Abstract
The gastric H,K-ATPase is the primary target for the treatment of acid-related diseases. Proton pump inhibitors (PPIs) are weak bases composed of two moieties, a substituted pyridine with a primary pKa of about 4.0, which allows selective accumulation in the secretory canaliculus of the parietal cell, and a benzimidazole with a second pKa of about 1.0. PPIs are acid-activated prodrugs that convert to sulfenic acids or sulfenamides that react covalently with one or more cysteines accessible from the luminal surface of the ATPase. Because of covalent binding, their inhibitory effects last much longer than their plasma half-life. However, the short half-life of the drug in the blood and the requirement for acid activation impair their efficacy in acid suppression, particularly at night. PPIs with longer half-life promise to improve acid suppression. All PPIs give excellent healing of peptic ulcers and produce good results in reflux esophagitis. PPIs combined with antibiotics eradicate Helicobacter pylori.
Introduction
When activated by stimuli such as histamine and acetylcholine, the parietal cell undergoes dramatic morphologic changes from the resting status to the stimulated state. The gastric H,K-ATPase, which pumps gastric acid, appears to be in cytoplasmic tubular membranes in the resting state and then in the microvilli of the expanded secretory canaliculus in the stimulated state of the parietal cell. This morphologic change is proposed to result from fusion of cytoplasmic vesicles with the rudimentary microvilli to form the elongated microvilli of the expanded secretory canaliculus [1,2]. The gastric H,K-ATPase moves from the tubulovesicles to the apical membrane in the canaliculus of the stimulated state and secretes gastric acid by an electroneutral, ATP-dependent hydrogen-potassium exchange [3]. The enzyme uses extracellular K+ in order to secrete acid by the exchange of cytoplasmic hydronium with this K+. The cation reaches the luminal surface of the ATPase by insertion of K+ Cl− (KCNQ1, Clic6) channels into the microvillus membrane.
Proton pump inhibitors (PPIs) block the gastric H,K-ATPase, inhibiting gastric acid secretion. This effect enables healing of peptic ulcers, gastroesophageal reflux disease (GERD), Barrett’s esophagus, and Zollinger-Ellison syndrome, as well as the eradication of Helicobacter pylori as part of combination regimens. This article reviews the structure and function of the gastric H,K-ATPase and the inhibitors of this enzyme, the PPIs.
The Gastric H,K-ATPase
The gastric ATPase is a member of the P2 type ATPases. The first step of the reaction is phosphorylation of the catalytic subunit by MgATP, with export of protons; this step is followed by luminal potassium-dependent dephosphorylation and potassium reabsorption. The result is electroneutral exchange of cytoplasmic protons for exoplasmic potassium [3]. The gastric H,K-ATPase is composed of two subunits: a catalytic α subunit and a β subunit. The primary structure of the gastric H,K-ATPase α subunit was elucidated in the rat [4] and then in the hog [5], rabbit [6], dog [7], and human [8]. This catalytic subunit consists of 1033 or 1034 amino acids with 10 transmembrane segments in all species. Functional studies demonstrated that ATP catalyzed an electroneutral exchange of H for K, with a variable stoichiometry of 2H/2K/ATP at pH 6.1, which fell to 1H/1K/ATP as luminal pH fell below 3.0 [9–11]. The β subunit consists of 291 amino acids and contains six or seven N-linked glycosylation sites with one trans-membrane segment [12–14]. The gastric H,K-ATPase is fully assembled during biosynthesis in the endoplasmic reticulum and is delivered to the apical membrane as a heterodimeric oligomer. N-glycosylation of the β subunit was identified as being responsible for trafficking to the canalicular membrane. The steady state distribution of the H,K-ATPase β subunit in polarized cells depends on the balance between direct sorting from the trans-Golgi network, secondary associative sorting with a partner protein, and selective trafficking [15–17].
In the α subunit, a cluster of intramembranal carboxylic amino acids, located in the middle of the transmembrane segments TM4, TM5, TM6, and TM8, contains the ion-binding domain in this enzyme, including a lysine 791. This lysine of the H,K-ATPase seems to characterize the H,K-enzyme specificity for outward transport of the hydronium ion [18•]. Movement of the R-NH3+ into the carboxylic ion-binding domain is thought to catalyze the export of protons to the luminal face of the pump. The functional form of the gastric H,K-ATPase is a [αβ]2 heterodimer oligomer [19,20•]. The large changes in conformation in the cytoplasmic domain probably account for the finding that the enzyme functions as an out-of-phase oligomeric heterodimer, as most clearly demonstrated by measuring the stoichiometry of ATP binding, acid-stable phosphorylation, and binding of acid pump antagonists (APAs) or PPIs [20•].
The E1 form of the enzyme allows access to the ion-binding domain from the cytoplasmic surface. Binding of two ATP moieties, along with two magnesium ions, occurs in this conformation. One stabilizes the αβ orientation of the first two phosphates of the nucleotide, and the second, in proximity to the acceptor aspartyl residue, allows transfer of the γ phosphate to the catalytic subunit of the protein and initiates the change of conformation from the E1 form to the E1P conformer with the ion sites binding the hydronium ions. This process is followed by conversion to the E2P form, in which the protons are released outward and K+ binds from the luminal surface. ATP has dual roles in the transport cycle of the gastric H,K-ATPase. ATP phosphorylates the enzyme and promotes the E2·K→E1 + K+ transition [21]. The potassium occlusion site shows distorted octahedral geometry, with K+ bound predominantly on the M4 helix, with ligands contributed by backbone carbonyl oxygens of V338, A339, and V341, and by side chain oxygens of E820 and E795 [18•]. Recently two hydronium transporting pathways were proposed [11]. The hydroniums in the binding sites are transported into the lumen during the conformational transition from E1P to E2P.
Chemistry and Biology of PPIs
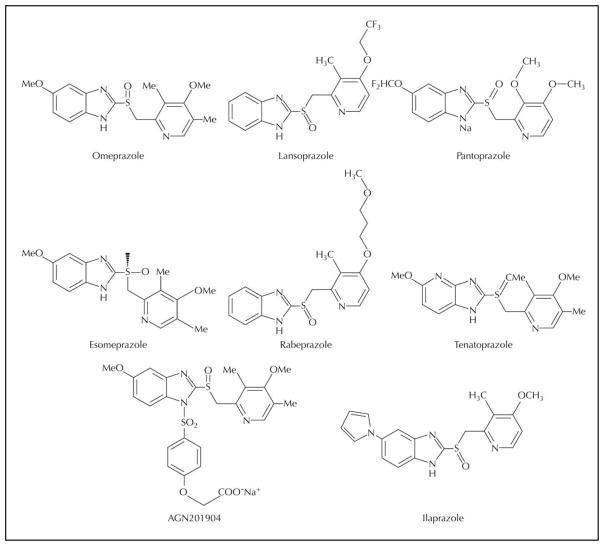
Because the H,K-ATPase is the final step of acid secretion, an inhibitor of this enzyme is more effective than receptor antagonists in suppressing gastric acid secretion [22]. Timoprazole is a compound that inhibited acid secretion in vivo regardless of the nature of the stimulus, whether ligands acting via extracellular receptors such as histamine or acetylcholine or the intracellular second messenger, cyclic adenosine monophosphate (cAMP). This compound, a pyridylmethylsulfinyl benzimidazole, was synthesized in 1975. It was found that the compound was ineffective in the absence of acid transport by the ATPase. With acid transport in gastric ATPase vesicles, the drug inhibited acid production and ATPase activity. It was therefore an acid-activated prodrug. Omeprazole was subsequently synthesized, and in 1989 it became the first drug of this class to be introduced into clinical use. Omeprazole (Losec; AstraZeneca, Wilmington, DE) was followed by lansoprazole (Prevacid; TAP Pharmaceuticals, Lake Forest, IL), pantoprazole (Protonix; Wyeth Pharmaceuticals, Madison, NJ) or rabeprazole (Aciphex; Eisai Company, Woodcliff, NJ) and more recently by the S-enantiomer of omeprazole (Nexium, AstraZeneca). Typical structures of PPIs are shown in Figure 1.
Figure 1.
Proton pump inhibitors.
PPIs are weak bases with a pKa1 between 3.8 and 4.9. This weak base pKa enables PPIs to accumulate selectively in the acidic space of the secretory canaliculus of the stimulated parietal cell, where the pH is about 1.0. This acid space–dependent concentration of PPIs is the first important property that determines their therapeutic index, giving a concentration at the luminal surface of the pump that is about 1000-fold higher than in the blood. The second step is acid-dependent conversion from the accumulated prodrug to the activated species, which is a highly reactive thiophilic reagent. A second protonation of these compounds is required for their activation to the compounds that form disulfides with luminally accessible cysteines of the H,K-ATPase. The actual inhibitory form of these prodrugs is a tetracyclic sulfenamide or sulfenic acid. The order of acid stability is tenatoprazole > pantoprazole > omeprazole > lansoprazole > rabeprazole [23].
Depending on the difference of the substituents on the pyridine or benzimidazole, PPIs bind to different cysteines. Omeprazole binds at cysteine 813 and cysteine 892. Lansoprazole binds at cysteine 813 and cysteine 321. Pantoprazole and tenatoprazole bind at cysteine 813 and cysteine 822. With acid transport by the ATPase, the second proton is added and then the compound converts to the sulfenic acid. If this occurs rapidly, as for omeprazole or lansoprazole, reaction with cysteine 813 and/or cysteine 321 takes place, and no drug can access cysteine 822. However, if the activation is delayed, the drug can access cysteine 822 before activation to the sulfenic acid. Then, when activated, both cysteine 813 and 822 are derivatized, as found for pantoprazole or tenatoprazole [23–27].
Differences of PPI binding sites modify biologic activity. When the PPI-bound enzyme was treated with glutathione, an endogenous reducing agent with a concentration of about 3 mM in the parietal cell, omeprazole and pantoprazole differed in loss of PPI binding. Pantoprazole binding resists glutathione reduction. These observations suggest that removal of binding of the drug to cysteine 813 accounts for the fast phase of recovery of acid secretion; the slow recovery occurs because of a delay in removal of the drug from cysteine 822. Both residues, cysteine 813 and 822, are equally labeled by pantoprazole in vivo. The small amount of cysteine 822 bound by omeprazole in vivo is not seen in vitro [26,28], presumably because acidification in isolated gastric vesicles is less than occurs in vivo. In vivo, it is likely that a minor fraction of the omeprazole remains protonated at both the pyridine and benzimidazole nitrogen and is slowly activated, allowing some access to cysteine 822.
Efficacy of Inhibition of Acid Secretion
All of these drugs inhibit the gastric H,K-ATPase by covalent binding, so the duration of their effect is longer than expected from their levels in the blood [28]. However, PPIs cannot inhibit all gastric acid pumps with oral dosing because not all pumps are active during the 90-minute half-life of the PPI in the blood. Because PPIs have a short half-life, only 70% of the pump enzymes are inhibited. It takes about 2 to 3 days to reach steady state inhibition of acid secretion. The pump protein has a half-life of about 54 hours in the rat [29] (and probably in humans). Thus about 20% of pumps are newly synthesized over a 24-hour period, and there may be greater pump synthesis at night than during the day. In addition, bedtime administration of PPIs will not add to inhibition of nocturnal acid breakthrough, because the drug will have disappeared by the time nighttime acid secretion is evident. Assuming that about 70% of pumps are activated by breakfast and that the PPI is given 30 to 60 minutes beforehand, it can be calculated that steady state inhibition on once-a-day dosing is about 66% of maximal acid output. Increasing the dose has virtually no effect once optimal dosage has been reached. Increasing the dose frequency does have some effect; a morning dose and an evening dose before meals results in about 80% inhibition of maximal acid output.
To improve acid inhibition, the plasma half-life of the PPI must be increased. One means is to replace the benzimidazole with imidazopyridine, slowing metabolism and prolonging the half-life of the drug, as found with tenatoprazole [30]. This PPI has an advantage in suppressing nighttime acid secretion, but its slow activation blunts its advantage for daytime acid suppression. An alternative approach was to synthesize a slowly absorbed derivative of omeprazole, which then increased the plasma half-life about threefold and produced a median pH of about 5 in initial studies [30].
Stability of Inhibition of Acid Secretion
Reversal of inhibition of the ATPase can occur either by de novo synthesis or reduction of the disulfide bond between the PPI and the protein. A rationale for examination of reversal of covalent binding to the H,K-ATPase was provided by measurement of the half-life of pump protein biosynthesis in rats treated for 7 days with omeprazole, which was 54 hours, and the half-time of restoration of ATPase activity, 15 hours. Such data suggest a more rapid recovery of ATPase activity and acid secretion than would occur if only de novo biosynthesis was responsible for restoration of ATPase activity [29]. In other experiments, the halftime of restoration of acid secretion in omeprazole-treated rats was 20 hours [31,32]. An analysis of the rate of restoration of acid secretion in humans suggested that the half-time was 24 hours following omeprazole inhibition, whereas after pantoprazole it was 46 hours [33]. Only pantoprazole appears to have a rate of recovery compatible with restoration of acid secretion due entirely to pump turnover [34,35].
Clinical Pharmacology of PPIs
In healthy humans, the half-life of PPIs is about 1 hour (9 hours for tenatoprazole), but the duration of acid inhibition is 48 hours because of irreversible binding to the H,K-ATPase. The maximal plasma drug concentration (Cmax) and the degree of acid suppression are poorly correlated, but the area under the plasma concentration–time curve (AUC) correlates well with acid suppression. Some pharmacokinetic parameters of the PPIs are summarized in Table 1.
Table 1.
Comparison of the pharmacokinetics of the proton pump inhibitors
| Drug, dose | AUC, μg×h/mL | Cmax, μg/mL | Half-life, h |
|---|---|---|---|
| Omeprazole, 20 mg | 0.2–1.2 [61] | 0.08–8 [61] | 0.6–1 [61] |
| 2.0 [62] | 1.5 [62] | ||
| Lansoprazole, 30 mg | 1.7–5 [61] | 0.6–1.2 [61] | 0.9–1.6 [61] |
| 5.2 [62] | 1.1 [62] | ||
| Pantoprazole, 40 mg | 2–5 [61] | 1.1–3.3 [61] | 0.9–1.9 [61] |
| 15.9 [62] | 1.2 [62] | ||
| Rabeprazole, 20 mg | 0.8 [61] | 0.41 [61] | 1 [61] |
| 2.2 [62] | 1.1 [62] | ||
| Esomeprazole, 40 mg | 7.3 [62] | 5.13 [36] | 1.1 [62] |
| 12.6 [36] | 3.46 [63] | 1.6 [36] | |
| 9.52 [63] | 1.45 [63] | ||
| Tenatoprazole, 40 mg | 75.2 [62] | 7.0 [52] | 8.7 [62] |
| 102.9 [52] | 9.28 [52] |
AUC—area under the curve; Cmax—maximal plasma drug concentration.
The oral bioavailability of PPIs is high: 77% for pantoprazole, 80% to 90% for lansoprazole, and 89% for esomeprazole, for example [36–39].
All the PPIs except tenatoprazole are rapidly metabolized in the liver by CYP enzymes (mostly by CYP2C19 and 3A4). Because of the sensitivity of PPIs to CYP enzymes, the pharmacokinetic profiles of PPIs are very different depending on the phenotypes of the metabolizers. Three phenotypes have been identified in various populations: extensive metabolizers (homEM), poor metabolizers (PM), and individuals carrying one wild-type and one mutant allele (hetEM). Poor metabolizers make up 3% of Caucasians and 15% to 20% of Asians. Systemic drug exposure (AUC) varies widely between these three populations: the AUC for omeprazole is about 7.5-fold higher in PM than in homEM, about 4.5-fold higher for lansoprazole, and about fourfold higher for rabeprazole. Because the pharmacodynamic response to PPIs is related to their AUC, intragastric pH is more elevated in PM (median pH ~6) and hetEM (median pH ~4–5) than in homEM (median pH ~3–4) [40]. Patients with hepatic impairment show a sevenfold increase in AUC for PPIs and a prolonged half-life. Esomeprazole was well tolerated across the spectrum of hepatic impairment, unlike other PPIs [41].
Comparing the Efficacy of PPIs
Suppressing gastric acid secretion enhances healing of acid-related diseases. Good healing of reflux esophagitis is achieved when the intragastric pH is greater than 4 for 16 hours per day, and peptic ulcer is optimally healed when the intragastric pH is greater than 3 for 16 hours per day. [42]. The best in vivo parameters to use in comparing PPIs with each other are the intragastric pH and total acid output. Generally, all PPIs provide good gastric acid suppression, but because they are used at different doses (omeprazole 20 mg, lansoprazole 30 mg, pantoprazole 40 mg, rabeprazole 20 mg, esomeprazole 40 mg, and tenatoprazole 40 mg), it is not easy to compare their efficacy.
One study compared rabeprazole (20 mg), lansoprazole (30 mg), pantoprazole (40 mg), and omeprazole (20-mg capsule vs 20-mg multiple unit pellet system tablet) [43]. Rabeprazole had the highest first-day median 24-hour pH. Another study compared gastric acid inhibition following the administration (30 minutes before breakfast) of rabeprazole (20 mg), esomeprazole (40 mg), omeprazole (20 mg), lansoprazole (30 mg) and pantoprazole (40 mg) for 5 consecutive days. At the end of the 5-day period, intragastric pH greater than 4 was maintained longer with esomeprazole, and more patients had a pH greater than 4 for more than 12 hours [44]. Esomeprazole (40 mg) gives good acid suppression (pH > 4 for 16.8 h/d) [45].
When lansoprazole (30 mg) was compared with omeprazole (20 mg), both taken orally on a daily basis, lansoprazole maintained the pH > 3 for a significantly greater time and produced a higher median 24-hour pH [46,47]. However, many other studies comparing omeprazole and lansoprazole have shown no significant difference overall in any pH parameters [48,49]. Pantoprazole (40 mg) has also been compared with omeprazole (20 mg); the results showed a significantly higher daytime and 24-hour pH with pantoprazole [50]. When the efficacy of each PPI is compared based on same dose, omeprazole, lansoprazole, and pantoprazole seem to produce similar acid suppression.
Tenatoprazole (40 mg) provided better nighttime acid suppression than other PPIs [51]. A significant difference was observed between tenatoprazole and esomeprazole during the nocturnal period; the mean pH was 4.64 with tenatoprazole versus 3.61 with esomeprazole, and the mean percentage of time with pH greater than 4 was significantly higher for tenatoprazole [52]. This difference is due to the prolonged half-life of tenatoprazole in the blood.
Many studies have compared healing rates of GERD. In comparisons of lansoprazole (30 mg) with omeprazole (20 mg), there was no significant difference in endoscopic healing rates at 4 and 8 weeks [53–55]. Again, when lansoprazole (30 mg) was compared with omeprazole (40 mg), no significant differences were found in healing rates or relief of symptoms [56]. Rabeprazole (20 mg) and omeprazole (20 mg) produced equivalent healing rates and relief of symptoms at 4 and 8 weeks [57].
More GERD patients (93.7%–94.1%) were healed at week 8 with the use of 40 mg of esomeprazole than with 20 mg of omeprazole (84.2%–86.9%) [58,59]. When 40 mg of esomeprazole was compared with 40 mg of pantoprazole, both gave good healing rates [60].
PPIs have been used successfully in triple-therapy regimens with clarithromycin and amoxicillin for the eradication of H. pylori. There was no significant difference between different PPI-based regimens.
Conclusions
The PPIs are prodrugs. These prodrugs require gastric acid secretion to be converted to the active sulfenamide or sulfenic acid that blocks gastric acid secretion. All PPIs except tenatoprazole have short half-lives (about 1 hour) and all have good oral bioavailability. Most PPIs are metabolized by CYP2C19 and 3A4. Hepatic impairment and old age reduce clearance of the PPIs, as do mutations in CYP2C19.
Acid suppression studies comparing omeprazole, lansoprazole, rabeprazole, and pantoprazole show equivalent efficacy. Most studies using standard doses have not shown a significant difference between the four PPIs for the healing of reflux esophagitis or duodenal ulcer. Esomeprazole and tenatoprazole have stronger acid suppression, with a longer period of intragastric pH greater than 4.
Acknowledgments
This work was supported by a US Veterans Administration Merit Grant and grants DK053642 and DK58333 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclosures
No potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Forte JG, Forte TM, Black JA, et al. Correlation of parietal cell structure and function. J Clin Gastroenterol. 1983;5(Suppl 1):17–27. doi: 10.1097/00004836-198312001-00003. [DOI] [PubMed] [Google Scholar]
- 2.Sawaguchi A, Aoyama F, Ide S, et al. The cryofixation of isolated rat gastric mucosa provides new insights into the functional transformation of gastric parietal cells: an in vitro experimental model study. Arch Histol Cytol. 2005;68:151–160. doi: 10.1679/aohc.68.151. [DOI] [PubMed] [Google Scholar]
- 3.Sachs G, Chang HH, Rabon E, et al. A nonelectrogenic H+ pump in plasma membranes of hog stomach. J Biol Chem. 1976;251:7690–7698. [PubMed] [Google Scholar]
- 4.Shull GE, Lingrel JB. Molecular cloning of the rat stomach (H+ + K+)-ATPase. J Biol Chem. 1986;261:16788–16791. [PubMed] [Google Scholar]
- 5.Maeda M, Ishizaki J, Futai M. cDNA cloning and sequence determination of pig gastric (H+ + K+)-ATPase. Biochem Biophys Res Commun. 1988;157:203–209. doi: 10.1016/s0006-291x(88)80033-0. [DOI] [PubMed] [Google Scholar]
- 6.Bamberg K, Mercier F, Reuben MA, et al. cDNA cloning and membrane topology of the rabbit gastric H+/K+-ATPase alpha-subunit. Biochim Biophys Acta. 1992;1131:69–77. doi: 10.1016/0167-4781(92)90100-e. [DOI] [PubMed] [Google Scholar]
- 7.Song I, Mortell MP, Gantz I, et al. Molecular cloning and structural analysis of canine gastric H+,K+-ATPase. Biochem Biophys Res Commun. 1993;196:1240–1247. doi: 10.1006/bbrc.1993.2385. [DOI] [PubMed] [Google Scholar]
- 8.Maeda M, Oshiman K, Tamura S, et al. Human gastric (H+ + K+)-ATPase gene. Similarity to (Na+ + K+)-ATPase genes in exon/intron organization but difference in control region. J Biol Chem. 1990;265:9027–9032. [PubMed] [Google Scholar]
- 9.Rabon EC, McFall TL, Sachs G. The gastric [H,K]ATPase: H+/ATP stoichiometry. J Biol Chem. 1982;257:6296–6299. [PubMed] [Google Scholar]
- 10.Munson K, Garcia R, Sachs G. Inhibitor and ion binding sites on the gastric H,K-ATPase. Biochemistry. 2005;44:5267–5284. doi: 10.1021/bi047761p. [DOI] [PubMed] [Google Scholar]
- 11.Morii M, Yamauchi M, Ichikawa T, et al. Involvement of the H3O+-Lys-164 -Gln-161-Glu-345 charge transfer pathway in proton transport of gastric H+,K+-ATPase. J Biol Chem. 2008;283:16876–16884. doi: 10.1074/jbc.M800563200. [DOI] [PubMed] [Google Scholar]
- 12.Reuben MA, Lasater LS, Sachs G. Characterization of a beta subunit of the gastric H+/K+-transporting ATPase. Proc Natl Acad Sci U S A. 1990;87:6767–6771. doi: 10.1073/pnas.87.17.6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shull GE. cDNA cloning of the beta-subunit of the rat gastric H,K-ATPase. J Biol Chem. 1990;265:12123–12126. [PubMed] [Google Scholar]
- 14.Toh BH, Gleeson PA, Simpson RJ, et al. The 60- to 90-kDa parietal cell autoantigen associated with autoimmune gastritis is a beta subunit of the gastric H+/K+-ATPase (proton pump) Proc Natl Acad Sci U S A. 1990;87:6418–6422. doi: 10.1073/pnas.87.16.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vagin O, Denevich S, Sachs G. Plasma membrane delivery of the gastric H,K-ATPase: the role of beta-subunit glycosylation. Am J Physiol Cell Physiol. 2003;285:C968–C976. doi: 10.1152/ajpcell.00068.2003. [DOI] [PubMed] [Google Scholar]
- 16.Vagin O, Turdikulova S, Sachs G. The H,K-ATPase beta subunit as a model to study the role of N-glycosylation in membrane trafficking and apical sorting. J Biol Chem. 2004;279:39026–39034. doi: 10.1074/jbc.M405453200. [DOI] [PubMed] [Google Scholar]
- 17.Vagin O, Turdikulova S, Yakubov I, et al. Use of the H,K-ATPase beta subunit to identify multiple sorting pathways for plasma membrane delivery in polarized cells. J Biol Chem. 2005;280:14741–14754. doi: 10.1074/jbc.M412657200. [DOI] [PubMed] [Google Scholar]
- 18•.Munson K, Law RJ, Sachs G. Analysis of the gastric H,K ATPase for ion pathways and inhibitor binding sites. Biochemistry. 2007;46:5398–5417. doi: 10.1021/bi062305h. This study showed how potassium ion moves across the membrane in the gastric H,K-ATPase. The new E2P model had increased separation between transmembrane segments M3 through M8, and addition of water in this space showed not only an inhibitor entry path to the luminal vestibule but also a channel leading to the ion binding site. Addition of K+ to the hydrated channel with molecular dynamics modeling of ion movement identified a pathway for K+ from the lumen to the ion binding site to give E2K. Autodock analyses of the new E2P model now correctly discriminate between high-affinity and low-affinity K+ competitive inhibitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe K, Kaya S, Taniguchi K, et al. Evidence for a relationship between activity and the tetraprotomeric assembly of solubilized pig gastric H/K-ATPase. J Biochem (Tokyo) 2005;138:293–301. doi: 10.1093/jb/mvi127. [DOI] [PubMed] [Google Scholar]
- 20•.Shin JM, Grundler G, Senn-Bilfinger J, et al. Functional consequences of the oligomeric form of the membrane-bound gastric H,K-ATPase. Biochemistry. 2005;44:16321–16332. doi: 10.1021/bi051342q. This study demonstrated that the gastric H,K-ATPase is an oligomeric structure composed of E1:E2. At < 10 μM MgATP, E1[ATP]·Mg·(H+):E2 is formed at a high-affinity site converting to E1P·Mg· (H+):E2 then to E2P·Mg:E1 with luminal proton extrusion. At high MgATP (> 0.1 mM), the oligomer forms E2P·Mg:E1[ATP]·Mg·(H+). The sum of maximal EP formation and ATP binding was 5.3 nmol/mg. An inhibitor, INT bound at the enzyme with 2.6 nmol/mg in the presence of MgATP. Binding of the inhibitor fixes half the oligomer in the E2 form with full inhibition of activity, whereas the other half of the oligomer is able to form E1P only when the inhibitor is bound. It appears that the catalytic subunits of the oligomer during turnover in intact gastric vesicles are restricted to a reciprocal E1:E2 configuration. [DOI] [PubMed] [Google Scholar]
- 21.Reenstra WW, Crothers J, Jr, Forte JG. The conformation of H,K-ATPase determines the nucleoside triphosphate (NTP) selectivity for active proton transport. Biochemistry. 2007;46:10145–10152. doi: 10.1021/bi700991n. [DOI] [PubMed] [Google Scholar]
- 22.Fellenius E, Berglindh T, Sachs G, et al. Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+ + K+)ATPase. Nature. 1981;290:159–161. doi: 10.1038/290159a0. [DOI] [PubMed] [Google Scholar]
- 23.Shin JM, Homerin M, Domagala F, et al. Characterization of the inhibitory activity of tenatoprazole on the gastric H+,K+-ATPase in vitro and in vivo. Biochem Pharmacol. 2006;71:837–849. doi: 10.1016/j.bcp.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 24.Sachs G, Shin JM, Besancon M, et al. The continuing development of gastric acid pump inhibitors. Aliment Pharmacol Ther. 1993;7(Suppl 1):4–12. doi: 10.1111/j.1365-2036.1993.tb00582.x. discussion 29–31. [DOI] [PubMed] [Google Scholar]
- 25.Shin JM, Besancon M, Simon A, et al. The site of action of pantoprazole in the gastric H+/K+-ATPase. Biochim Biophys Acta. 1993;1148:223–233. doi: 10.1016/0005-2736(93)90133-k. [DOI] [PubMed] [Google Scholar]
- 26.Shin JM, Sachs G. Differences in binding properties of two proton pump inhibitors on the gastric H+,K+-ATPase in vivo. Biochem Pharmacol. 2004;68:2117–2127. doi: 10.1016/j.bcp.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 27.Besancon M, Shin JM, Mercier F, et al. Membrane topology and omeprazole labeling of the gastric H+,K+-adenosine-triphosphatase. Biochemistry. 1993;32:2345–2355. doi: 10.1021/bi00060a028. [DOI] [PubMed] [Google Scholar]
- 28.Shin JM, Sachs G. Restoration of acid secretion following treatment with proton pump inhibitors. Gastroenterology. 2002;123:1588–1597. doi: 10.1053/gast.2002.36593. [DOI] [PubMed] [Google Scholar]
- 29.Gedda K, Scott D, Besancon M, et al. Turnover of the gastric H+,K+-adenosine triphosphatase alpha subunit and its effect on inhibition of rat gastric acid secretion. Gastroenterology. 1995;109:1134–1141. doi: 10.1016/0016-5085(95)90571-5. [DOI] [PubMed] [Google Scholar]
- 30.Hunt RH, Armstrong D, Yaghoobi M, et al. Predictable prolonged suppression of gastric acidity with a novel proton pump inhibitor, AGN 201904-Z. Aliment Pharmacol Ther. 2008;28:187–199. doi: 10.1111/j.1365-2036.2008.03725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallmark B, Larsson H, Humble L. The relationship between gastric acid secretion and gastric H+,K+-ATPase activity. J Biol Chem. 1985;260:13681–13684. [PubMed] [Google Scholar]
- 32.Im WB, Blakeman DP, Davis JP. Irreversible inactivation of rat gastric (H+-K+)-ATPase in vivo by omeprazole. Biochem Biophys Res Commun. 1985;126:78–82. doi: 10.1016/0006-291x(85)90573-x. [DOI] [PubMed] [Google Scholar]
- 33.Ferron GM, McKeand W, Mayer PR. Pharmacodynamic modeling of pantoprazole’s irreversible effect on gastric acid secretion in humans and rats. J Clin Pharmacol. 2001;41:149–156. doi: 10.1177/00912700122009953. [DOI] [PubMed] [Google Scholar]
- 34.Dammann HG, Burkhardt F. Pantoprazole versus omeprazole: influence on meal-stimulated gastric acid secretion. Eur J Gastroenterol Hepatol. 1999;11:1277–1282. [PubMed] [Google Scholar]
- 35.Katashima M, Yamamoto K, Tokuma Y, et al. Comparative pharmacokinetic/pharmacodynamic analysis of proton pump inhibitors omeprazole, lansoprazole and pantoprazole, in humans. Eur J Drug Metab Pharmacokinet. 1998;23:19–26. doi: 10.1007/BF03189822. [DOI] [PubMed] [Google Scholar]
- 36.Lind T, Rydberg L, Kyleback A, et al. Esomeprazole provides improved acid control vs. omeprazole in patients with symptoms of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2000;14:861–867. doi: 10.1046/j.1365-2036.2000.00813.x. [DOI] [PubMed] [Google Scholar]
- 37.Landes BD, Petite JP, Flouvat B. Clinical pharmacokinetics of lansoprazole. Clin Pharmacokinet. 1995;28:458–470. doi: 10.2165/00003088-199528060-00004. [DOI] [PubMed] [Google Scholar]
- 38.Huber R, Hartmann M, Bliesath H, et al. Pharmacokinetics of pantoprazole in man. Int J Clin Pharmacol Ther. 1996;34:185–194. [PubMed] [Google Scholar]
- 39.Gerloff J, Mignot A, Barth H, et al. Pharmacokinetics and absolute bioavailability of lansoprazole. Eur J Clin Pharmacol. 1996;50:293–297. doi: 10.1007/s002280050111. [DOI] [PubMed] [Google Scholar]
- 40.Klotz U. Clinical impact of CYP2C19 polymorphism on the action of proton pump inhibitors: a review of a special problem. Int J Clin Pharmacol Ther. 2006;44:297–302. doi: 10.5414/cpp44297. [DOI] [PubMed] [Google Scholar]
- 41.Sjovall H, Bjornsson E, Holmberg J, et al. Pharmacokinetic study of esomeprazole in patients with hepatic impairment. Eur J Gastroenterol Hepatol. 2002;14:491–496. doi: 10.1097/00042737-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Bell NJ, Burget D, Howden CW, et al. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion. 1992;51(Suppl 1):59–67. doi: 10.1159/000200917. [DOI] [PubMed] [Google Scholar]
- 43.Pantoflickova D, Dorta G, Ravic M, et al. Acid inhibition on the first day of dosing: comparison of four proton pump inhibitors. Aliment Pharmacol Ther. 2003;17:1507–1514. doi: 10.1046/j.1365-2036.2003.01496.x. [DOI] [PubMed] [Google Scholar]
- 44.Miner P, Jr, Katz PO, Chen Y, et al. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five-way crossover study. Am J Gastroenterol. 2003;98:2616–2620. doi: 10.1111/j.1572-0241.2003.08783.x. [DOI] [PubMed] [Google Scholar]
- 45.Kalaitzakis E, Bjornsson E. A review of esomeprazole in the treatment of gastroesophageal reflux disease (GERD) Ther Clin Risk Manag. 2007;3:653–663. [PMC free article] [PubMed] [Google Scholar]
- 46.Blum RA, Shi H, Karol MD, et al. The comparative effects of lansoprazole, omeprazole, and ranitidine in suppressing gastric acid secretion. Clin Ther. 1997;19:1013–1023. doi: 10.1016/s0149-2918(97)80053-7. [DOI] [PubMed] [Google Scholar]
- 47.Tolman KG, Sanders SW, Buchi KN, et al. The effects of oral doses of lansoprazole and omeprazole on gastric pH. J Clin Gastroenterol. 1997;24:65–70. doi: 10.1097/00004836-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Geus WP, Mulder PG, Nicolai JJ, et al. Acid-inhibitory effects of omeprazole and lansoprazole in Helicobacter pylori-negative healthy subjects. Aliment Pharmacol Ther. 1998;12:329–335. doi: 10.1046/j.1365-2036.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- 49.Janczewska I, Sagar M, Sjostedt S, et al. Comparison of the effect of lansoprazole and omeprazole on intragastric acidity and gastroesophageal reflux in patients with gastro-esophageal reflux disease. Scand J Gastroenterol. 1998;33:1239–1243. doi: 10.1080/00365529850172304. [DOI] [PubMed] [Google Scholar]
- 50.Hartmann M, Theiss U, Huber R, et al. Twenty-four-hour intragastric pH profiles and pharmacokinetics following single and repeated oral administration of the proton pump inhibitor pantoprazole in comparison to omeprazole. Aliment Pharmacol Ther. 1996;10:359–366. doi: 10.1111/j.0953-0673.1996.00359.x. [DOI] [PubMed] [Google Scholar]
- 51.Galmiche JP, Bruley Des Varannes S, et al. Tenatoprazole, a novel proton pump inhibitor with a prolonged plasma half-life: effects on intragastric pH and comparison with esomeprazole in healthy volunteers. Aliment Pharmacol Ther. 2004;19:655–662. doi: 10.1111/j.1365-2036.2004.01893.x. [DOI] [PubMed] [Google Scholar]
- 52.Hunt RH, Armstrong D, James C, et al. Effect on intra-gastric pH of a PPI with a prolonged plasma half-life: comparison between tenatoprazole and esomeprazole on the duration of acid suppression in healthy male volunteers. Am J Gastroenterol. 2005;100:1949–1956. doi: 10.1111/j.1572-0241.2005.41956.x. [DOI] [PubMed] [Google Scholar]
- 53.Castell DO, Richter JE, Robinson M, et al. Efficacy and safety of lansoprazole in the treatment of erosive reflux esophagitis. The Lansoprazole Group. Am J Gastroenterol. 1996;91:1749–1757. [PubMed] [Google Scholar]
- 54.Mee AS, Rowley JL. Rapid symptom relief in reflux oesophagitis: a comparison of lansoprazole and omeprazole. Aliment Pharmacol Ther. 1996;10:757–763. doi: 10.1046/j.1365-2036.1996.56198000.x. [DOI] [PubMed] [Google Scholar]
- 55.Hatlebakk JG, Berstad A, Carling L, et al. Lansoprazole versus omeprazole in short-term treatment of reflux oesophagitis. Results of a Scandinavian multicentre trial. Scand J Gastroenterol. 1993;28:224–228. doi: 10.3109/00365529309096076. [DOI] [PubMed] [Google Scholar]
- 56.Mulder CJ, Dekker W, Gerretsen M. Lansoprazole 30 mg versus omeprazole 40 mg in the treatment of reflux oesophagitis grade II, III and IVa (a Dutch multicentre trial). Dutch Study Group. Eur J Gastroenterol Hepatol. 1996;8:1101–1106. doi: 10.1097/00042737-199611000-00013. [DOI] [PubMed] [Google Scholar]
- 57.Dekkers CP, Beker JA, Thjodleifsson B, et al. Double-blind comparison [correction of Double-blind, placebo-controlled comparison] of rabeprazole 20 mg vs. omeprazole 20 mg in the treatment of erosive or ulcerative gastro-oesophageal reflux disease. The European Rabeprazole Study Group. Aliment Pharmacol Ther. 1999;13:49–57. doi: 10.1046/j.1365-2036.1999.00438.x. [DOI] [PubMed] [Google Scholar]
- 58.Kahrilas PJ, Falk GW, Johnson DA, et al. Esomeprazole improves healing and symptom resolution as compared with omeprazole in reflux oesophagitis patients: a randomized controlled trial. The Esomeprazole Study Investigators. Aliment Pharmacol Ther. 2000;14:1249–1258. doi: 10.1046/j.1365-2036.2000.00856.x. [DOI] [PubMed] [Google Scholar]
- 59.Richter JE, Kahrilas PJ, Johanson J, et al. Efficacy and safety of esomeprazole compared with omeprazole in GERD patients with erosive esophagitis: a randomized controlled trial. Am J Gastroenterol. 2001;96:656–665. doi: 10.1111/j.1572-0241.2001.3600_b.x. [DOI] [PubMed] [Google Scholar]
- 60.Labenz J, Armstrong D, Lauritsen K, et al. A randomized comparative study of esomeprazole 40 mg versus pantoprazole 40 mg for healing erosive oesophagitis: the EXPO study. Aliment Pharmacol Ther. 2005;21:739–746. doi: 10.1111/j.1365-2036.2005.02368.x. [DOI] [PubMed] [Google Scholar]
- 61.Stedman CA, Barclay ML. Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:963–978. doi: 10.1046/j.1365-2036.2000.00788.x. [DOI] [PubMed] [Google Scholar]
- 62.Scarpignato C, Pelosini I. Review article: the opportunities and benefits of extended acid suppression. Aliment Pharmacol Ther. 2006;23(Suppl 2):23–34. doi: 10.1111/j.1365-2036.2006.02945.x. [DOI] [PubMed] [Google Scholar]
- 63.Katz PO, Castell DO, Chen Y, et al. Intragastric acid suppression and pharmacokinetics of twice-daily esomeprazole: a randomized, three-way crossover study. Aliment Pharmacol Ther. 2004;20:399–406. doi: 10.1111/j.1365-2036.2004.02079.x. [DOI] [PubMed] [Google Scholar]