Abstract
Since defects in renal autoregulation may contribute to renal barotrauma in chronic kidney disease, we tested the hypothesis that the myogenic response is diminished by reduced renal mass. Kidneys from 5/6 nephrectomized mice had only a minor increase in the glomerular sclerosis index. The telemetric mean arterial pressure (108±10 mmHg) was unaffected after 3 months of high salt intake (6% salt in chow) or reduced renal mass. Afferent arterioles from 5/6 nephrectomized mice and sham-operated controls were perfused ex vivo during step changes in pressure from 40 to 134 mmHg. Afferent arterioles developed a constriction and a linear increase in active wall tension above a perfusion pressure of 36±6 mmHg, without a plateau. The slope of active wall tension vs perfusion pressure defined the myogenic response which was similar in sham mice fed normal or high salt diets for 3 months (2.90±0.22 vs 3.22±0.40 dynes·cm−1/mmHg, ns). The myogenic response was unaffected after 3 days of reduced renal mass on either salt diet (3.39±0.61 vs 4.04±0.47) but was reduced (p<0.05) in afferent arterioles from reduced renal mass groups fed normal and high salt at 3 months (2.10±0.28 and 1.35±0.21 dynes·cm−1/mmHg). In conclusion, mouse renal afferent arterioles develop a linear increase in myogenic tone around the range of ambient perfusion pressures. This myogenic response is impaired substantially in the mouse model of prolonged reduced renal mass, especially during high salt intake.
Keywords: Kidney, renal autoregulation, chronic kidney disease, hypertension, salt-sensitivity
Introduction
Renal autoregulation implies a proportionate increase in renal vascular resistance with increasing perfusion pressure. Autoregulation is mediated predominantly by myogenic and tubuloglomerular feedback components. Loutzenhiser et al demonstrated a myogenic response in the renal afferent arteriole of hydronephrotic kidneys from rats 1,2 that contributed 31% to the autoregulation of renal blood flow 3. Takenaka et al reported a one third decrease in afferent arteriolar diameter and a maintained blood flow velocity in juxtamedullary nephrons of the rat during increases in renal perfusion pressure 4. Impaired autoregulation and systemic hypertension in chronic kidney disease (CKD) have been proposed to cause the elevated glomerular capillary pressure that has been linked to progressive glomerular injury 5. Dietary salt restriction reduced glomerulosclerosis and renal damage in rats with reduced renal mass (RRM) 6,7 and reduced proteinuria in patients with chronic kidney disease 8. A preliminary study reported that a high salt intake impaired autoregulation of the juxtamedullary afferent arterioles in the rat 9. However, the myogenic response of isolated afferent arterioles has not been studied in models of CKD or during changes in salt intake. Renal afferent arterioles are the main renal resistance vessels and, unlike the arcuate or interlobular arteries 10, can have strong myogenic contractions 11,12. We tested the hypothesis that the myogenic responses of renal cortical afferent arterioles were impaired by prolonged RRM and by dietary salt loading. The aim was to study the myogenic responses in isolated perfused afferent arterioles where the perfusion pressure could be controlled and changes in the luminal diameter measured directly without confounding effects of the tubuloglomerular feedback or circulating factors. We evaluated myogenic responses at 3 days, 3 weeks and 3 months after RRM or sham operations during normal or high levels of dietary salt intake.
Methods and protocols
Male C57BL6 mice weighing 22 g to 30 g (Jackson Laboratory, Bar Harbor, Maine) were fed a 0.4% NaCl control test diet (normal salt, or NS; Harlan Teklad, CA) or an equivalent 6% NaCl diet (high salt, or HS; TD92055, Harlan Teklad, CA) and allowed free access to tap water. All procedures conformed to the Guide for Care and Use of Laboratory Animals prepared by The Institute for Laboratory Animal Research (ILAR). Studies were approved by the Georgetown University Animal Care and Use Committee.
Animal preparation and surgery
A two-step surgical 5/6 nephrectomy procedure was used to create RRM under inhalational anesthesia with 2% isoflurane and oxygen mixed with room air in a vaporizer. Two-thirds of the mass of the left kidneys was ablated by stitching off each pole using an absorbable hemostat (Ethicon, INC. NJ). At a second surgery after one week, the right renal vessels were dissected, clipped and cut and the kidney removed. Sham-operated control mice (Sham) were subject to a similar two stage procedure without removal of kidneys. After a one week recovery period, mice were randomized to a normal or high salt intake for 3 days, 3 weeks or 3 months.
Parallel groups of RRM and sham mice fed normal or high salt intakes were equipped with telemetric blood pressure recorders (Data Sciences International, St. Paul, MN) connected to a cannula in the carotid artery 13. The telemeters were implanted during the second surgery and were switched on one week later. The data for the first and last three weeks corresponding to the times of assessment of myogenic responses (3 weeks and 3 months) were averaged.
Dissection and microperfusion of afferent arterioles
Renal afferent arterioles were dissected, mounted and perfused as described in detail 14,15. Briefly, the kidneys were sliced along the corticomedullary axis immediately after sacrifice, placed in 4°C dissection solution and an afferent arteriole with glomerulus attached was microdissected under a stereomicroscope using sharpened forceps, transferred to a thermoregulated chamber on the stage of an inverted microscope (Olympus IX70, Olympus America, Inc., NY) and perfused using a micromanipulator system (Vestavia Scientific, Vestavia Hills, AL) with concentric holding and perfusion pipettes. The arteriole was aspirated into the holding pipette which had an outer diameter (OD) 2.13 mm, inner diameter (ID) 1.63 mm and a tip aperture of approximately 20 μm. The inner perfusion pipette had an OD 1.19 mm, an ID 1.02 mm and a tip diameter of 6 μm. It was advanced into the arteriolar lumen. The pressure at its tip was calibrated using a closed chamber connected to a DPM-1B pneumatic transducer calibrator (Bio-Tek Instruments, INC., Winooski, VT). Microdissection and cannulation were completed within 120 min, after which the bath was gradually warmed to 37°C and the arteriole stabilized for 20 minutes. The cannulated afferent arteriole was perfused with Dulbecco's Modified Eagle's Medium/Nutrient Mixture F-12 Ham (DMEM, Sigma, St. Louis, MO) at 40 mmHg. For calibrating the pressure in the lumen of the perfused afferent arteriole, a special holding pipette was applied at the end of the arteriole near the glomerulus and a sucking pressure applied to occlude flow. The intraluminal pressures during free flow were calibrated from the stop flow pressures. DMEM bubbled with 95% O2 and 5% CO2 and pH adjusted to 7.4 was used for dissection, bath and perfusion. The microperfused arteriole was displayed at ×400 magnification (Nomarski optics; Olympus Corp., Melville, NY) on a video monitor via a camera (model NC 70; Dage-MTI, Inc., Michigan, IN) on an inverted microscope and recorded on VHS tape. Arterioles were selected according to the criteria of basal tone and a rapid constriction with KCl (100 mmol · l−1) as described previously 14,15. Only one arteriole was studied from each mouse.
Renal glomerulosclerosis score
Kidney sections were stained with hematoxylin-eosin (HE) and periodic acid-Schiff (PAS). Glomerulosclerosis was scored in all glomeruli of each section as described 16 as G0 for a normal glomerulus; G1, mild sclerosis (<25%); G2, moderate segmental sclerosis (25% to 50%); G3, severe segmental sclerosis (50% to 75%); and G4, global sclerosis. The score was calculated in 6 mice in each group as (0×number of S0+1×number of S1+2×number of S2+3×number of S3+4×number of S4)/(number of S0+S1+S2+S3+S4), where S represents glomerulosclerosis index.
Measurement of myogenic tone
Data were analyzed by proprietary Windows based advanced software. The experiments were recorded by a video system of Panasonic VHS linked to an analog analysis system, digitized, and monitored in real time. Wall tension (T) was calculated from:
where Pi was the intravascular perfusion pressure and R was the internal radius 10. A set of pressure steps from 40 to 134 mmHg were undertaken in each arteriole in physiologic solution and in a perfusate without Ca++ and containing 5 × 10−3M ethylene glycol-bis(2 aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA, Sigma, St. Louis, MO) to abolish active tone. The active wall tension (AWT) was calculated as the difference between the tensions measured during perfusion with these two solutions. Since AWT increased linearly with perfusion pressure above about 40 mmHg (Fig 1), the myogenic response of each arteriole was calculated as the slope of the regression of AWT on perfusion pressure. The calculated intercept on the x-axis defined the threshold pressure that initiated an AWT response.
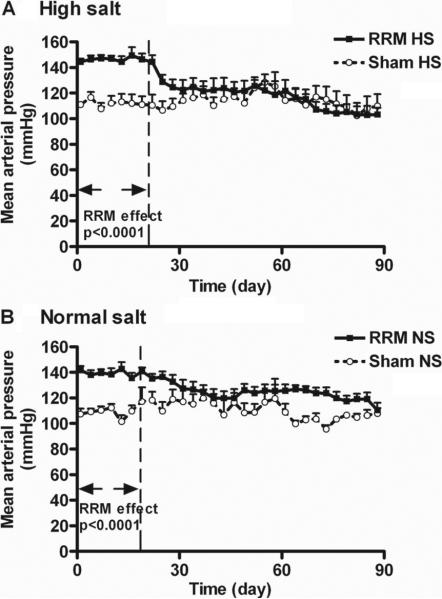
Figure 1.
Mean ± SEM values for mean arterial pressure (MAP) recorded telemetrically from conscious mice with reduced renal mass or sham mice fed normal salt or high salt diets. The MAP was significantly higher in rats with RRM during the first 3 weeks of recording, but not during the last 3 weeks.
Chemical methods: plasma and urinary creatinine, and urinary albumin
Plasma and urinary creatinine were measured with High Performance Capillary Electrophoresis using a P/ACE MDQ system (Beckman Coulter, Fullerton, CA) equipped with UV detection. Briefly, 5 nL of plasma ultrafiltrate was injected under vacuum at the short end of an uncoated fused silica capillary (Polymicro, Phoenix, AZ) with an effective length of 10.2 cm, total length of 60.2 cm, and internal diameter of 50 μm. The background buffer contained 40 mmol/L of sodium phosphate at pH 2.35. The peaks detected at 200 nm wavelength were confirmed by spiking with known amount of creatinine. The urine samples were diluted ten-fold. All samples were run in duplicate. The values for plasma creatinine and creatinine clearance for normal mice were in good agreement with a prior report using tandem mass spectrometry 17.
Urinary albumin was measured by a murine microalbuminuria ELISA using a microplate reader equipped to determine absorbance at 450 nm (Albuwell M kit, Exocell).
Statistics
Data are expressed as mean ± SEM. GraphPad Software Prism 3.0 was used for statistical analysis (GraphPad Prism, GraphPad Software). A 2 × 2 analyses of variance (ANOVA) was used to compare effects of RRM and salt intake and any interaction (i.e. effects of salt intake on the response to RRM). When appropriate, these calculations were followed by Bonferroni post hoc Student's t tests. Changes were analyzed using parametric statistics. P<0.05 was considered statistically significant.
Results
A high salt intake increased body and kidney weights whereas RRM reduced total kidney weight (Table 1). There was an increase in albuminuria in mice with RRM. Plasma creatinine increased more in mice with RRM fed high salt intake. There was no effect of RRM or salt on the basal diameter of the afferent arteriole perfused at 40 mmHg without activating the myogenic response. There was a small but significant increase in the glomerular sclerosis index of mice with RRM. Morphological changes were correspondingly mild in all groups (data not shown) as described previously 18.
Table 1.
Body and total kidney weights, kidney function, afferent arteriolar diameter and glomerulosclerosis index
| Parameter | Normal Salt (3 months) |
High Salt (3 months) |
Effect of |
||||
|---|---|---|---|---|---|---|---|
| Sham | RRM | Sham | RRM | Salt | RRM | Interaction | |
| Body weight (g) | 25.86 ± 0.51 | 25.30 ± 0.97 | 27.10 ± 0.9 | 27.00 ± 0.38 | p<0.05 | ns | ns |
| Kidney weight (g) | 0.32 ± 0.01 | 0.18 ± 0.02 | 0.35 ± 0.01 | 0.20 ± 0.01 | p<0.0001 | p<0.0001 | ns |
| Pcr (mg/dl) | 0.082 ± 0.003 | 0.102 ± 0.005 | 0.077 ± 0.007 | 0.175 ± 0.021 | p<0.001 | p<0.0001 | p<0.001 |
| Albuminuria (ug/day/g-bwt) | 6.01 ± 0.52 | 8.53 ± 1.68 | 9.72 ± 1.48 | 12.35 ± 0.98 | p<0.01 | p<0.05 | ns |
| Aff diameter (μm) at 40 mmHg | 9.41 ± 0.53 | 10.48 ± 0.97 | 10.38 ± 0.64 | 10.74 ± 0.99 | ns | ns | ns |
| Sclerosis index (Arbitrary unit) | 0.02 ± 0.002 | 0.073 ± 0.024 | 0.044 ± 0.008 | 0.081 ± 0.012 | ns | p<0.01 | ns |
Mean ± SEM values for mice with a sham operation (sham) or with reduced renal mass (RRM) fed a high salt (HS) or a normal salt (NS) diet for 3 months (n=6-7 per group).
The mean arterial pressure (MAP) was measured telemetrically starting one week after the second surgery, corresponding to the allocation to normal or high salt intakes (Fig 1). There were no differences in the MAP of sham mice on NS or HS diets. However, the MAP of mice with RRM during high salt (n=9) and normal salt diets (n=7) were significantly (p<0.0001) higher than the MAP of sham high salt (n=8) and normal salt (n=6) mice during the first three weeks of recording. Thereafter, the pressure differences between the RRM and sham groups became less prominent or disappeared. When assessed over the last 3 weeks of the RRM protocol, corresponding to the period of the 3-month myogenic response measurements, there were no significant differences among the 4 groups. The heart rates and the activity records were not different among the 4 groups (data not shown).
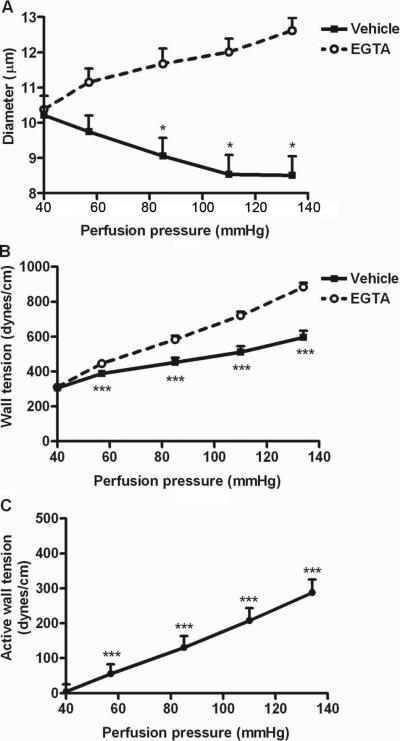
Figure 2 depicts data in isolated perfused afferent arterioles from 9 normal mice fed NS diet. When perfused in a bath with a calcium-free solution and EGTA to abolish active tone, there was an increase in luminal diameter with perfusion pressure but when perfused in a physiologic calcium-containing solution (vehicle), the diameter of the afferent arterioles decreased significantly (p< 0.05) by 11% from 10.22 ± 0.55 to 9.06 ± 0.51 μm during an increase renal perfusion pressure from 40 to 80 mmHg (Panel A). The corresponding wall tensions are shown in Panel B and the active wall tension (the difference between the two sets of data in Panel B) is shown in Panel C. The slope of the line in Panel C gives the myogenic response that averaged 2.98 ± 0.37 dynes · cm−1/mmHg.
Figure 2.
Mean ± SEM values from studies in normal mice (n=9) fed a normal salt intake of the afferent arteriolar diameter (panel A), the wall tensions (panel B) and the active wall tension (panel C) during pressure steps from 40 to 134 mmHg. Studies were undertaken in a physiologic solution and in a perfusate with a calcium-free solution containing EGTA to abolish active tone. Compared to data at 40 mmHg; *, p<0.05; ***, p<0.005.
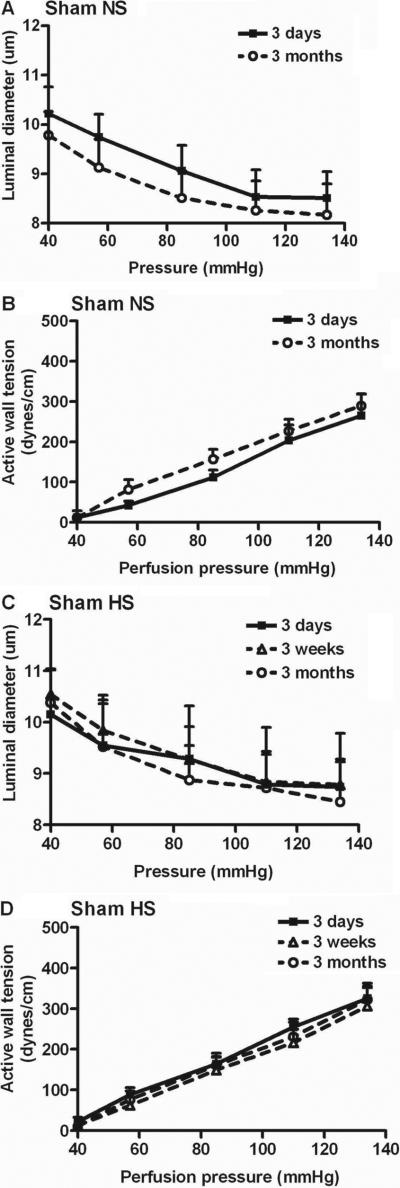
Figure 3 depicts data of sham mice fed normal or high salt diets for 3 days, 3 weeks or 3 months. Neither the changes in luminal diameter nor the active wall tension with increasing perfusion pressure were different from data in normal mice fed normal salt (Table 2).
Figure 3.
Mean ± SEM values for the afferent arteriolar diameters and active wall tensions from sham mice fed a normal salt for 3 days (n=5) or 3 months (n=6) (panels A, B) or sham mice fed a high salt for 3 days (n=5), 3 weeks (n=5) or 3 months (n=5) (panels C, D).
Table 2.
The slope and threshold pressure of active wall tension as a function of perfusion pressure
| Group | Number of mice | Salt intake | Time | Slope (dynes·cm−1/mmHg) | Threshold pressure (mmHg) |
|---|---|---|---|---|---|
| Normal | 9 | Normal | 2.98±0.37 | 38.52±8.19 | |
| Sham | 5 | Normal | 3 days | 2.78±0.62 | 36.28 ±6.35 |
| Sham | 6 | Normal | 3 months | 2.90±0.22 | 32.71 ±6.23 |
| Sham | 5 | High | 3 days | 3.19±0.25 | 32.16 ±3.26 |
| Sham | 5 | High | 3 weeks | 3.08±0.42 | 36.96±3.71 |
| Sham | 5 | High | 3 months | 3.22±0.40 | 35.50 ±3.60 |
| RRM | 5 | Normal | 3 days | 3.39±0.61 | 35.04 ±1.64 |
| RRM | 5 | Normal | 3 months | 2.10±0.28*‡ | 34.75 ±1.68 |
| RRM | 6 | High | 3 days | 4.04±0.47 | 36.65 ±4.43 |
| RRM | 6 | High | 3 weeks | 2.38±0.23§ | 33.02±5.68 |
| RRM | 6 | High | 3 months | 1.35±0.21†§ | 23.09 ±7.69*‡ |
Mean ± SEM values for the slopes and threshold pressures of arteriolar active wall tensions. Significance of differences for the effects of RRM compared to sham at equivalent levels of salt intake and time:
p<0.05
p<0.01 and the effects at 3 weeks or 3 months compared to 3 days of salt intake or RRM:
p<0.05
p<0.01.
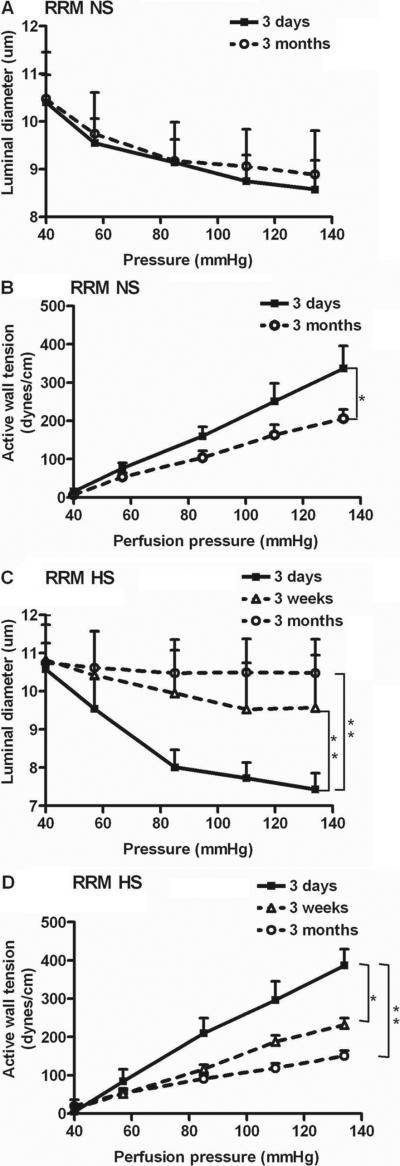
The pressure-induced decreases in luminal diameters and the increases in active wall tension (myogenic responses) of afferent arterioles from mice with RRM fed normal salt for 3 days did not differ from the responses of arterioles from normal salt sham control mice. Although there was no significant difference in a arteriolar luminal diameters of RRM mice fed NS diet at 3 days and 3 months (Fig 4A), there was a significant (p<0.05) but modest impairment of myogenic responses at 3 months whether compared to sham mice (Table 2; 28±3% reduction in slope) or mice with RRM at 3 days (Fig 4B).
Figure 4.
Mean ± SEM values for the afferent arteriolar diameters and active wall tensions from mice with reduced renal mass fed normal salt for 3 days (n=5), or 3 months (n=5) (panels A, B), or high salt for 3 days (n=6), 3 weeks (n=6) or 3 months (n=6) (panels C, D). Significance of differences between groups: *, p<0.05; **, p<0.01.
The pressure-induced reductions in luminal diameters (Fig 4C) of mice with RRM fed a high salt diet were reduced at 3 weeks and reduced further at 3 months (Table 2). There were corresponding reductions in pressure-induced active wall tension which averaged 60± 7% reduction at 3 months (Fig 4D). At 3 months of HS, the luminal diameters of arterioles from mice with RRM were not reduced as the perfusion pressure was incurred from 40 to 80 mmHg (10.75 ± 0.88 to 10.48 ± 0.79; ns) whereas all other groups had a significant (p<0.05) reduction.
The calculated threshold pressures to initiate myogenic contractions were similar among groups except for a significant reduction in the threshold of arterioles from mice with RRM fed high salt for 3 months (Table 2).
Data analysis by ANOVA at 3 months showed no significant effects of RRM or salt intake on MAP but a significant (p<0.0001) effect of RRM on the myogenic response with a significant (p<0.05) interaction.
Discussion
These results demonstrate a consistent and significant myogenic response of renal afferent arterioles isolated from the mouse and extend studies in the rat 12. The main new findings were that there was a linear increase in active wall tension of the mouse afferent arteriole with perfusion pressure above a threshold of about 40 mmHg with no evidence of a plateau up to 134 mmHg. A 15-fold increase in dietary salt intake over three months did not modify myogenic responses from arterioles of sham operated mice, but these responses were reduced over 3 weeks to 3 month after surgical reduction in renal mass. Unlike the rat model of RRM 19,20 and many human subjects with CKD 8,21, the mouse model of RRM, even when studied with prolonged telemetric recording, did not display salt sensitivity. However, mice with RRM had a higher MAP than sham-operated controls for the first three weeks of recording (four weeks total after RRM), with no apparent effects of salt intake. This might relate to activation of renal afferent reflexes initiated by the renal injury and the healing process from the two thirds nephrectomy 22, but this was not studied further. The mouse model of RRM had an adaptive increase in the size of the remaining kidney tissue but only modest albuminuria. There were corresponding modest renal morphologic changes, confirming a prior report 18 and only a minor increase in the glomerular sclerosis index. Since salt intake did not affect the blood pressure, the defective myogenic responses of renal afferent arterioles in this strain of mice with RRM, and the enhancing effects of salt, should be related primarily to the RRM and not to hypertension.
A preliminary study reported that a high salt diet reduced the autoregulatory responses of rat juxtamedullary arterioles and impaired responsiveness to purinergic receptor stimulation 9. The failure of a high salt intake to modify myogenic responses of afferent arterioles from sham-operated mice in the present study suggests that the diminished autoregulation in the rat study may relate to additional factors, such as a diminished tubuloglomerular feedback response 23, which contributes to autoregulation in intact kidneys, but this requires study.
Human chronic kidney disease is usually accompanied by a normal or low level of plasma renin activity unless it is due to segmental renal ischemia 24. This has been modeled in the rat by surgical reduction of renal mass in one kidney and contralateral nephrectomy which leads to a low renin, low angiotensin, salt-sensitive form of hypertension accompanied by an adaptive increase in single nephron glomerular filtration rate (GFR) followed by a slowly progressive loss of kidney function 25,26. The mouse model described here develops an initial modest hypertension of uncertain cause that is independent of salt intake and lasts about three or four weeks. Thereafter, the BP returns to normal even during rather severe dietary salt loading. These mice developed only modest albuminuria and glomerular injury, confirming prior studies in this mouse strain 18. Mice with RRM had a 3.4-fold increase in weight of the partially nephrectomized kidney. This demonstrates considerable adaptive responses to the reduction in renal mass and dissociates glomerular injury in this model from the adaptive growth of remaining nephrons. Studies in rat models led to the conclusion that glomerular injury results from the combination of systemic hypertension and impaired autoregulation 5,27 that permits transmission to the elevated pressure into the glomerulus 28. Thus, the absence of hypertension might explain the absence of significant glomerular injury in this study.
Autoregulation preserves a constant renal blood flow and glomerular filtration rate over a physiologic range of renal perfusion pressures. It is mediated predominantly by an afferent arteriolar myogenic and tubuloglomerular feedback (TGF) response 29. The myogenic response entails a proportionate contraction of the vascular smooth muscle cells with increasing stretch. The TGF response translates flow-dependent changes in the composition of the tubular fluid at the macula densa into proportionate changes in the resistance of afferent arterioles. Recent studies have identified additional mechanisms that contribute to autoregulation of renal blood flow 29-31. The mechanism of the afferent arteriolar myogenic response is incompletely understood, and might be clarified by future studies in gene deleted mice. Inscho et al demonstrated that ATP-sensitive purinoceptors linked to Rho-kinase regulated myogenic responsiveness of preglomerular microvascular elements 9,32. Preglomerular resistance is regulated by vascular smooth muscle cell calcium influx- and calcium mobilization-dependent mechanisms 33.
The present study is the first to quantify the myogenic component in isolation in the afferent arteriole of the mouse. A doubling of perfusion pressure in the afferent arterioles from normal mice within a physiologically relevant range of 40 to 80 mmHg elicited an 11% reduction in diameter (Fig 1A). This compares with a 32% 34 and a 15 to 30% 4 reduction in diameter of rat intact juxtaglomerular afferent arterioles with a doubling of perfusion pressure but, if the tubuloglomerular feedback response was blocked (as in the isolated arteriole), the response was reduced to 0 4 or 8 to 10% 34. Studies with the hydronephrotic kidney of normal rats (which lacks a TGF response) report a 7 to 23% response during a doubling of perfusion in pressure from 80 to 160 mmHg 1, whereas studies in isolated afferent arterioles from normal rats or rabbits report no significant contraction with a doubling of perfusion pressure 35,36. However, there was a 34% reduction in the diameter of afferent arterioles isolated from spontaneously hypertensive rats 12. Measurement in the rat juxtamedullary nephron preparation show that at 20% reduction in luminal diameter during a 40 mmHg pressure increase maintained a stable arteriolar blood flow (perfect autoregulation). Clearly, there is much variability in response that may relate to technical differences between preparations. Since resistance to flow is proportional to the fourth power of the radius, there would be more than a doubling in the calculated resistance from the reduction in afferent arteriolar radius recorded in the present study in normal mice across the pressure range of 40 to 80 mmHg. The impaired myogenic response in vessels from mice with RRM may contribute to the impaired ability to autoregulate glomerular capillary pressure 37 and whole kidney hemodynamics 38 that have been described in rats with RRM.
The glomerular capillary pressure (PGc), which approximates the pressure at the end of the afferent arteriole, can be calculated indirectly from the sum of the plasma protein oncotic pressure and the proximal tubule stop flow pressure (PSF). The oncotic pressure of mouse plasma averaged 20.9 ± 1.8 mmHg 39. The PSF in anesthetized mice was 32 to 42 mmHg (average 36 mmHg, unpublished data from our lab) 40,41. Thus, the calculated PGc in the mouse is approximately 57 mmHg. Since this is above the threshold pressure to elicit a myogenic response, which we found to average 36 mmHg, the afferent arteriolar myogenic response could contribute to the maintenance of renal blood flow at perfusion pressures above and below the normal range. Indeed, whole kidney blood flow in response to 20 mmHg pressure changes was well autoregulated in the mouse. The myogenic component contributed more to overall autoregulation during reductions than increases in renal perfusion pressure 29.
In summary, renal afferent arterioles from mice develop a contraction and a linear increase in myogenic tone above ambient perfusion pressure. This myogenic response is impaired substantially in the mouse model of prolonged RRM, especially during high salt intake.
Perspectives
Rats with RRM develop hypertension, proteinuria and glomerulosclerosis that are exacerbated by a high salt intake 7. In contrast, the mouse model of RRM developed hypertension over three weeks, but thereafter maintained a normal blood pressure and developed only modest albuminuria and glomerular injury even at three months of high salt intake. The myogenic response has been considered to protect the kidney from barotrauma during hypertension 5. A reset tubuloglomerular feedback response 42 and an impaired autoregulation of glomerular capillary pressure 37 or renal blood flow 43 have been related to renal damage in rat models 5. However, renal damage in the rat RRM model, or in patients with chronic kidney disease, is generally slight, as in the present and previous study 18 in this strain of mice, unless they develop hypertension 5. Thus, one might speculate that the delayed development of an impaired afferent arteriolar myogenic response in mice after three weeks might even be protective if it permitted better transmission of the arterial pressure into the kidneys and thereby allowed correction of the hypertension that developed during the first month. Development of a transient salt-resistant hypertension early after induction of RRM was not apparent in studies in the rat 7.
Acknowledgements
We thank Ms Emily Wing Kam Chan for preparing and editing the manuscript.
Sources of funding
The work described in this study was supported by research grants to Christopher S. Wilcox from the NIDDK (DK-049870 and DK-036079) and from the NHLBI (HL-68686) and by funds from the George E. Schreiner Chair of Nephrology.
Footnotes
Conflict of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hayashi K, Epstein M, Loutzenhiser R. Pressure-induced vasoconstriction of renal microvessels in normotensive and hypertensive rats. Studies in the isolated perfused hydronephrotic kidney. Circ Res. 1989;65:1475–1484. doi: 10.1161/01.res.65.6.1475. [DOI] [PubMed] [Google Scholar]
- 2.Loutzenhiser R, Epstein M, Horton C. Inhibition by diltiazem of pressure-induced afferent vasoconstriction in the isolated perfused rat kidney. Am J Cardiol. 1987;59:72A–75A. doi: 10.1016/0002-9149(87)90180-9. [DOI] [PubMed] [Google Scholar]
- 3.Cupples WA, Loutzenhiser RD. Dynamic autoregulation in the in vitro perfused hydronephrotic rat kidney. Am J Physiol. 1998;275:F126–F130. doi: 10.1152/ajprenal.1998.275.1.F126. [DOI] [PubMed] [Google Scholar]
- 4.Takenaka T, Harrison-Bernard LM, Inscho EW, Carmines PK, Navar LG. Autoregulation of afferent arteriolar blood flow in juxtamedullary nephrons. Am J Physiol. 1994;267:F879–F887. doi: 10.1152/ajprenal.1994.267.5.F879. [DOI] [PubMed] [Google Scholar]
- 5.Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertens. 2009;54:393–398. doi: 10.1161/HYPERTENSIONAHA.109.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dworkin LD, Benstein JA, Tolbert E, Feiner HD. Salt restriction inhibits renal growth and stabilizes injury in rats with established renal disease. J Am Soc Nephrol. 1996;7:437–442. doi: 10.1681/ASN.V73437. [DOI] [PubMed] [Google Scholar]
- 7.Li P, Mendonca M, Welch WJ, Wilcox CS. Salt-sensitive hypertension in a model of chronic renal failure is ameliorated by tempol. J Am Soc Nephrol. 2007;18:846A. Ref Type: Abstract. [Google Scholar]
- 8.Weir MR, Dengel DR, Behrens MT, Goldberg AP. Salt-induced increases in systolic blood pressure affect renal hemodynamics and proteinuria. Hypertens. 1995;25:1339–1344. doi: 10.1161/01.hyp.25.6.1339. [DOI] [PubMed] [Google Scholar]
- 9.Inscho EW, Pollock DM, Pollock JS, Cook AK. Attenuation of afferent arteriolar autoregulatory behavior by a high-salt diet involves activation of ETB receptors. Hypertens. 2009;54:e27. Ref Type: Abstract. [Google Scholar]
- 10.Navar LG. Integrating multiple paracrine regulators of renal microvascular dynamics. Am J Physiol. 1998;274:F433–F444. doi: 10.1152/ajprenal.1998.274.3.F433. [DOI] [PubMed] [Google Scholar]
- 11.Carmines PK, Inscho EW, Gensure RC. Arterial pressure effects on preglomerular microvasculature of juxtamedullary nephrons. Am J Physiol. 1990;258:F94–F102. doi: 10.1152/ajprenal.1990.258.1.F94. [DOI] [PubMed] [Google Scholar]
- 12.Ito S, Juncos LA, Carretero OA. Pressure-induced constriction of the afferent arteriole of spontaneously hypertensive rats. Hypertens. 1992;19:II164–II167. doi: 10.1161/01.hyp.19.2_suppl.ii164. [DOI] [PubMed] [Google Scholar]
- 13.Kawada N, Solis G, Ivey N, Connors S, Dennehy K, Modlinger P, Hamel R, Kawada JT, Imai E, Langenbach R, Welch W, Wilcox CS. Cyclooxygenase-1-deficient mice have high sleep-to-wake blood pressure ratios and renal vasoconstriction. Hypertens. 2005;45:1131–1138. doi: 10.1161/01.HYP.0000166141.69081.80. [DOI] [PubMed] [Google Scholar]
- 14.Lai EY, Martinka P, Fahling M, Mrowka R, Steege A, Gericke A, Sendeski M, Persson PB, Persson AE, Patzak A. Adenosine restores angiotensin II-induced contractions by receptor-independent enhancement of calcium sensitivity in renal arterioles. Circ Res. 2006;99:1117–1124. doi: 10.1161/01.RES.0000249530.85542.d4. [DOI] [PubMed] [Google Scholar]
- 15.Patzak A, Lai EY, Mrowka R, Steege A, Persson PB, Persson AEG. AT1 receptors mediate angiotensin II-induced release of nitric oxide in afferent arterioles. Kidney Int. 2004;66:1949–1958. doi: 10.1111/j.1523-1755.2004.00981.x. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi N, Hara K, Tojo A, Onozato ML, Honda T, Yoshida K, Mita S, Nakano S, Tsubokou Y, Matsuoka H. Eplerenone shows renoprotective effect by reducing LOX-1-mediated adhesion molecule, PKCepsilon-MAPK-p90RSK, and Rho-kinase pathway. Hypertens. 2005;45:538–544. doi: 10.1161/01.HYP.0000157408.43807.5a. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi N, Boysen G, Li F, Li Y, Swenberg JA. Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int. 2007;71:266–271. doi: 10.1038/sj.ki.5002033. [DOI] [PubMed] [Google Scholar]
- 18.Ma LJ, Fogo AB. Model of robust induction of glomerulosclerosis in mice: importance of genetic background. Kidney Int. 2003;64:350–355. doi: 10.1046/j.1523-1755.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 19.Ylitalo P, Hepp R, Mohring J, Gross F. Effects of varying sodium intake on blood pressure and renin-angiotensin system in subtotally nephrectomized rats. J Lab Clin Med. 1976;88:807–816. [PubMed] [Google Scholar]
- 20.Smith S, Anderson S, Ballermann BJ, Brenner BM. Role of atrial natriuretic peptide in adaptation of sodium excretion with reduced renal mass. J Clin Invest. 1986;77:1395–1398. doi: 10.1172/JCI112447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koomans HA, Roos JC, Dorhout Mees EJ, Delawi IM. Sodium balance in renal failure. A comparison of patients with normal subjects under extremes of sodium intake. Hypertens. 1985;7:714–721. doi: 10.1161/01.hyp.7.5.714. [DOI] [PubMed] [Google Scholar]
- 22.Campese VM, Krol E. Neurogenic factors in renal hypertension. Curr Hypertens Rep. 2002;4:256–260. doi: 10.1007/s11906-002-0016-3. [DOI] [PubMed] [Google Scholar]
- 23.Welch WJ, Wilcox CS. Macula densa arginine delivery and uptake in the rat regulates glomerular capillary pressure: effects of salt intake. J Clin Invest. 1997;100:2235–2242. doi: 10.1172/JCI119761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitch WE, Wilcox CS. Disorders of body fluids, sodium and potassium in chronic renal failure. Am J Med. 1982;72:536–550. doi: 10.1016/0002-9343(82)90523-x. [DOI] [PubMed] [Google Scholar]
- 25.Correa-Rotter R, Hostetter TH, Manivel JC, Rosenberg ME. Renin expression in renal ablation. Hypertens. 1992;20:483–490. doi: 10.1161/01.hyp.20.4.483. [DOI] [PubMed] [Google Scholar]
- 26.Hofbauer KG, Zschiedrich H, Gross F. Regulation of renin release and intrarenal formation of angiotensin. Studies in the isolated perfused rat kidney. Clin Exp Pharmacol Physiol. 1976;3:73–93. doi: 10.1111/j.1440-1681.1976.tb00593.x. [DOI] [PubMed] [Google Scholar]
- 27.Griffin KA, Picken M, Bidani AK. Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J Am Soc Nephrol. 1994;4:2023–2031. doi: 10.1681/ASN.V4122023. [DOI] [PubMed] [Google Scholar]
- 28.Bidani AK, Mitchell KD, Schwartz MM, Navar LG, Lewis EJ. Absence of glomerular injury or nephron loss in a normotensive rat remnant kidney model. Kidney Int. 1990;38:28–38. doi: 10.1038/ki.1990.163. [DOI] [PubMed] [Google Scholar]
- 29.Just A, Arendshorst WJ. A novel mechanism of renal blood flow autoregulation and the autoregulatory role of A1 adenosine receptors in mice. Am J Physiol Renal Physiol. 2007;293:F1489–F1500. doi: 10.1152/ajprenal.00256.2007. [DOI] [PubMed] [Google Scholar]
- 30.Just A, Olson AJ, Whitten CL, Arendshorst WJ. Superoxide mediates acute renal vasoconstriction produced by angiotensin II and catecholamines by a mechanism independent of nitric oxide. Am J Physiol Heart Circ Physiol. 2007;292:H83–H92. doi: 10.1152/ajpheart.00715.2006. [DOI] [PubMed] [Google Scholar]
- 31.Just A, Arendshorst WJ. Dynamics and contribution of mechanisms mediating renal blood flow autoregulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R619–R631. doi: 10.1152/ajpregu.00766.2002. [DOI] [PubMed] [Google Scholar]
- 32.Inscho EW, Ohishi K, Navar LG. Effects of ATP on pre- and postglomerular juxtamedullary microvasculature. Am J Physiol. 1992;263:F886–F893. doi: 10.1152/ajprenal.1992.263.5.F886. [DOI] [PubMed] [Google Scholar]
- 33.Fellner SK, Arendshorst WJ. Voltage-gated Ca2+ entry and ryanodine receptor Ca2+-induced Ca2+ release in preglomerular arterioles. Am J Physiol Renal Physiol. 2007;292:F1568–F1572. doi: 10.1152/ajprenal.00459.2006. [DOI] [PubMed] [Google Scholar]
- 34.Moore LC, Casellas D. Tubuloglomerular feedback dependence of autoregulationin rat juxtamedullary afferent arterioles. Kidney Int. 1990;37:1402–1408. doi: 10.1038/ki.1990.129. [DOI] [PubMed] [Google Scholar]
- 35.Yuan BH, Robinette JB, Conger JD. Effect of angiotensin II and norepinephrine on isolated rat afferent and efferent arterioles. Am J Physiol. 1990;258:F741–F750. doi: 10.1152/ajprenal.1990.258.3.F741. [DOI] [PubMed] [Google Scholar]
- 36.Edwards RM. Segmental effects of norepinephrine and angiotensin II on isolated renal microvessels. Am J Physiol. 1983;244:F526–F534. doi: 10.1152/ajprenal.1983.244.5.F526. [DOI] [PubMed] [Google Scholar]
- 37.Pelayo JC, Westcott JY. Impaired autoregulation of glomerular capillary hydrostatic pressure in the rat remnant nephron. J Clin Invest. 1991;88:101–105. doi: 10.1172/JCI115264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bidani AK, Schwartz MM, Lewis EJ. Renal autoregulation and vulnerability to hypertensive injury in remnant kidney. Am J Physiol. 1987;252:F1003–F1010. doi: 10.1152/ajprenal.1987.252.6.F1003. [DOI] [PubMed] [Google Scholar]
- 39.Stohrer M, Boucher Y, Stangassinger M, Jain RK. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000;60:4251–4255. [PubMed] [Google Scholar]
- 40.Loutzenhiser R, Bidani A, Chilton L. Renal myogenic response: kinetic attributes and physiological role. Circ Res. 2002;90:1316–1324. doi: 10.1161/01.res.0000024262.11534.18. [DOI] [PubMed] [Google Scholar]
- 41.Anderson S, Meyer TW, Rennke HG, Brenner BM. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1985;76:612–619. doi: 10.1172/JCI112013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salmond R, Seney FD., Jr. Reset tubuloglomerular feedback permits and sustains glomerular hyperfunction after extensive renal ablation. Am J Physiol. 1991;260:F395–F401. doi: 10.1152/ajprenal.1991.260.3.F395. [DOI] [PubMed] [Google Scholar]
- 43.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1153–R1167. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]