Abstract
This paper provides the first evidence of a clinical response to gene therapy in human arthritis. Two subjects with rheumatoid arthritis received ex vivo, intraarticular delivery of human interleukin-1 receptor antagonist (IL-1Ra) cDNA. To achieve this, autologous synovial fibroblasts were transduced with a retrovirus, MFG-IRAP, carrying IL-1Ra as the transgene, or remained as untransduced controls. Symptomatic metacarpophalangeal (MCP) joints were injected with control or transduced cells. Joints were clinically evaluated on the basis of pain; the circumference of MCP joint 1 was also measured. After 4 weeks, joints underwent surgical synovectomy. There were no adverse events in either subject. The first subject responded dramatically to gene transfer, with a marked and rapid reduction in pain and swelling that lasted for the entire 4 weeks of the study. Remarkably, joints receiving IL-1Ra cDNA were protected from flares that occurred during the study period. Analysis of RNA recovered after synovectomy revealed enhanced expression of IL-1Ra and reduced expression of matrix metalloproteinase-3 and IL-1β. The second subject also responded with reduced pain and swelling. Thus, gene transfer to human, rheumatoid joints can be accomplished safely to produce clinical benefit, at least in the short term. Using this ex vivo procedure, the transgene persisted within the joint for at least 1 month. Further clinical studies are warranted.
Introduction
Although novel biologic therapies (“biologics”) greatly improve the treatment of patients with rheumatoid arthritis (RA), the disease remains incurable and the need for improved therapies remains (Smolen et al., 2007). Biologics are expensive and need to be administered repeatedly by injection or infusion. The majority of patients mount an incomplete therapeutic response and the issue of side effects remains unresolved. The introduction of antiarthritic genes into individual diseased joints promises to provide a superior therapeutic effect at much lower cost while minimizing the exposure of nontarget sites, potentially reducing side effects (Evans et al., 2006b). Because gene delivery offers the prospect of sustained, endogenous, intraarticular synthesis of the transgene product (Gouze et al., 2007), frequent readministration of the gene therapeutic may not be required.
There is overwhelming, preclinical proof of principle that local gene therapy is effective in animal models of RA (Evans et al., 2006a). In the first human study (Evans et al., 2005), autologous synovial fibroblasts were genetically modified with a recombinant retrovirus (MFG-IRAP) carrying the human interleukin-1 receptor antagonist (IL-1Ra) cDNA. In a dose-escalation, double-blind fashion genetically modified or control cells were injected into the metacarpophalangeal (MCP) joints of nine subjects with RA. One week later, the MCP joints underwent unilateral sialastic implant arthroplasty; synovia were retrieved and examined for evidence of transgene persistence and expression.
This phase 1 protocol (Evans et al., 1996, 2005) confirmed that genes could be safely transferred to human rheumatoid joints in an ex vivo fashion and expressed intraarticularly, but it was not possible to address efficacy or whether the transgene persisted within the recipient joints for longer than 1 week. Here we report results from a subsequent study that demonstrate a clinical response to gene transfer, with persistence of the transgene for at least 1 month.
Approval was given for the treatment of six subjects, but the study was terminated after only two individuals had completed the protocol because of serious adverse events in an unrelated clinical trial for X-linked severe combined immunodeficiency (Kohn et al., 2003) that used the same retroviral vector backbone (MFG). Data from these two subjects are presented here.
Materials and Methods
Study design
This protocol is based on that of Evans and colleagues (1996), which uses an ex vivo, retrovirus-based strategy for transferring IL-1Ra cDNA to individual joints (Bandara et al., 1992, 1993). The protocol was approved by the ethics committee of the University of Düsseldorf (Düsseldorf, Germany). Subjects participating in the study gave their informed consent.
Briefly, subjects were postmenopausal females under the age of 75 years with a diagnosis of RA according to American College of Rheumatology (ACR, Atlanta, GA) criteria (Arnett et al., 1988). They required surgical synovectomy of MCP joints 1–3 on both hands. Synovectomy of the MCP joints on one hand provided autologous synovial fibroblasts for genetic modification and injection into MCP joints of the contralateral hand. Nontransduced, autologous cells served as intrapatient controls. Surgeons and patients were blinded and joints were randomly assigned to receive transduced or untransduced cells by an individual external to the study.
At baseline, the circumference of MCP joint 1 was measured and all experimental joints were assigned a pain score of 100% by each study subject. Subjects were required to keep a “pain diary” and, in particular, to record pain levels at the time points indicated in Fig. 1, with 0% indicating a pain-free joint. Subjects were reexamined at 2 weeks and for each the circumference of MCP joint 1 was remeasured. Four weeks after the injection of cells, the MCP joints underwent surgical synovectomy. Just before surgery, the circumference of MCP joint 1 was again measured. Fibroblasts were cultured from synovium harvested from subject 1 at the time of synovectomy, and RNA was examined for expression of IL-1Ra, matrix metalloproteinase-3 (MMP-3; stromelysin-1), and interleukin-1β (IL-1β) by Northern blotting.
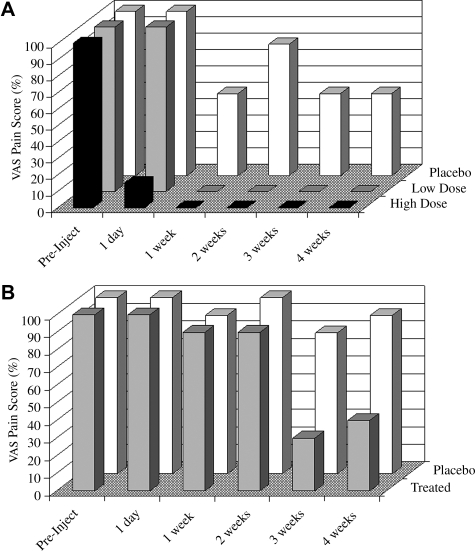
FIG. 1.
Effect of gene transfer on pain in metacarpophalangeal joints. (A) Subject 1 received intraarticular injection of 3 × 106 genetically modified synoviocytes (high dose; solid columns) in MCP joint 1 and 106 genetically modified synoviocytes (low dose; gray columns) in MCP joint 2. The control joint, MCP joint 3, received 106 unmodified synoviocytes (placebo; open columns). Pain was assessed on a visual analog scale (VAS), with 100% representing the level of pain immediately before injection. (B) Subject 2 received 2 × 106 genetically modified synoviocytes in MCP joint 1 (treated; gray columns) and 106 unmodified synoviocytes in MCP joint 2 (placebo; open columns). All injected joints underwent surgical synovectomy after 4 weeks.
Laboratory methods
Culture and transduction of synovial cells
Synovial tissue recovered at the time of surgery was digested with collagenase and the liberated cells were seeded into plastic culture flasks. Adherent, fibroblastic cells were grown in Ham's F-12 medium supplemented with 10% fetal bovine serum and expanded by passage. Half the cultures from each subject were genetically modified with the amphotropic retrovirus, MFG-IRAP, which carries the full-length coding sequence for human IL-1Ra under the transcriptional regulation of the viral long terminal repeat (Bandara et al., 1993; Evans et al., 2006c). Cultures were transduced at 60% confluence by incubation with 4 ml of retroviral supernatant containing 1,5-dimethyl-1,5-diazaundecamethylene polymethobromide (Polybrene, 8 μg/ml). Cells were centrifuged at 2000 × g for 2 hr during retroviral transduction (Del Vecchio et al., 2001) and incubated for 18 hr before adding fresh growth medium. At confluence, media were changed and the amount of IL-1Ra secreted by transduced and untransduced cells was measured by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN). The release criteria required the synthesis by the transduced cells of at least 30 ng of IL-1Ra per 106 cells per 48 hr (Evans et al., 1996). This amount was based on an estimated minimal effective dose derived from preclinical data in rabbits (Otani et al., 1996).
Northern blot
RNA was recovered from cells with TRIzol solution (Invitrogen, Carlsbad, CA), extracted with chloroform, precipitated with isopropanol, resuspended in 10 mM Tris-HCl buffer (pH 8) containing 1 mM EDTA and measured spectrophotometrically at a wavelength of 260 nm. RNA was subjected to 1% agarose gel electrophoresis (10 μg of RNA per lane). Bands were viewed after ethidium bromide staining, blotted onto nylon, and cross-linked by ultraviolet irradiation. Radiolabeled probe was generated by random primer extension and used to visualize bands by autoradiography.
Results
Patient demographics are shown in Table 1. Both subjects were female with extensive use of disease-modifying antirheumatic drugs (DMARDs) and steroids that failed to provide adequate control of their clinical symptoms, thus necessitating surgical intervention. Subject 1 had undergone ovariectomy and was thus surgically postmenopausal. Her RA was difficult to manage pharmacologically and she had active, highly inflammatory disease. She voluntarily stopped taking methylprednisolone shortly before receiving injections of cells. Subject 2, unlike subject 1, had established disease and her joints were less acutely inflamed. Cultures of synovial fibroblasts produced no IL-1Ra spontaneously, but synthesized sufficient amounts to satisfy the release criteria after transduction with MFG-IRAP (Table 1).
Table 1.
Characteristics and Disease History of Subjects
| Subject 1 | Subject 2 | |
|---|---|---|
| Gender | Female | Female |
| Age (years) | 35 | 60 |
| Duration of disease (years) | 4 | 8 |
| Rheumatoid factor | + | + |
| Prior DMARD and steroid use | MethylprednisoloneDiclofenacResochinMethotrexate | PrednisoloneMeloxicamMethotrexate |
| Therapy at time of study | NeurofenacMonoflamTramalNovalgin | PrednisoneTramalMobic |
| IL-1Ra productiona per 106 autologous synoviocytes per 48 hr (ng) | ||
| Before transduction | 0 | 0 |
| After transduction | 56 | 97 |
Abbreviation: DMARD; disease-modifying antirheumatic drug; IL-1Ra, interleukin-1 receptor antagonist.
IL-1Ra production was measured by ELISA of conditioned medium.
Subject 1 mounted a dramatic clinical response to gene therapy (Fig. 1A). Pain in MCP joint 1, which received 3 × 106 genetically modified cells, was reduced by 85% within 1 day. After 1 week, this joint was pain-free and remained so for the entire 4 weeks until synovectomy; the circumference of MCP joint 1 was reduced by 6 mm at 2 weeks and by 5 mm at 4 weeks. MCP joint 2, which received 106 genetically modified cells, responded more slowly, but it, too, was pain-free from 1 week onward. Pain in MCP joint 3, which received unmodified cells, was reduced up to 50%. Of note, this subject experienced major flares of her RA during the study period, probably because of methylprednisolone withdrawal. Her MCP joints 1 and 2, unlike other joints, were largely protected from the effects of the flares.
Subject 2 also responded to gene therapy (Fig. 1B) but less dramatically than subject 1. Pain in MCP joint 1, which received 2 × 106 genetically modified cells, fell by 70% between weeks 2 and 3; it remained at 40% of preinjection levels at 4 weeks. The circumference of this joint was reduced by 4 mm at week 2 and by 5 mm at week 4. The control, MCP joint 2 that received unmodified cells showed only small pain improvements at weeks 3 and 4.
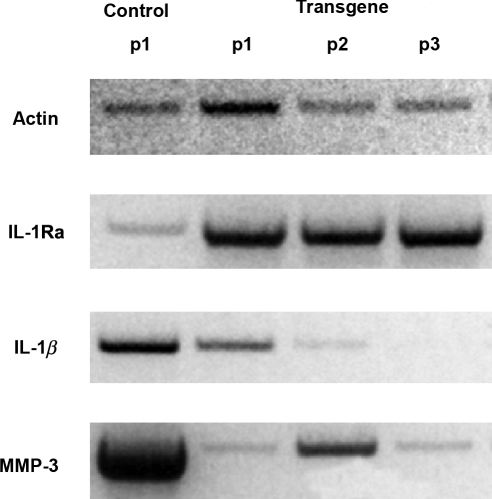
Synovial tissue was recovered from subject 1 at the time of synovectomy. RNA was extracted from synovial fibroblasts grown from the control joint and the joint receiving the high dose of genetically modified cells; expression of certain relevant genes was determined by Northern blotting. Expression of IL-1Ra was greatly elevated in the joint that received this cDNA, and high levels of expression persisted for at least three passages in vitro, as expected from the use of an integrating vector. Expression of IL-1β and MMP-3 was strongly inhibited by the transgene (Fig. 2). No RNA analysis was performed on samples from subject 2.
FIG. 2.
Northern blot analysis of gene expression by synoviocytes recovered from joints of subject 1. Four weeks after gene transfer, synovium was retrieved from the placebo joint receiving unmodified cells (control) and the joint receiving the high dose of genetically modified cells (transgene). Cells were placed into monolayer culture and RNA was extracted at passage 1 (p1). Cultures of cells recovered from the joints receiving the transgene were also examined at passage 2 (p2) and passage 3 (p3). MMP-3, matrix metalloproteinase-3.
Discussion
These data provide the first documented, clinical evidence that local gene therapy can provide symptomatic relief in human RA. Clearly, these findings need to be confirmed in greater numbers of subjects and joints, but, for the reasons stated earlier, we were limited to just two individuals. Nevertheless, their response is noteworthy because, in both patients, the disease was difficult to manage pharmacologically, which is why they were candidates for surgical synovectomy.
Outcome measures used in efficacy trials of RA treatments document global changes in multiple joints (Felson et al., 1995). Such measures are of little use when attempting to assess efficacy in individual joints, and no standardized outcome instruments for detecting such changes exist. For this reason, we established criteria based on pain and, where possible, swelling. Pain was assessed in individual joints according to each subject's pretreatment assignment of 100%. This approach has been validated previously (Gaston-Johansson and Gustafsson, 1990). During the course of the study, each subject kept a “pain diary” to aid this evaluation. In addition, it was possible to quantify swelling of MCP joint 1 by measuring the circumference of the joint. To obviate the large placebo effect encountered in clinical trials of arthritis, one of each subject's own joints served as an internal control.
On the basis of these outcome criteria, the subject who mounted the more striking response to gene transfer had active, highly inflammatory joint disease and her RA flared in untreated joints during the course of this study. It is possible that IL-1 is the major mediator of acute inflammatory events, including flares, in RA, and is thus an important target early in the disease process. The second subject had established disease; although her genetically modified synovial cells produced more IL-1Ra than those of the first subject, she responded more slowly and, by 4 weeks, less completely to gene transfer. The control joint of the first, but not the second, subject also experienced a reduction in pain. This could be a placebo effect or one related to the contralateral response observed in preclinical studies (Ghivizzani et al., 1998).
As a safety precaution, the protocol required that the MCP joints undergo synovectomy 4 weeks after gene transfer. Thus it is impossible to know whether clinical improvement would have persisted beyond this time point. The molecular data suggest that, at least in subject 1, there was carriage of the transferred IL-1Ra cDNA for the entire 4 weeks with evidence of reduced expression of IL-1β and MMP-3, consistent with reduced inflammation and tissue destruction. Reduced expression of IL-1β in response to IL-1Ra presumably reflects the interruption of an autocrine induction loop (Abe et al., 1997). The reduced expression of MMP-3 confirms the importance of IL-1 as an inducer of this enzyme in rheumatoid joints (Jeong et al., 2004). Additional studies, using more precise outcome measures and larger numbers of subjects who do not undergo early synovectomy, are needed to evaluate these matters further.
Although this is the first arthritis gene therapy trial to report a clinical response, we are aware of its shortcomings. In particular, it would have been informative to perform additional biological, histologic, and molecular studies on the retrieved synovia. Also, for technical reasons related to the growth of the individual synovial fibroblast cultures, it was not possible to standardize the number of cells across subjects and joints, and there was no empty vector control. This illustrates one of the complications of ex vivo gene delivery, a strategy that is also expensive and time-consuming. For these reasons, in vivo gene transfer to joints has become increasingly popular, with adeno-associated virus (AAV) as the favored vector. The death of a subject with RA shortly after receiving an intraarticular injection of AAV has forced a reevaluation of this approach, although the most recent data suggest that gene transfer was probably not to blame (Evans et al., 2008).
The findings we report here suggest that there are patients who mount a robust clinical response to IL-1Ra when sufficiently high concentrations of this protein are continuously present within the joint. This observation is relevant to the unresolved question of why recombinant IL-1Ra (anakinra; Kineret), delivered by daily subcutaneous injection, has limited efficacy in RA. It is possible that subcutaneous injections of Kineret fail to achieve sustained, therapeutic concentrations of IL-1Ra within joints. Local gene transfer, in contrast, is uniquely capable of doing this.
A larger study, involving greater numbers of subjects using more refined outcome measures, is warranted.
IL-1 is likely be an important mediator in joints with osteoarthritis, in which case this form of arthritis may also respond to IL-1Ra gene transfer (Evans et al., 2004). Preclinical data support this possibility for human and veterinary medicine (Pelletier et al., 1997; Frisbie et al., 2002).
Acknowledgments
This study was partly supported by NIH grant RO1 AR 43623 and by Orthogen.
Author Disclosure Statement
C.H.E. and P.D.R. are on the scientific advisory board of TissueGene, for which they receive an honorarium but no stock. TissueGene is developing gene therapies for osteoarthritis. They are also on the scientific advisory board of Orthogen. Neither individual receives an honorarium, but C.H.E. owns stock in the company. Orthogen is not developing gene therapies for arthritis. P.W. is CEO of Orthogen. P.D.R. and S.C.G. are cofounders of Molecular Orthopaedics. M.O.I. is developing gene therapies for osteoarthritis. The authors are developing a clinical protocol using AAV to treat osteoarthritis by gene therapy.
References
- Abe M. Tanaka Y. Saito K. Shirakawa F. Koyama Y. Goto S. Eto S. Regulation of interleukin (IL)-1β gene transcription induced by IL-1β in rheumatoid synovial fibroblast-like cells, E11, transformed with simian virus 40 large T antigen. J. Rheumatol. 1997;24:420–429. [PubMed] [Google Scholar]
- Arnett F.C. Edworthy S.M. Bloch D.A. McShane D.J. Fries J.F. Cooper N.S. Healey L.A. Kaplan S.R. Liang M.H. Luthra H.S. Medsger T.A., Jr. Mitchell D.M. Neustadt D.H. Pinals R.S. Schaller J.G. Sharp J.T. Wilder R.L. Hunder G.G. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bandara G. Robbins P.D. Georgescu H.I. Mueller G.M. Glorioso J.C. Evans C.H. Gene transfer to synoviocytes: Prospects for gene treatment of arthritis. DNA Cell Biol. 1992;11:227–231. doi: 10.1089/dna.1992.11.227. [DOI] [PubMed] [Google Scholar]
- Bandara G. Mueller G.M. Galea-Lauri J. Tindal M.H. Georgescu H.I. Suchanek M.K. Hung G.L. Glorioso J.C. Robbins P.D. Evans C.H. Intraarticular expression of biologically active interleukin 1-receptor-antagonist protein by ex vivo gene transfer. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10764–10768. doi: 10.1073/pnas.90.22.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio M.A. Georgescu H.I. McCormack J.E. Robbins P.D. Evans C.H. Approaches to enhancing the retroviral transduction of human synoviocytes. Arthritis Res. 2001;3:259–263. doi: 10.1186/ar311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.H. Robbins P.D. Ghivizzani S.C. Herndon J.H. Kang R. Bahnson A.B. Barranger J.A. Elders E.M. Gay S. Tomaino M.M. Wasko M.C. Watkins S.C. Whiteside T.L. Glorioso J.C. Lotze M.T. Wright T.M. Clinical trial to assess the safety, feasibility, and efficacy of transferring a potentially anti-arthritic cytokine gene to human joints with rheumatoid arthritis. Hum. Gene Ther. 1996;7:1261–1280. doi: 10.1089/hum.1996.7.10-1261. [DOI] [PubMed] [Google Scholar]
- Evans C.H. Gouze J.N. Gouze E. Robbins P.D. Ghivizzani S.C. Osteoarthritis gene therapy. Gene Ther. 2004;11:379–389. doi: 10.1038/sj.gt.3302196. [DOI] [PubMed] [Google Scholar]
- Evans C.H. Robbins P.D. Ghivizzani S.C. Wasko M.C. Tomaino M.M. Kang R. Muzzonigro T.A. Vogt M. Elder E.M. Whiteside T.L. Watkins S.C. Herndon J.H. Gene transfer to human joints: Progress toward a gene therapy of arthritis. Proc. Natl. Acad. Sci. U.S.A. 2005;102:8698–8703. doi: 10.1073/pnas.0502854102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.H. Ghivizzani S.C. Robbins P.D. Arthritis gene therapy: What next? Arthritis Rheum. 2006a;54:1714–1729. doi: 10.1002/art.21886. [DOI] [PubMed] [Google Scholar]
- Evans C.H. Ghivizzani S.C. Robbins P.D. Will arthritis gene therapy become a clinical reality? Nat. Clin. Pract. 2006b;2:344–345. doi: 10.1038/ncprheum0215. [DOI] [PubMed] [Google Scholar]
- Evans C.H. Ghivizzani S.C. Wehling P. Robbins P.D. Gene Therapy with the interleukin-1 receptor antagonist for the treatment of arthritis. Future Rheumatol. 2006c;1:173–178. [Google Scholar]
- Evans C.H. Ghivizzani S.C. Robbins P.D. Arthritis gene therapy's first death. Arthritis Res. Ther. 2008;10:110–119. doi: 10.1186/ar2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson D.T. Anderson J.J. Boers M. Bombardier C. Furst D. Goldsmith C. Katz L.M. Lightfoot R., Jr. Paulus H. Strand V. Tugwell P. Weinblatt M. Williams H.J. Wolfe F. Kieszak S. American College of Rheumatology: Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–735. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- Frisbie D.D. Ghivizzani S.C. Robbins P.D. Evans C.H. McIlwraith C.W. Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene. Gene Ther. 2002;9:12–20. doi: 10.1038/sj.gt.3301608. [DOI] [PubMed] [Google Scholar]
- Gaston-Johansson F. Gustafsson M. Rheumatoid arthritis: Determination of pain characteristics and comparison of RAI and VAS in its measurement. Pain. 1990;41:35–40. doi: 10.1016/0304-3959(90)91106-S. [DOI] [PubMed] [Google Scholar]
- Ghivizzani S.C. Lechman E.R. Kang R. Tio C. Kolls J. Evans C.H. Robbins P.D. Direct adenovirus-mediated gene transfer of interleukin 1 and tumor necrosis factor α soluble receptors to rabbit knees with experimental arthritis has local and distal anti-arthritic effects. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4613–4618. doi: 10.1073/pnas.95.8.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouze E. Gouze J.N. Palmer G.D. Pilapil C. Evans C.H. Ghivizzani S.C. Transgene persistence and cell turnover in the diarthrodial joint: Implications for gene therapy of chronic joint diseases. Mol. Ther. 2007;15:1114–1120. doi: 10.1038/sj.mt.6300151. [DOI] [PubMed] [Google Scholar]
- Jeong J.G. Kim J.M. Cho H. Hahn W. Yu S.S. Kim S. Effects of IL-1β on gene expression in human rheumatoid synovial fibroblasts. Biochem. Biophys. Res. Commun. 2004;324:3–7. doi: 10.1016/j.bbrc.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Kohn D.B. Sadelain M. Glorioso J.C. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat. Rev. Cancer. 2003;3:477–488. doi: 10.1038/nrc1122. [DOI] [PubMed] [Google Scholar]
- Otani K. Nita I. Macaulay W. Georgescu H.I. Robbins P.D. Evans C.H. Suppression of antigen-induced arthritis in rabbits by ex vivo gene therapy. J. Immunol. 1996;156:3558–3562. [PubMed] [Google Scholar]
- Pelletier J.P. Caron J.P. Evans C. Robbins P.D. Georgescu H.I. Jovanovic D. Fernandes J.C. Martel-Pelletier J. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997;40:1012–1019. doi: 10.1002/art.1780400604. [DOI] [PubMed] [Google Scholar]
- Smolen J.S. Aletaha D. Koeller M. Weisman M.H. Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–1864. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]