Abstract
Background
We aimed to estimate the prevalence, correlates and impact of dementia in Havana and Matanzas, Cuba.
Methods
A 1-phase catchment area survey of all over 65-year-old residents of 7 catchment areas in Havana and 1 in Matanzas was conducted. Dementia diagnosis was established according to DSM-IV and our own, pre-validated10/66 criteria. The impact of dementia was assessed through associations with needs for care, cutting back on work to care and caregiver psychological morbidity.
Results
We interviewed 2,944 older people, a response proportion of 96.4%. The prevalence of DSM-IV dementia was 6.4% and that of 10/66 dementia 10.8%. Both dementia outcomes were associated with older age, less education, a family history of dementia, shorter leg length and smaller skull circumference. Dementia, rather than physical health problems or depression, was the main contributor to needs for care (population-attributable prevalence fraction = 64.6%) and caregiver cutting back on work (population-attributable prevalence fraction = 57.3%).
Conclusion
The prevalence of dementia in Cuba is similar to Europe. Among health conditions, dementia is the major contributor to dependency and caregiver economic and psychological strain. More attention needs to be given to it and other chronic diseases associated more with disability than premature mortality.
Key Words: Dementia, risk factors; Population-based studies; Prevalence study; Comorbidity; Caregiving; Caregiver burden; Developing countries
Background
Over 24 million people live with dementia worldwide, with 4.6 million new cases annually [1], similar to the annual global incidence of non-fatal stroke. The prevalence is generally lower in developing countries than in the developed north [1, 2], strikingly so in some studies [3, 4]. However, most people with dementia live in low- and middle-income countries; 60% in 2001, rising to 71% by 2040. The numbers will double every 20 years to over 80 million by 2040, with more rapid increases in developing than developed regions [1]. The proportionate increases in Latin America, where the prevalence of dementia seems to be similar to that in high-income countries, will exceed those of any other world region [1]. Well-designed epidemiological research can generate awareness, inform policy and encourage service development. Such evidence has been lacking in many world regions and has been patchy in others, with few studies and widely varying estimates [1]. In Latin America, new data on the prevalence of dementia have recently become available from studies in Brazil [5, 6] and Venezuela [7]. There are also publications in Spanish relating to surveys conducted in the 1990s in Colombia [8], Uruguay [9] and Cuba [10], and a conference abstract describing a survey from the same period in Chile [11].
The 10/66 Dementia Research Group's population-based cross-sectional surveys in 11 low- and middle-income countries (India, China, Nigeria, Cuba, Dominican Republic, Puerto Rico, Brazil, Venezuela, Mexico, Peru and Argentina) will provide a resource for the comparative study of prevalence, impact and cost. In this paper we report the prevalence and impact of dementia in Cuba, one of the first completed 10/66 surveys. Cuba is a developing country with a health profile akin to developed countries. Health care is centrally planned and financed by the state. There is comprehensive coverage and free access; 99% of the population is registered with the catchment-area-organized family doctor system, with 1 doctor to every 160 households. The infant mortality rate is only 7 per 1000, fertility is low and stable and 1.1 million Cubans (10% of the population) are aged over 65 [12]. The main causes of death, in order of frequency, are coronary heart disease, cancer and cerebrovascular disease [12].
Method
Comprehensive details of the protocol for the 10/66 population-based cross-sectional surveys are provided in an open-access online journal publication [13].
Setting and Study Design
We conducted a 1-phase cross-sectional catchment area survey of all over 65-year-old residents of 7 catchment areas in 5 urban districts in Havana, Cuba (Lisa, Luyano, Marianao, Playa and Plaza) and 1 catchment area in Matanzas (Milanes), a port city 120 km along the coast from Havana. We supplemented health service registers with systematic door-knocking, recording the genders and ages of all residents and the names of those aged 65 years or over on the census date. Precision calculations indicated that a sample of 3,000 would allow estimation of a typical dementia prevalence of 4.5% [14] with a precision of ±0.7%.
Preparation and Training
A 1-week project planning meeting was held for all 10/66 investigators. A standardized operating procedure manual covers every aspect of training and field procedures. All assessments were translated into Ibero-American Spanish with country-specific adaptations, where necessary. In Cuba, the interviews were carried out by polyclinic doctors (psychiatrists, geriatricians or general medical specialists). All were rigorously trained in study protocol and procedures, and structured interviewing techniques with an additional 2-day training for the Geriatric Mental State clinical assessment and the neurological/physical examination.
Recruitment and Interview Procedures
Age and eligibility were determined on revisit for interview, using the participant's stated and documented ages, their age according to an informant, and, if discrepant, that according to an event calendar. The participants were recruited with informed signed consent or a relative's signed agreement if lacking capacity. The interviews were carried out in the participants’ own homes. Data were collected directly onto laptop computers using computerized Spanish questionnaires driven by EPIDATA software, including conditional skips and interactive checking. This was a comprehensive 1-phase survey – all participants received the full assessment lasting approximately 2–3 h, comprising participant interview, physical examination and phlebotomy, and an informant interview.
Measures
The 10/66 interview generates information on dementia diagnosis, mental disorders, physical health, anthropometry, demographics, an extensive non-communicable disease and dementia risk factor questionnaire, disability and functioning, health service utilization, care arrangements and caregiver strain [13]. Only the assessments relevant to the current analysis of dementia prevalence, correlates and impact are listed here.
Our 10/66 dementia diagnosis algorithm [15] requires: (i) a structured clinical interview, the Geriatric Mental State, which applies a computer algorithm (AGECAT) [16], identifying organicity (probable dementia), depression, anxiety and psychosis; (ii) a cognitive test battery comprising (a) the Community Screening Instrument for Dementia (CSI-D) COGSCORE[17] [incorporating the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) animal naming verbal fluency task], and (b) the modified CERAD 10-word list learning task with delayed recall [18], and (iii) an informant interview, the CSI-D RELSCORE [17], for evidence of cognitive and functional decline.
Additional information for DSM-IV dementia diagnosis and Clinical Dementia Rating (CDR) [19] is obtained from (iv) an extended informant interview, the modified (Dementia Diagnosis and Subtype) History and Aetiology Schedule [20], providing information on dementia onset and course; (v) the NEUROEX, a structured neurological assessment of lateralizing signs, parkinsonism, ataxia, apraxia and primitive ‘release’ reflexes [21, 22], and (vi) behavioural and psychological symptoms of dementia (BPSD) assessed using an informant questionnaire, the Neuropsychiatric Inventory (NPI-Q) [23].
The participants were allocated to the category of 10/66 dementia when they scored above a cutpoint of predicted probability of dementia estimated from the logistic regression equation developed and validated cross-culturally in the 10/66 international pilot study, using coefficients from the Geriatric Mental State, CSI-D informant and cognitive test interviews and the modified CERAD 10-word list learning tasks [15]. DSM-IV dementia is a widely used criterion-based diagnosis requiring impairment in memory and at least 1 other domain of cognitive function, linked to social or occupational impairment, not better accounted for by delirium or other mental disorders [24]. DSM-IV dementia criteria were applied directly using a computerized algorithm; full details are available in an open-access online journal publication [25].
Assessments of care arrangements and caregiver strain [13] were refined in the 10/66 pilot studies [26, 27]. Here, we have concentrated on 3 indicators of the impact of dementia; (i) dependency: an interviewer assessment, after a series of open-ended questions on care arrangements, as to whether the participant needed care, none, some or much of the time; (ii) caregiver economic strain: whether the caregiver had cut back or stopped work to care, and (iii) caregiver psychological strain; the Self-Reporting Questionnaire 20, a 20-item scale of symptoms of common mental disorder (anxiety, depression and somatization) [28], a score of 8 or above indicating clinically significant morbidity.
Analyses
We reported the prevalence of 10/66 and DSM-IV dementia by age and gender with robust 95% confidence intervals, adjusted for household clustering. We compared the prevalence of DSM-IV dementia in Cuba with that from the EURODEM (Community Concerted Action on the Epidemiology and Prevention of Dementia Group) meta-analysis of 12 European studies [14], indirectly standardizing for age and gender.
We tested for associations between both 10/66 dementia and DSM-IV dementia and correlates of possible aetiologic significance. We concentrated upon those for which associations were unlikely to have been explained by reverse causality; age, rural or urban birthplace [29], education, family history of dementia, history of head injury with loss of consciousness [30, 31], history of depression [32, 33], leg length [34, 35], skull circumference [36] and left-handedness [37,38,39,40]. Associations were estimated using Poisson regression generating prevalence ratios with 95% confidence intervals, adjusting for household clustering.
We estimated, using Poisson regression adjusted for household clustering, the independent contributions of 10/66 dementia, DSM-IV major depression and physical health conditions (self-reported clinician-diagnosed stroke and number of physical impairments [41]) to needing much care, caregiver psychological morbidity and caregiver needing to cut back or give up on paid work to care. Population-attributable prevalence fractions were calculated, estimating the proportion of the prevalence of the outcome that could be avoided if each of these health conditions were eliminated, assuming a causal relationship between the health condition and the outcome and that the associations are unconfounded. We also tested for mediation of the effect of dementia through the severity of BPSD.
All analyses were carried out using STATA version 9.2 and release 1.0 of the 10/66 data archive (May 2007). The numbers of missing values are described for each variable used in the analyses. For the multivariate analyses, only the participants with non-missing data for all independent variables were included.
Results
Of the 3,000 older people enumerated, 2,944 interviews were completed (response proportion = 96.4%); 2,043 in Havana (97.6%) and 901 in Matanzas (92.7%). The sociodemographic characteristics are summarized in table 1. The median age was 74 years, with an interquartile range of 69–79; 25.4% of the sample was aged 80 years or older, and 65.0% were female. Levels of education were relatively high, with only 2.6% having no education and 16.9% having tertiary education. There was a high prevalence of cardiovascular risk factors and chronic non-communicable disease; 51.0% of the participants had been told that they were hypertensive, and 55.7% met European Society of Hypertension criteria for hypertension, 18.5% had received a diagnosis of diabetes, and 7.8% reported a stroke diagnosed by a clinician. The Havana and Matanzas samples were homogenous with respect to sociodemographic, health and lifestyle characteristics (details provided on request).
Table 1.
Sociodemographic characteristics and associations with 10/66 dementia and DSM-IV dementia (n = 2,944)
| Exposure | Exposure prevalence | Associations with 10/66 dementia |
Associations with DSM-IV dementia |
||||
|---|---|---|---|---|---|---|---|
| 10/66 dementia prevalence in exposed, % | crude prevalence ratio | multiply adjusted prevalence ratioa (n = 2,735) | DSM-IV dementia prevalence in exposed, % | crude prevalence ratio | multiply adjusted prevalence ratioa (n = 2,747) | ||
| Age | 7 missing values | ||||||
| 65–69 years | 760 (25.8) | 2.9 (1.9–4.4) | 1 (ref.) | 1 (ref.) | 1.6 (0.9–2.8) | 1 (ref.) | 1 (ref.) |
| 70–74 years | 789 (26.8) | 6.0 (4.5–7.9) | 2.06 [1.25–3.38] | 2.09 [1.21–3.61] | 3.4 (2.3–4.9) | 2.17 [1.11–4.25] | 2.22 [1.06–4.65] |
| 75–79 | 639 (21.7) | 8.6 (6.6–11.0) | 2.92 [1.80–4.74] | 2.89 [1.68–4.96] | 5.1 (3.7–7.2) | 3.27 [1.70–6.28] | 3.08 [1.48–6.41] |
| ≥80 years | 749 (25.4) | 25.7 (22.7–29.0) | 8.77 [5.71–13.48] | 8.01 [4.90–13.11] | 15.6 (13.2–18.4) | 9.89 [5.51–17.77] | 9.42 [4.88–18.2] |
| Gender | |||||||
| Female | 1,913 (65.0) | 11.7 (10.3–13.2) | 1 (ref.) | 1 (ref.) | 7.1 (6.1–8.4) | 1 (ref.) | – |
| Male | 1,031 (35.0) | 9.2 (7.6–11.2) | 0.79 [0.63–1.00] | 1.03 [0.81–1.33] | 5.2 (4.0–6.7) | 0.90 [0.73–1.12] | |
| Education | 8 missing values | ||||||
| None | 75 (2.6) | 27.0 (18.2–28.2) | 1 (ref.) | 1 (ref.) | 12.0 (6.3–21.5) | 1 (ref.) | 1 (ref.) |
| Minimal | 655 (22.3) | 15.9 (13.3–18.9) | 0.59 [0.38–0.89] | 0.59 [0.37–0.94] | 9.9 (7.9–12.5) | 0.83 [0.43–1.59] | 0.93 [0.44–1.97] |
| Primary | 979 (33.3) | 11.8 (9.9–14.0) | 0.44 [0.29–0.66] | 0.55 [0.35–0.87] | 6.4 (5.1–8.2) | 0.54 [0.28–1.04] | 0.73 [0.35–1.54] |
| Secondary | 728 (24.8) | 6.5 (4.9–8.5) | 0.24 [0.15–0.38] | 0.41 [0.25–0.69] | 4.4 (3.1–6.1) | 0.37 [0.18–0.74] | 0.70 [0.32–1.54] |
| Tertiary | 499 (17.0) | 5.8 (4.1–8.3) | 0.22 [0.13–0.36] | 0.36 [0.20–0.64] | 3.8 (2.4–5.9) | 0.32 [0.15–0.68] | 0.55 [0.23–1.31] |
| Residence at birth | 8 missing values | ||||||
| City | 1,431 (48.7) | 9.7 (8.3–11.4) | 1 (ref.) | – | 5.5 (4.4–6.8) | 1 (ref.) | – |
| Town | 715 (24.4) | 11.9 (9.7–14.4) | 1.22 [0.95–1.57] | – | 7.8 (6.1–10.0) | 1.43 [1.04–1.99] | – |
| Country | 790 (26.9) | 11.7 (9.6–14.2) | 1.20 [0.93–1.55] | – | 7.0 (5.4–9.0) | 1.28 [0.91–1.79] | – |
| Head injury | 14 missing values | ||||||
| No | 2,764 (94.3) | 10.7 (9.6–11.9) | 1 (ref.) | – | 6.5 (5.6–7.5) | 1 (ref.) | – |
| Yes | 166 (5.7) | 12.6 (8.3–18.7) | 1.18 [0.77–1.80] | – | 6.0 (3.3–10.8) | 0.93 [0.50–1.73] | – |
| Handedness | 14 missing values | ||||||
| Right-handed | 2,820 (96.2) | 10.7 (9.6–11.9) | 1 (ref.) | – | 6.3 (5.4–7.2) | 1 (ref.) | – |
| Left-handed | 110 (3.8) | 8.3 (4.3–15.1) | 0.77 [0.44–1.46] | – | 3.6 (1.4–9.3) | 0.58 [0.22–1.53] | – |
| Family history of dementia | 10 missing values | ||||||
| No | 2,385 (81.3) | 9.9 (8.7–11.2) | 1 (ref.) | 1 (ref.) | 5.6 (4.8–6.6) | 1 (ref.) | 1 (ref.) |
| Yes | 549 (18.7) | 14.6 (1.8–17.8) | 1.47 [1.16–1.87] | 1.60 [1.26–2.03] | 9.8 (7.6–12.7) | 1.75 [1.29–2.38] | 1.73 [1.25–2.40] |
| Treated depression | 10 missing values | ||||||
| No | 2,537 (86.5) | 11.0 (9.9–12.3) | 1 (ref.) | – | 6.4 (5.5–7.4) | 1 (ref.) | – |
| Yes | 397 (13.5) | 9.7 (7.1–13.0) | 0.88 [0.64–1.21] | – | 6.5 (4.5–9.4) | 1.02 [0.68–1.51] | – |
| Anthropometry | |||||||
| Leg length | 127 missing values | case: 83.8±7.5b | 0.98 [0.96–0.99]c | 1.00 [0.99–1.01]c | case: 84.3±7.8b | 0.98 [0.97–1.00]c | 1.01 [0.99–1.03]c |
| non-case: 85.4±7.4b | non-case: 85.3±7.4b | ||||||
| Skull circumference | 57 missing values | case: 55.1±1.9b | 0.85 [0.80–0.89]c | 0.89 [0.84–0.95]c | case: 55.1±1.9b | 0.83 [0.78–0.90c | 0.89 [0.82–0.97]c |
| non-case: 55.8±1.9b | non-case: 55.8±1.9b | ||||||
Figures in parentheses are percentages and values in square brackets indicate 95% confidence intervals.
Adjusted for all other covariates in the model.
Mean ± standard deviation.
Change in prevalence per 1 cm increment in the anthropometric index.
Dementia Prevalence
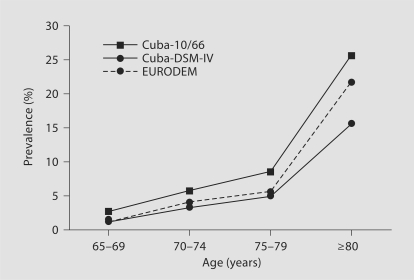
The overall prevalence of 10/66 dementia was 10.8% (95% confidence intervals = 9.7–12.0) and that of DSM-IV dementia 6.4% (5.6–7.4). The distribution of dementia cases according to CDR severity was, for 10/66 dementia 22% questionable, 38% mild, 23% moderate and 17% severe, and for DSM-IV dementia 1% questionable, 44% mild, 31% moderate and 24% severe. The prevalence of both 10/66 dementia and DSM-IV dementia increases with age (table 2). It is higher in women than in men, particularly among the oldest old. The age-specific prevalence of DSM-IV dementia in Cuba is similar but slightly higher than that reported in the EURODEM meta-analysis of European studies [14] (see fig. 1), with an age- and gender-standardized morbidity ratio of 108.
Table 2.
The prevalence (percent) of dementia by age group and gender, with 95% confidence intervals derived from robust standard errors, adjusted for household clustering
| 65–69 years (n = 760) | 70–74 years (n = 789) | 75–79 years (n = 639) | 80–84 years (n = 420) | 85–89 years (n = 223) | =90 years (n = 106) | All ages (n = 2,944) | |
|---|---|---|---|---|---|---|---|
| 10/66 dementia (13 missing values) | |||||||
| Female | 2.9 (1.7–4.8) | 6.1 (4.3–8.5) | 9.9 (7.3–13.2) | 18.8 (14.7–23.7) | 34.0 (26.9–41.9) | 38.3 (28.5–49.1) | 11.6 (10.3–13.1) |
| Male | 2.9 (1.5–5.8) | 5.9 (3.7–9.3) | 6.7 (4.1–10.8) | 16.2 (11.0–23.2) | 28.8 (19.2–40.8) | 48.0 (29.6–66.9) | 9.2 (7.6–11.1) |
| Total | 2.9 (1.9–4.3) | 6.0 (4.5–7.9) | 8.7 (6.7–11.1) | 17.9 (14.5–21.8) | 33.0 (27.2–39.4) | 40.6 (31.8–50.0) | 10.8 (9.7–12.0) |
| DSM-IV dementia | |||||||
| Female | 1.9 (1.0–3.5) | 3.6 (2.3–5.7) | 5.9 (4.0–8.6) | 11.2 (8.0–15.4) | 20.7 (15.0–27.7) | 27.2 (18.5–38.0) | 7.1 (6.1–8.4) |
| Male | 1.1 (0.4–3.3) | 3.1 (1.6–5.8) | 4.0 (2.1–7.4) | 9.9 (5.9–16.0) | 18.2 (10.6–29.4) | 24.0 (11.2–44.2) | 5.2 (4.0–6.7) |
| Total | 1.6 (0.9–2.8) | 3.4 (2.3–4.9) | 5.1 (3.7–7.2) | 10.7 (8.1–14.1) | 19.7 (15.0–25.5) | 26.4 (18.8–35.7) | 6.4 (5.6–7.4) |
Fig. 1.
The prevalence of dementia by age, comparing 10/66 and DSM-IV dementias in the current study with DSM-IV dementia in the EURODEM meta-analysis [14].
Correlates of Dementia
Associations with dementia were similar for the 10/66 and DSM-IV dementia outcomes. For each outcome (table 1), in the univariate analyses, dementia was related to increasing age, lower levels of education, shorter leg length and smaller skull circumference, and to a family history of dementia. There were no associations between dementia and handedness, rural/urban origins at birth, head injury or a history of depression treated by a doctor. 10/66 dementia, but not DSM-IV dementia, was associated with female gender. In the multiply adjusted multivariate models the association between leg length and dementia was no longer apparent. On inspection of the models this was attributable to negative confounding by age and education. The relationship between female gender and 10/66 dementia was confounded by age and skull circumference.
The Impact of Dementia
10/66 dementia was strongly associated with needing much care (45.2% of those with dementia, 2.3% of those with physical impairments but no dementia and 0.8% of others needed much care), with the caregiver needing to cut back on work to care (19.0% of those with dementia, 1.4% of those with physical impairments but no dementia and 0.4% of others), and with clinically significant caregiver psychological morbidity (22.5% of those with dementia, 9.5% of those with physical impairments but no dementia and 7.5% of others). We estimated the independent effects of health conditions (10/66 dementia, major depression, number of physical impairments and stroke) upon each of the indicators of impact controlling for participant age and gender, and household assets (table 3). Those with 10/66 dementia were 17.8 times more likely to need much care, while their caregivers were 13.4 times more likely to have cut back on work to care and 2.1 times more likely to have clinically significant psychological morbidity. The effect of dementia predominated for each of these outcomes. For needing much care the population-attributable prevalence fractions were: dementia 64.6%, depression 1.5% and physical health conditions 23.1%. For cutting back work to care the proportions were: dementia 57.3%, depression 2.3% and physical health conditions 17.5%. For caregiver psychological morbidity they were: dementia 10.6%, depression 1.2% and physical health conditions 8.6%. The severity of BPSD was an independent predictor and mediated some of the effect of dementia for all 3 outcomes.
Table 3.
Associations (prevalence ratios from Poisson regression models) between health conditions and 3 indicators of impact
| Participant health status | Exposure prevalence, % | Needing much care (348 missing values) |
Cutting back work to care |
Caregiver psychological morbidity (19 missing values) |
|||
|---|---|---|---|---|---|---|---|
| basic modela (n = 2,574) | including BPSD (n = 2,561) | basic modela (n = 2,915) | including BPSD (n = 2,881) | basic modelb (n = 2,766) | including BPSD (n = 2,748) | ||
| 10/66 dementia (13 missing values) | 10.8 | 17.8 (11.8–27.0) | 15.1 (9.7–23.6) | 13.4 (7.4–24.5) | 10.6 (5.5–20.5) | 2.1 (1.6–2.8) | 1.5 (1.1–2.0) |
| Major depression | 1.5 | 2.0 (1.0–4.2) | 1.8 (0.9–3.8) | 2.6 (1.0–6.6) | 2.4 (1.0–5.9) | 1.8 (1.1–2.9) | 1.3 (0.8–2.1) |
| Stroke (9 missing values) | 7.8 | 2.5 (1.8–3.3) | 2.4 (1.8–3.2) | 1.7 (1.0–2.9) | 1.8 (1.1–3.0) | 1.3 (0.9–1.7) | 1.2 (0.9–1.7) |
| Physical impairments (6 missing values) | |||||||
| 0 | 43.9 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1–2 | 46.2 | 1.1 (0.9–1.6) | 1.1 (0.8–1.5) | 1.2 (0.8–1.9) | 1.2 (0.7–1.9) | 1.2 (0.9–1.5) | 1.1 (0.9–1.5) |
| ≥3 | 9.9 | 1.9 (1.2–2.6) | 1.7 (1.2–2.5) | 1.4 (0.7–2.5) | 1.2 (0.6–2.3) | 1.4 (1.0–2.0) | 1.2 (0.9–1.8) |
| BPSD (per 1-point change in NPI-Q severity; 39 missing values) | – | – | 1.03 (1.01–1.06) | – | 1.05 (1.01–1.09) | – | 1.08 (1.06–1.11) |
Mutually adjusted for all health conditions, for participant age and gender and household assets.
Mutually adjusted for all health conditions, for participant age and gender, household assets, carer age, carer marital status, carer gender and carer/informant coresidence (yes/no).
Discussion
This is one of the first outputs from the 10/66 Dementia Research Group's programme of population-based studies in 7 countries in Latin America, as well as in India and China. The whole catchment area sampling strategy enabled us to foster links within the communities studied, improving response and facilitating later follow-up. Prevalence estimates may not generalize beyond these and similar communities, but this is unlikely to lead to bias in estimates of association. Our 1-phase dementia diagnostic assessment has some advantages over the 2-phase approach used in most previous dementia cohort studies [42]. Attrition is generally marked between the first and second phases [2]; participants with probable dementia are particularly likely to refuse, to move away or to die, leading to informative censoring. The problem is compounded when no random sample of screen negatives is selected for second-phase assessment [42,43,44], as was the case with several previous studies from the Latin American region (see table 4). We were also able to gather detailed information on mental health diagnoses, physical health, risk exposures and care arrangements on all participants, permitting us to study the independent impact of dementia, relative to that of other health conditions, on outcomes relevant to public policy. This has been a neglected research topic. The 2.5-hour assessments were well tolerated, as indicated by the high levels of participation.
Table 4.
Design features, and observed and directly standardized prevalence estimates for studies of the prevalence of dementia from Latin America
| Study, setting | Reference | Sample size of those aged ≥65 | Screening procedure | Screen negatives sampled in phase 2? | Dementia criterion | Dementia preva-lence (≥65), % |
Dementia prevalence (≥70), % |
||
|---|---|---|---|---|---|---|---|---|---|
| observed | directly standardized to the sample or the current Cuban study | observed | directly standardized to the sample for the current Cuban study | ||||||
| Cuba, Havana and Matanzas | current study | 2,944 (2,184) | 1 phase | n/a | DSM-IV | 6.4 | 6.4 | 8.1 | 8.1 |
| Previous studies | |||||||||
| Chile, Concepcion | Albala et al. 1997 [11] | 2,449 | MMSE <22 | yes, 2% | DSM-III-R | 6.0a | |||
| Chile (rural) | 2,240 | PFAQ >5 | 5.5a | ||||||
| Venezuela, Maracaibo | Molero et al. 2007 [7] | 1,364 (941) | 1 phase | n/a | CDR ≥1 | 13.3 | 14.8b,c | 17.2d | 18.6b,c |
| Brazil, Cantanduva | Herrera et al. 2002 [5] | 1,656 (1,042) | MMSE (education-specific cutpoints) | no | DSM-IV | 7.1 | 9.5e | 10.4 | 12.3e |
| PFAQ >5 | |||||||||
| Brazil, São Paulo | Scazufca et al. 2008 [6] | 2,072 (1,183) | 1 phase | n/a | DSM-IV | 5.1 | 7.2b | 7.2 | 8.9b |
| Colombia, 5 regions | Pradilla et al. 2003 [8] | data not provided | WHO screen and MMSE | no | DSM-IV | – | – | 3.0a | |
| Uruguay, Villa del Cerro | Ketzoian et al. 1997 [9] | data not provided | ‘suspected dementia’ (method not specified) | no | not specified | - | - | 70–79 years: 2.7f | 5.2b |
| ≥80 years: 9.6f | |||||||||
| Cuba, Marianao | Llibre et al. 1997 [10] | 619 (409) | 1 phasea | n/a | DSM-III-R | 10.0 | 11.5b | 14.4a | 15.1b |
Figures in parentheses represent results for the participants aged ≥70 years. PFAQ = Pfeffer Functional Activities Questionnaire.
No further breakdown of prevalence by age or gender within this broad age group, therefore standardization was not possible.
Standardized for age and gender.
The prevalence of dementia according to the same criterion (CDR >1) in the current Cuban study was 9.8% for those aged ≥65 and 11.7% for those aged ≥70.
Unpublished data, provided by the authors.
Standardized for age only.
The age-specific prevalence was estimated from a bar chart, as no numbers are provided in paper. Also, denominators were not provided, hence it was not possible to aggregate the observed prevalence across these age groups.
The prevalence of DSM-IV dementia in our study was similar to that previously reported in Europe. How does it compare with estimates from earlier Latin American studies? We identified 7 surveys (see background and table 4 for details) and facilitated comparison, where feasible, by directly standardizing the reported prevalence for age and gender, using our Cuban sample as the standard population. Our Cuban DSM-IV prevalence estimates were most consistent with DSM-IV estimates from the 10/66 Dementia Research Group study in São Paulo, Brazil [6], which used identical 1-phase methods, and a Chilean study [11] that featured a correct application of 2-phase methodology. The other 2-phase studies did not sample screen-negative participants in the second phase and would have underestimated dementia prevalence if the screen was less than perfectly sensitive. Of these, the Brazilian Cantanduva study [5] nevertheless reported a slightly higher prevalence; the relatively high MMSE cutpoints probably maximized the sensitivity. The Colombian [8] and Uruguayan [9] surveys screened for a range of neurological disorders in a large sample of all ages; the strikingly low dementia prevalence in these studies may be explained by insensitive screening procedures. An investigation in Kashmir that used a similar methodology identified no cases of dementia [45]. The highest age- and gender-standardized prevalences were recorded in two 1-phase studies; from Marianao, Cuba [10], and Maracaibo, Venezuela [7]. For Maracaibo, this may be explained partly by the outcome definition, CDR [19] of at least mild severity. In the current sample we demonstrated that the DSM-IV criterion missed many of the CDR mild dementia cases [25]. The current study adds to the evidence that the prevalence of DSM-IV dementia in Latin American settings is at least as high as that seen in high-income countries in Europe and North America. The one caveat is that most Latin American studies published to date, including our own, have sampled from urban rather than rural settings and from countries with relatively low child and adult mortalities.
The age-specific prevalence of 10/66 dementia in Cuba was consistently higher than that of DSM-IV dementia, raising the possibility that the use of the DSM-IV criterion may underestimate the prevalence of clinically significant dementia. The 10/66 dementia algorithm has been carefully validated in 26 low- and middle-income country centres [15], including Cuba. While the sensitivity (94%) and specificity (97% in high-education controls and 94% in low-education controls) were both excellent against the gold standard of a local clinician's DSM-IV diagnosis, the false-positive rate, which varied between 1 and 10% across regions and levels of education, can be expected to result in a higher prevalence of 10/66 dementia. Conversely, the DSM-IV criterion, with its stringent requirement for multiple domains of cognitive function to be affected with clear evidence for social and occupational impairment, may be insensitive to mild yet clinically relevant cases [46]. In a separate paper we have shown that 10/66 dementia corresponded more closely to Cuban clinical interviewer dementia diagnoses than did the more restrictive DSM-IV criterion [25]. The predictive validity of the 2 diagnoses will be determined in the 10/66 incidence phase; true dementia cases would have progressed, and failure to do so would suggest misclassification.
The cross-sectional correlates of dementia (older age, lower education and family history of dementia) are generally similar to those previously and widely reported. We confirm earlier reports of shorter leg length [34, 35] and smaller skull circumferences [36, 47] among those with dementia. Interestingly both of these exposures were inversely linearly associated with age, suggesting the presence of cohort effects; older people, from earlier birth cohorts, having poorer nutrition and hence having developed less successfully in early life. Adjusting for age, which diminishes the strength of both associations, may be inappropriate. We did not replicate previous reports of relationships between handedness [37,38,39,40], head injury [30, 31] or history of depression [32, 33] and dementia. Data on these exposures were collected from participants or from their relatives if they were considered to give more reliable information. The lack of an association might be explained by random or systematic misclassification because of poor recall by those with dementia or their relatives.
Conclusion
Dementia is at least as common in Cuba as in developed countries. In what we believe to be an original observation for the Latin American region, we have demonstrated that dementia, rather than physical health conditions or depression, is the main contributor to needs for care, to the caregiver needing to give up work to care and to caregiver psychological strain. Chronic diseases in developing countries are now receiving more attention, but this is focused upon conditions linked to premature mortality; cardiovascular disease and cancer [48]. Dementia has a uniquely devastating impact on the capacity for independent living, and care for people with dementia places a huge burden on social and health care budgets in developed countries [49]. Yet its public health significance is underappreciated.
Acknowledgements
This study was funded by the Wellcome Trust Health Consequences of Population Change Programme (GR066133). The clinician interviewers were seconded to the project with the support of the Cuban government.
Footnotes
The 10/66 Dementia Research Group works closely with Alzheimer's Disease International, the non-profit federation of 77 Alzheimer associations around the world. Alzheimer's Disease International is committed to strengthening Alzheimer associations worldwide, raising awareness regarding dementia and Alzheimer's disease and advocating for more and better services for people with dementia and their caregivers. Alzheimer's Disease International is supported in part by grants from GlaxoSmithKline, Novartis, Lundbeck, Pfizer and Eisai.
References
- 1.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.10/66 Dementia Research Group Methodological issues in population-based research into dementia in developing countries: a position paper from the 10/66 Dementia Research Group. Int J Geriatr Psychiatry. 2000;15:21–30. doi: 10.1002/(sici)1099-1166(200001)15:1<21::aid-gps71>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Chandra V, Ganguli M, Pandav R, Johnston J, Belle S, DeKosky ST. Prevalence of Alzheimer's disease and other dementias in rural India: the Indo-US study. Neurology. 1998;51:1000–1008. doi: 10.1212/wnl.51.4.1000. [DOI] [PubMed] [Google Scholar]
- 4.Hendrie HC, Osuntokun BO, Hall KS, Ogunniyi AO, Hui SL, Unverzagt FW, Gureje O, Rodenberg CA, Baiyewu O, Musick BS, et al. Prevalence of Alzheimer's disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry. 1995;152:1485–1492. doi: 10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- 5.Herrera E, Jr, Caramelli P, Silveira AS, Nitrini R. Epidemiologic survey of dementia in a community-dwelling Brazilian population. Alzheimer Dis Assoc Disord. 2002;16:103–108. doi: 10.1097/00002093-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Scazufca M, Menezes PR, Vallada HP, Crepaldi AL, Pastor-Valero M, Coutinho LM, Di Rienzo VD, Almeida OP. High prevalence of dementia among older adults from poor socioeconomic backgrounds in São Paulo, Brazil. Int Psychogeriatr. 2008;20:394–405. doi: 10.1017/S1041610207005625. [DOI] [PubMed] [Google Scholar]
- 7.Molero AE, Pino-Ramirez G, Maestre GE. High prevalence of dementia in a Caribbean population. Neuroepidemiology. 2007;29:107–112. doi: 10.1159/000109824. [DOI] [PubMed] [Google Scholar]
- 8.Pradilla AG, Vesga AB, Leon-Sarmiento FE. National neuroepidemiological study in Colombia (EPINEURO) Rev Panam Salud Publica. 2003;14:104–111. doi: 10.1590/s1020-49892003000700005. [DOI] [PubMed] [Google Scholar]
- 9.Ketzoian C, Rega I, Caseres R, Dieguez E, Coirolo G, Scaramelli A, Salamano R, Caamano JL, Romero S, Carrasco L, Pizzarossa C, Chouza C. Estudio de la prevalencia de las principales enfermedades neurológicas en una población del Uruguay. Prensa Med Urug. 1997;17:9–26. [Google Scholar]
- 10.Llibre JJ, Guerra MA, Perez-Cruz H. Dementia syndrome and risk factors in adults aged over 60 residing in La Habana. Rev Neurol. 1999;29:908–911. [PubMed] [Google Scholar]
- 11.Albala C, Quiroga P, Klaasen G, Rioseco P, Pérez H, Calvo C: Prevalence of dementia and cognitive impairment in Chile (abstract 483). Conf Proc World Congr Gerontol, Adelaide, 1997.
- 12.National Health Statistics Bureau: Demography, Mortality, Morbidity, Personnel and Health Services Resources. Annual Health Statistics Report 2006. Havana, Ministry of Public Health of Cuba, 2006.
- 13.Prince M, Ferri CP, Acosta D, Albanese E, Arizaga R, Dewey M, Gavrilova SI, Guerra M, Huang Y, Jacob KS, Krishnamoorthy ES, McKeigue P, Rodrigues JL, Salas A, Sosa AL, Sousa R, Stewart R, Uwakwe R. The protocols for the 10/66 Dementia Research Group population-based research programme. BMC Public Health. 2007;7:165. doi: 10.1186/1471-2458-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM. Neurologic Diseases in the Elderly Research Group: Prevalence of dementia and major subtypes in Europe: a collaborative study of population- based cohorts. Neurology. 2000;54:S4–S9. [PubMed] [Google Scholar]
- 15.Prince M, Acosta D, Chiu H, Scazufca M, Varghese M. Dementia diagnosis in developing countries: a cross-cultural validation study. Lancet. 2003;361:909–917. doi: 10.1016/S0140-6736(03)12772-9. [DOI] [PubMed] [Google Scholar]
- 16.Copeland JRM, Dewey ME, Griffith-Jones HM. A computerised psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychol Med. 1986;16:89–99. doi: 10.1017/s0033291700057779. [DOI] [PubMed] [Google Scholar]
- 17.Hall KS, Hendrie HH, Brittain HM, Norton JA, Rodgers DD, Prince CS, Pillay N, Blue AW, Kaufert JN, Nath A, Shelton P, Postl BD, Osuntokun BO. The development of a dementia screeing interview in two distinct languages. Int J Methods Psychiatr Res. 1993;3:1–28. [Google Scholar]
- 18.Ganguli M, Chandra V, Gilbey J. Cognitive test performance in a community-based nondemented elderly sample in rural India: the Indo-US cross-national dementia epidemiology study. Int Psychogeriatr. 1996;8:507–524. doi: 10.1017/s1041610296002852. [DOI] [PubMed] [Google Scholar]
- 19.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 20.Dewey ME, Copeland JR. Diagnosis of dementia from the history and aetiology schedule. Int J Geriatr Psychiatry. 2001;16:912–917. doi: 10.1002/gps.446. [DOI] [PubMed] [Google Scholar]
- 21.Broe GA, Akhtar AJ, Andrews GR, Caird FI, Gilmore AJ, McLennan WJ. Neurological disorders in the elderly at home. J Neurol Neurosurg Psychiatry. 1976;39:361–366. doi: 10.1136/jnnp.39.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broe GA, Jorm AF, Creasey H, Grayson D, Edelbrock D, Waite LM, Bennett H, Cullen JS, Casey B. Impact of chronic systemic and neurological disorders on disability, depression and life satisfaction. Int J Geriatr Psychiatry. 1998;13:667–673. doi: 10.1002/(sici)1099-1166(1998100)13:10<667::aid-gps839>3.0.co;2-g. (erratum appears in Int J Geriatr Psychiatry 1999;14:497–498). [DOI] [PubMed] [Google Scholar]
- 23.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, ed 4. Washington, AMA, 1994.
- 25.Prince M, Rodriguez JL, Noriega L, Lopez A, Acosta D, Albanese E, Arizaga R, Copeland J, Dewey M, Ferri CP, Guerra M, Huang Y, Jacob KS, Krishnamoorthy ES, McKeigue P, Sousa R, Stewart R, Salas A, Sosa AL, Uwakwe R. The 10/66 Dementia Research Group's fully operationalised DSM-IV dementia computerized diagnostic algorithm, compared with the 10/66 dementia algorithm and a clinician diagnosis: a population validation study. BMC Public Health. 2008;8:219. doi: 10.1186/1471-2458-8-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferri CP, Ames D, Prince M. Behavioral and psychological symptoms of dementia in developing countries. Int Psychogeriatr. 2004;16:441–459. doi: 10.1017/s1041610204000833. [DOI] [PubMed] [Google Scholar]
- 27.Prince M. Care arrangements for people with dementia in developing countries. Int J Geriatr Psychiatry. 2004;19:170–177. doi: 10.1002/gps.1046. [DOI] [PubMed] [Google Scholar]
- 28.Mari JJ, Williams P. A comparison of the validity of two psychiatric screening questionnaires (GHQ-12 and SRQ-20) in Brazil, using Relative Operating Characteristic (ROC) analysis. Psychol Med. 1985;15:651–659. doi: 10.1017/s0033291700031500. [DOI] [PubMed] [Google Scholar]
- 29.Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand. 1987;76:465–479. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 30.Jellinger KA. Head injury and dementia. Curr Opin Neurol. 2004;17:719–723. doi: 10.1097/00019052-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, Jorm AF, Kokmen E, Kondo K, Rocca WA, et al. EURODEM Risk Factors Research Group Head trauma as a risk factor for Alzheimer's disease: a collaborative re-analysis of case-control studies. Int J Epidemiol. 1991;20(suppl 2):S28–S35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- 32.Devanand DP, Sano M, Tang MX, Taylor S, Gurland BJ, Wilder D, Stern Y, Mayeux R. Depressed mood and the incidence of Alzheimer's disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 33.Jorm AF, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, Kokmen E, Kondo K, Mortimer JA, Rocca WA, et al. EURODEM Risk Factors Research Group Psychiatric history and related exposures as risk factors for Alzheimer's disease: a collaborative re-analysis of case-control studies. Int J Epidemiol. 1991;20(suppl 2):):S43–S47. doi: 10.1093/ije/20.supplement_2.s43. [DOI] [PubMed] [Google Scholar]
- 34.Kim JM, Stewart R, Shin IS, Yoon JS. Limb length and dementia in an older Korean population. J Neurol Neurosurg Psychiatry. 2003;74:427–432. doi: 10.1136/jnnp.74.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mak Z, Kim JM, Stewart R. Leg length, cognitive impairment and cognitive decline in an African-Caribbean population. Int J Geriatr Psychiatry. 2006;21:266–272. doi: 10.1002/gps.1458. [DOI] [PubMed] [Google Scholar]
- 36.Schofield PW, Logroscino G, Andrews HF, Albert S, Stern Y. An association between head circumference and Alzheimer's disease in a population-based study of aging and dementia. Neurology. 1997;49:30–37. doi: 10.1212/wnl.49.1.30. [DOI] [PubMed] [Google Scholar]
- 37.Raiha I, Kaprio J, Koskenvuo M, Rajala T, Sourander L. Environmental differences in twin pairs discordant for Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1998;65:785–787. doi: 10.1136/jnnp.65.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li G, Shen YC, Li YT, Chen CH, Zhau YW, Silverman JM. A case-control study of Alzheimer's disease in China. Neurology. 1992;42:1481–1488. doi: 10.1212/wnl.42.8.1481. [DOI] [PubMed] [Google Scholar]
- 39.De Leon MJ, la Regina ME, Ferris SH, Gentes CI, Miller JD. Reduced incidence of left-handedness in clinically diagnosed dementia of the Alzheimer type. Neurobiol Aging. 1986;7:161–164. doi: 10.1016/0197-4580(86)90037-0. [DOI] [PubMed] [Google Scholar]
- 40.Seltzer B, Burres MJ, Sherwin I. Left-handedness in early and late onset dementia. Neurology. 1984;34:367–369. doi: 10.1212/wnl.34.3.367. [DOI] [PubMed] [Google Scholar]
- 41.Duke University Centre for the Study of Aging and Human Development . The OARS Methodology. Durham: Duke University; 1978. Multidimensional Functional Assessment. [Google Scholar]
- 42.Prince M. Commentary: two-phase surveys – a death is announced; no flowers please. Int J Epidemiol. 2003;32:1078–1080. doi: 10.1093/ije/dyg321. [DOI] [PubMed] [Google Scholar]
- 43.Prince M. Dementia in developing countries (guest editorial) Int Psychogeriatr. 2001;13:389–393. doi: 10.1017/s1041610201007797. [DOI] [PubMed] [Google Scholar]
- 44.Dunn G, Pickles A, Tansella M, Vazquez-Barquero JL. Two-phase epidemiological surveys in psychiatric research. Br J Psychiatry. 1999;174:95–100. doi: 10.1192/bjp.174.2.95. [DOI] [PubMed] [Google Scholar]
- 45.Razdan S, Kaul RL, Motta A, Kaul S, Bhatt RK. Prevalence and pattern of major neurological disorders in rural Kashmir (India) in 1986. Neuroepidemiology. 1994;13:113–119. doi: 10.1159/000110368. [DOI] [PubMed] [Google Scholar]
- 46.Erkinjuntti T, Ostbye T, Steenhuis R, Hachinski V. The effect of different diagnostic criteria on the prevalence of dementia. N Engl J Med. 1997;337:1667–1674. doi: 10.1056/NEJM199712043372306. [DOI] [PubMed] [Google Scholar]
- 47.Mortimer JA, Snowdon DA, Markesbery WR. Head circumference, education and risk of dementia: findings from the Nun Study. J Clin Exp Neuropsychol. 2003;25:671–679. doi: 10.1076/jcen.25.5.671.14584. [DOI] [PubMed] [Google Scholar]
- 48.Strong K, Mathers C, Leeder S, Beaglehole R. Preventing chronic diseases: how many lives can we save? Lancet. 2005;366:1578–1582. doi: 10.1016/S0140-6736(05)67341-2. [DOI] [PubMed] [Google Scholar]
- 49.Langa KM, Chernew ME, Kabeto MU, Herzog AR, Ofstedal MB, Willis RJ, Wallace RB, Mucha LM, Straus WL, Fendrick AM. National estimates of the quantity and cost of informal caregiving for the elderly with dementia. J Gen Intern Med. 2001;16:770–778. doi: 10.1111/j.1525-1497.2001.10123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]