. Ann Surg. 2003 Apr;237(4):509–514. doi: 10.1097/01.SLA.0000059981.13160.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Heek NT, Kuhlmann KF, Scholten RJ, et al. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005 Dec;242(6):781–788. doi: 10.1097/01.sla.0000188462.00249.36. discussion 788-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birkmeyer JD, Sun Y, Goldfaden A, Birkmeyer NJ, Stukel TA. Volume and process of care in high-risk cancer surgery. Cancer. 2006 Jun 1;106(11):2476–2481. doi: 10.1002/cncr.21888. [DOI] [PubMed] [Google Scholar]
- 38.Kotwall CA, Maxwell JG, Brinker CC, Koch GG, Covington DL. National estimates of mortality rates for radical pancreaticoduodenectomy in 25,000 patients. Ann Surg Oncol. 2002 Nov;9(9):847–854. doi: 10.1007/BF02557520. [DOI] [PubMed] [Google Scholar]
- 39.Gouma DJ, van Geenen RC, van Gulik TM, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000 Dec;232(6):786–795. doi: 10.1097/00000658-200012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordon TA, Bowman HM, Tielsch JM, Bass EB, Burleyson GP, Cameron JL. Statewide regionalization of pancreaticoduodenectomy and its effect on in-hospital mortality. Ann Surg. 1998 Jul;228(1):71–78. doi: 10.1097/00000658-199807000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riall TS, Eschbach KA, Townsend CM, Jr, Nealon WH, Freeman JL, Goodwin JS. Trends and disparities in regionalization of pancreatic resection. J Gastrointest Surg. 2007 Oct;11(10):1242–1251. doi: 10.1007/s11605-007-0245-5. discussion 1251-1242. [DOI] [PubMed] [Google Scholar]
- 42.Sosa JA, Bowman HM, Gordon TA, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg. 1998 Sep;228(3):429–438. doi: 10.1097/00000658-199809000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher ES, Whaley FS, Krushat WM, et al. The accuracy of Medicare's hospital claims data: progress has been made, but problems remain. Am J Public Health. 1992 Feb;82(2):243–248. doi: 10.2105/ajph.82.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bathe OF, Caldera H, Hamilton KL, et al. Diminished benefit from resection of cancer of the head of the pancreas in patients of advanced age. J Surg Oncol. 2001 Jun;77(2):115–122. doi: 10.1002/jso.1081. [DOI] [PubMed] [Google Scholar]


