Abstract
There is an urgent need for innovative therapies against ovarian cancer, one of the leading causes of death from gynecological cancers in the United States. Immunotherapy employing Toll-like receptor (TLR) ligands, such as CpG oligodeoxynucleotides (CpG-ODN), may serve as a potentially promising approach in the control of ovarian tumors. The CpG-ODN requires intracellular delivery into the endosomal compartment, where it can bind to TLR9 in order to activate the immune system. In the current study, we aim to investigate whether the antimicrobial polypeptide from the cathelicidin family, LL-37, could enhance the immunostimulatory effects of CpG-ODN by increasing the uptake of CpG-ODN into the immune cells, thus enhancing the antitumor effects against ovarian cancer. We found that treatment with the combination of CpG-ODN and LL-37 generated significantly better therapeutic antitumor effects and enhanced survival in murine ovarian tumor-bearing mice compared with treatment with CpG-ODN or LL-37 alone. We also observed that treatment with the combination of CpG-ODN and LL-37 enhanced proliferation and activation of natural killer (NK) cells, but not CD4+ or CD8+ T cells, in the peritoneal cavity. Furthermore, in vivo antibody depletion experiments indicated that peritoneal NK cells played a critical role in the observed antitumor effects. Thus, our data suggest that the combination of CpG-ODN with LL-37 peptide may lead to the control of ovarian tumors through the activation of innate immunity.
Introduction
Ovarian cancer is distinguished as the most lethal gynecologic cancer in the United States, with approximately 22,000 new cases and 16,000 deaths occurring annually (Jemal et al., 2007). Despite maximal cytoreductive surgery followed by first-line paclitaxel-based chemotherapy, most women with advanced ovarian cancer will eventually relapse (Markman, 2007). In the recurrent setting, response rates to second-line chemotherapy are substantially diminished (Fung-Kee-Fung et al., 2007), highlighting the crucial need to develop novel therapeutic strategies for the control of ovarian cancer.
Cancer immunotherapy has emerged as an attractive approach to cancer treatment, with high specificity for cancer cells and less toxicity to the host (Rosenberg et al., 2004). For advanced ovarian cancer, direct intraperitoneal seeding is the most common pathway of tumor metastases, although lymphatic and hematogenous spread can also occur (Gerber et al., 2006). Therefore, for immunotherapy to be successful, harnessing the peritoneal immune system for antitumor response is a critical step in the treatment of advanced ovarian cancer. However, systemic investigations of the peritoneal immune system are relative scarce in the literature as compared with other immune systems (Funda et al., 1993; Kubicka et al., 1996; Broche and Tellado, 2001). Thus, it is essential to develop immunotherapeutic strategies targeting the peritoneal immune system for the treatment of ovarian cancer.
One approach for the treatment of ovarian cancer is the employment of “alert” signals such as Toll-like receptor (TLR) ligands that stimulate dendritic cells to mature and differentiate into potent activators of antigen-specific T cells (for a review, see Guermonprez et al., 2002). It is clear that TLRs play a crucial role in enhancing innate and adaptive immune responses (for reviews, see Aderem and Ulevitch, 2000; Akira et al., 2001; Akira and Takeda, 2004; Iwasaki and Medzhitov, 2004). TLR ligands have emerged as a promising new class of vaccine adjuvants, particularly the oligodeoxynucleotides containing one or more unmethylated CpG dinucleotides (CpG-ODN), which target TLR9 (Krieg, 2006). It has been shown that CpG-ODN can stimulate innate immunity and confer protection against a variety of bacterial and viral infections (Deng et al., 2004; Rees et al., 2005). In the presence of antigen, CpG-ODN can trigger the development of predominantly helper T cell type 1 (Th1)-promoting adaptive immune responses (Ioannou et al., 2002). However, although CpG-ODN has the potential to eradicate tumor in the monotherapy setting, it generally needs to be combined with other therapeutic modalities (e.g., surgery, chemotherapy, or radiation) to successfully control large tumors (Weigel et al., 2003; Speiser et al., 2005). This may be because, since TLR9 is located in the endosomal compartment, it is difficult for the CpG-ODN to efficiently penetrate the cell membrane and bind to its receptor (Krieg, 2007). Thus, it is important to develop strategies to improve the uptake of CpG-ODN into the effector cells in order to enhance the antitumor function of CpG-ODN.
One strategy that could potentially improve the uptake of CpG-ODN by immune cells is the coadministration of CpG-ODN with the LL-37 peptide. LL-37 is a member of the cathelicidin family of antimicrobial polypeptides, which are characterized by a highly conserved region (cathelin domain) and a highly variable cathelicidin peptide domain. Cathelicidins serve a critical role in mammalian innate immune defense against invasive bacterial infection (Nizet et al., 2001). LL-37 is a 37-residue helical peptide found throughout the body and has been shown to exhibit a broad spectrum of antimicrobial activity (Durr et al., 2006). LL-37, when complexed with self-DNA or CpG-ODN, has been shown to promote DNA translocation and can significantly increase interferon (IFN)-α production in plasmacytoid dendritic cells (DCs) (Lande et al., 2007).
In the current study, we aimed to investigate whether LL-37 can enhance the immunostimulatory effects of CpG-ODN and thus increase the antitumor effects against ovarian cancer, using a murine ovarian cancer model. We found that treatment with the combination of CpG-ODN and LL-37 generated significant therapeutic antitumor effects and enhanced survival in mouse ovarian surface epithelial cell (MOSEC) tumor-bearing mice. We also observed that treatment with the combination of CpG-ODN and LL-37 enhanced proliferation and activation of natural killer (NK) cells in the peritoneal cavity. Peritoneal NK cells were also shown to play a critical role in the antitumor effects on MOSEC/luc tumors generated by treatment with the combination of CpG-ODN and LL-37 in antibody depletion experiments. The clinical implications of the current study are discussed.
Materials and Methods
Animals and cell lines
Female C57BL/6 mice (H-2Kb and I-Ab), 5 to 6 weeks of age, were purchased from the National Cancer Institute (Frederick, MD) and kept in the oncology animal facility of the Johns Hopkins Hospital (Baltimore, MD). Animals were used in compliance with institutional animal health care regulations, and all animal experimental procedures were approved by the Johns Hopkins Institutional Animal Care and Use Committee. The mouse ovarian MOSEC cell line (clone ID8), generated as described previously (Roby et al., 2000), was originally derived from mouse ovarian surface epithelial cells. The luciferase-expression MOSEC cell line (MOSEC/luc) was generated as previously described (Hung et al., 2007). MOSEC/luc cells were maintained in RPMI 1640, supplemented with 10% (v/v) fetal bovine serum, penicillin–streptomycin (50 U/ml), 2 mM l-glutamine, 1 mM sodium pyruvate, 2 mM nonessential amino acids, and G418 (0.4 mg/ml) at 37°C with 5% CO2.
Reagents
CpG-ODN 1826 (CpG-B no. 1826, TCCATGACGTTCCTGACGTT), with a fully phosphorothioated backbone, was synthesized by Invitrogen (Carlsbad, CA). Fluorescein isothiocyanate (FITC)-conjugated CpG-ODN 1826 was purchased from InvivoGen (San Diego, CA). CpG-ODN 1826 is denoted CpG-ODN hereafter. The antimicrobial peptide, LL-37, was purchased from Sigma-Aldrich (St. Louis, MO).
In vitro cell-labeling assay
For the in vitro cell-labeling assay, single-cell suspensions (1 × 106/ml) were made from peritoneal cells harvested from naive mice and seeded into a 24-well plate. Phosphate-buffered saline (PBS), FITC-conjugated CpG-ODN (10 μg/ml), or FITC-conjugated CpG-ODN combined with LL-37 (50 μg/ml) was added to each of triplicate wells and incubated at 37°C for 1 hr. Cells were then recovered, washed with PBS, and analyzed by flow cytometry for the number of FITC+ cells.
In vivo MOSEC/luc therapeutic model
C57BL/6 mice (five per group) were injected intraperitoneally with 2 × 105 MOSEC/luc cells per mouse to induce advanced ovarian cancer (Chang et al., 2007). Four days after tumor inoculation, mice were injected intraperitoneally with CpG-ODN (30 μg/mouse) and LL-37 (100 μg/mouse) either alone or in combination, starting 4 days after MOSEC/luc inoculation. The injection was repeated three times at 4-day intervals (i.e., on days 4, 8, and 12 after MOSEC/luc inoculation). Mice were imaged with the IVIS-200 system (Xenogen, Alameda, CA) at baseline and every 7 days to monitor for therapeutic effects. The bioluminescence signals were analyzed with Living Image software (Xenogen). To evaluate intraperitoneal tumor seeding, additional mice (five per group) were treated with the same reagents and were killed 50 days after tumor inoculation. Mice were killed when clinical signs of massive tumor burden (e.g., massive ascites, bowel obstruction, and cachexia) occurred.
In vitro cytotoxicity assay
Groups of naive C57BL/6 mice (three per group) were treated with CpG-ODN (30 μg/mouse) and LL-37 (100 μg/mouse) either alone or in combination. Two days after the last treatment, mice were killed by CO2 asphyxiation, their abdomens were wiped with 70% alcohol, 10 ml of cold sterile PBS was injected into the peritoneal cavity, and peritoneal exudate cells (PECs) were harvested by syringe. Contaminating red blood cells were lysed with ACK buffer (Quality Biological, Gaithersburg, MD). The viability of cells was checked by trypan blue exclusion test. MOSEC/luc cells (when 60–80% confluent) were seeded into a round-bottom 96-well microplate at 5 × 103/well in complete medium. After 2 hr, counted PECs were added to each well in triplicate at titrated effector-to-target ratios. The plates were imaged for bioluminescence activity after 16 hr of culture in an incubator (at 37°C with 5% CO2). For parallel experiments, some mice also received in vivo depletion of peritoneal macrophages or NK cells as indicated. For in vivo depletion of peritoneal macrophages, mice were injected intraperitoneally, 1 day before and 3 days after the first time of CpG-ODN and LL-37 treatment, with 0.2 ml of clodronate liposomes. For in vivo NK depletion, mice were injected intraperitoneally, 1 day before and 3 days after first time of combined CpG-ODN with LL-37 treatment, with anti-mouse NK1.1 monoclonal antibody (PK136, 200 μg/mouse). Depletion efficiency was checked by flow cytometry, with >90% depletion of target cells (data not shown).
Antibodies and flow cytometric analysis
Groups of C57BL/6 mice (three per group) were treated with CpG-ODN (30 μg/mouse) and LL-37 (100 μg/mouse) either alone or in combination as described earlier. PECs were harvested 2 days after the last treatment. PECs were then washed once in FACScan buffer and stained with surface markers for innate and adaptive effectors including phycoerythrin (PE)-conjugated anti-CD4 (L3T4), PE-conjugated anti-CD8 (53-6.7), FITC-conjugated anti-GR-1 (RB6-8C5), PE-conjugated anti-CD19 (1D3), PE-conjugated anti-NK1.1 (PK136), and PE–Cy5-conjugated anti-F4/80 (BM8). All antibodies were purchased from eBioscience (San Diego, CA).
For the analysis of interferon-γ and CD69 expression with Cp-ODN and LL-37 administration either alone or in combination, splenocytes from naive mice were harvested and made into single-cell suspension in complete medium (1 × 106/ml). Splenocytes were then seeded into a 24-well microplate and cultured with CpG-ODN 1826 (10 μg/ml) and/or LL-37 (50 μg/ml) for 16 hr. GolgiStop (BD Biosciences, San Jose, CA) was added 6 hr before harvesting the cells from the culture. Cells were then washed once in FACScan buffer and stained with PE-conjugated anti-mouse NK1.1 (PK136) and FITC-conjugated anti-mouse CD69 (H1.2F3). For intracellular cytokine staining, cells were subjected to intracellular cytokine staining, using a Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's instructions. FITC-conjugated anti-IFN-γ antibodies and the immunoglobulin isotype control antibody (Rat IgG1) were purchased from BD Biosciences. Analysis was performed with a FACScan equipped with CellQuest software (BD Biosciences). Interferon-γ and CD69 histograms are presented on gated NK1.1+ cells.
In vivo antibody depletion experiments
C57BL/6 mice (five per group) were inoculated with MOSEC/luc cells (2 × 105 per mouse) at baseline, and were administered PBS or combined treatment (LL-37 plus CpG-ODN). For mice given combined treatment, two separate groups of mice also underwent either peritoneal NK cell or peritoneal macrophage depletion. For in vivo depletion of peritoneal macrophages, mice were injected intraperitoneally, 1 day before and 3 days after the first time of CpG-ODN and LL-37 treatment, with 0.2 ml of clodronate liposomes and thereafter once per week with 0.1 ml of clodronate liposomes until the end of follow-up. For in vivo depletion of peritoneal NK cells, mice were injected intraperitoneally, 1 day before and 3 days after the first time of combined CpG-ODN with LL-37 treatment, with anti-mouse NK1.1 monoclonal antibody (PK136, 200 μg/mouse) and thereafter once per week until the end of follow-up. Depletion was performed according to standard protocols as described earlier (van Rooijen et al., 1997; Chen et al., 2000). Depletion efficiency was tested by flow cytometry, with more than 90% depletion of both peritoneal macrophages and NK cells.
Statistical analysis
Statistical analysis was performed with Prism 3.0 software (GraphPad, San Diego, CA). Cellular proliferation and both CD69 and IFN-γ expression among PECs in the various groups were analyzed by Pearson's χ2 test. The difference in bioluminescence signal between groups was analyzed by Mann–Whitney test. Survival curves were plotted by the Kaplan–Meier method and compared by log-rank test. For all analyses p < 0.05 was considered statistically significant. Data are presented as means ± standard deviation (SD) unless otherwise specified.
Results
Coadministration of LL-37 peptide with CpG-ODN leads to the formation of a complex
Evidence suggests that antimicrobial peptide LL-37 can significantly enhance the intracellular transport of self-DNA into early endocytic compartments containing TLR9 in dendritic cells (Lande et al., 2007). To determine whether LL-37 forms a complex with CpG-ODN, we performed a gel retardation assay. LL-37 was mixed with CpG at a 1:5 (peptide–DNA) mass ratio and incubated for 30 min. Complex formation was assayed by running samples on gels. CpG alone was loaded as a control. As shown in Fig. 1, we observed that the addition of LL-37 to CpG-ODN leads to retardation of the migration of CpG-ODN. This suggests that the addition of the LL-37 peptide to CpG-ODN leads to the formation of a complex.
FIG. 1.
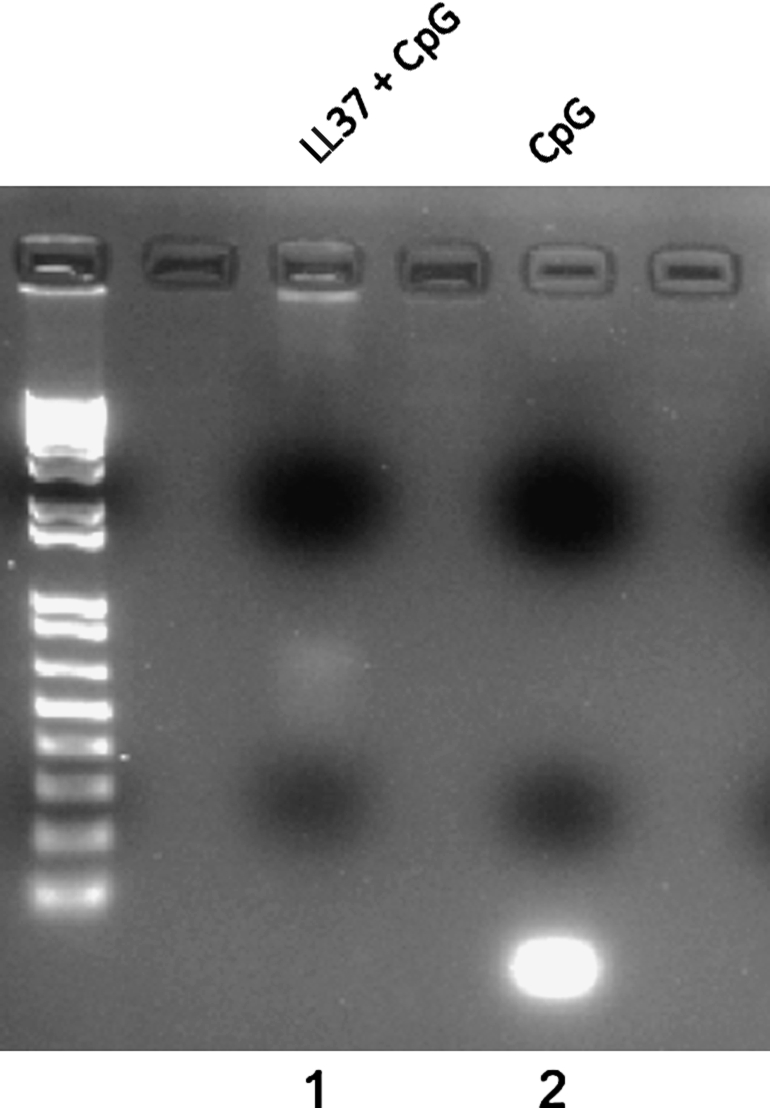
Gel retardation assay demonstrating that LL-37 forms a complex with CpG. LL-37 was mixed with CpG at a 1:5 (peptide–DNA) mass ratio (lane 1) and incubated for 30 min. Complex formation was assayed by running samples on gels. CpG alone was loaded as a control (lane 2). Note: Addition of LL-37 to CpG-ODN leads to retardation of the migration of CpG-ODN.
Coadministration of LL-37 can enhance the delivery of CpG-ODN into peritoneal cells
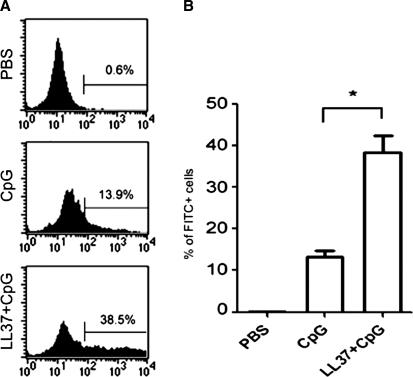
To determine whether LL-37 could enhance the delivery of CpG-ODN (non-self-DNA) into immune cells derived from the peritoneal cavity, peritoneal cells harvested from naive mice were incubated with PBS, FITC-conjugated CpG-ODN, or FITC-conjugated CpG-ODN combined with LL-37 peptide. Peritoneal cells were washed and analyzed by flow cytometry on the gated lymphocyte population. As shown in Fig. 2, we observed that incubation with CpG in combination with the LL-37 peptide demonstrated a significantly higher percentage of FITC+ cells compared with cells incubated with CpG alone (p < 0.05). Taken together, our results indicate that the addition of LL-37 to CpG-ODN leads to the formation of a complex, which may facilitate enhanced delivery of CpG-ODN into peritoneal cells compared with CpG-ODN alone.
FIG. 2.
Flow cytometric analysis to characterize the uptake of FITC-labeled CpG into peritoneal effector cells with or without LL-37. Single-cell suspensions (1 × 106/ml) were made from peritoneal cells harvested from naive mice and seeded into a 24-well plate. PBS, FITC-conjugated CpG-ODN (10 μg/ml), or FITC-conjugated CpG-ODN combined with LL-37 (50 μg/ml) were added to each of triplicate wells and incubated at 37°C for 1 hr. Cells were then recovered, washed with phosphate-buffered saline, and analyzed by flow cytometry. Analysis was performed on gated lymphocytes. (A) Representative flow cytometry for each indicated reagent. (B) Bar graph depicting the percentage of FITC-positive cells (*p < 0.05). Data shown are representative of two separate experiments performed (mean ± SD).
Treatment with the combination of CpG-ODN and LL-37 generates the best therapeutic antitumor effects and enhanced survival in MOSEC/luc tumor-bearing mice
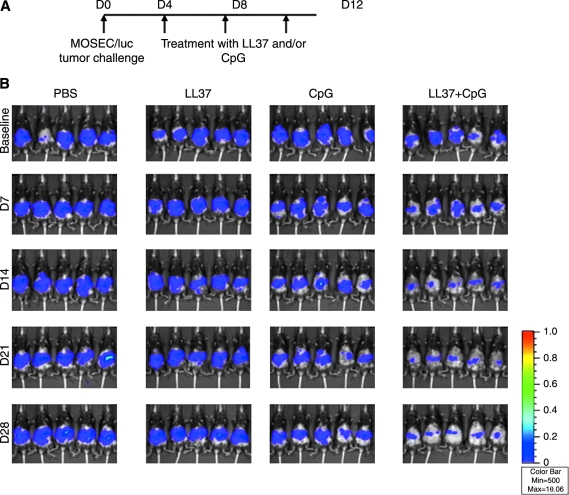
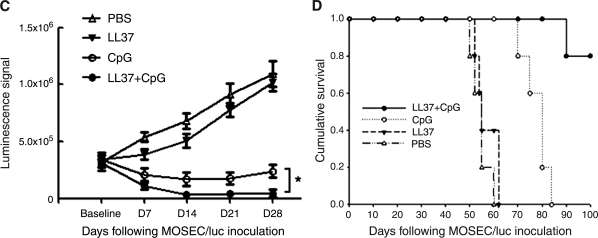
We next investigated whether LL-37 could increase the therapeutic antitumor effects of CpG-ODN. Groups of C57BL/6 mice (five per group) were inoculated with murine ovarian cancer cells that express luciferase (MOSEC/luc). Four, 8, and 12 days after tumor inoculation, mice were intraperitoneally administered PBS, LL-37 alone, CpG-ODN alone, or CpG-ODN in combination with LL-37 as depicted in Fig. 3A. Mice were imaged with the IVIS imaging system series 200. As shown in Fig. 3B, MOSEC/luc tumor-bearing mice treated with the combination of CpG-ODN and LL-37 demonstrated a significant reduction in luminescence intensity over time compared with tumor-bearing mice treated with CpG alone or LL-37 alone (*p < 0.05). A graphical representation of the luminescence intensity in the treated mice is demonstrated in Fig. 3C. Furthermore, tumor-bearing mice treated with the combination of CpG-ODN and LL-37 showed improved survival compared with tumor-bearing mice treated with CpG alone or LL-37 alone (Fig. 3D). In addition, mice treated with PBS or LL-37 alone showed signs of bloody ascites as compared with mice treated with CpG-ODN alone or with CpG plus LL-37 on day 50 after tumor inoculation (see Supplementary Fig. 1A at www.liebertonline.com/hum). Furthermore, the size of the tumor in the peritoneal cavity was significantly smaller in mice treated with CpG plus LL-37 or with CpG-ODN alone compared with the bulky tumor observed in the peritoneal cavity of mice treated with PBS or LL-37 alone (see Supplementary Fig. 1B). Thus, our data suggest that treatment with the combination of CpG-ODN and LL-37 produces the best therapeutic antitumor effects and long-term survival in MOSEC/luc tumor-bearing mice.
FIG. 3.
In vivo tumor treatment experiments. Groups of C57BL/6 mice (five per group) were inoculated with murine ovarian cancer cells (2 × 105 per mouse) that express luciferase (MOSEC/luc). Four, 8, and 12 days after tumor inoculation, each mouse was intraperitoneally administered PBS, LL-37 alone (100 μg/dose per mouse), CpG-ODN alone (30 μg/dose per mouse), or CpG-ODN plus LL-37. Mice were imaged with the IVIS imaging system series 200 to monitor tumor growth. Bioluminescence signals were acquired for 1 min. (A) Schematic diagram of the treatment regimen. (B) Luminescence images of representative mice challenged with MOSEC/luc cells and treated according to the various treatment regimens. (C) Line graph depicting the luminescence intensity in MOSEC/luc tumor-bearing mice treated according to the various treatment regimens (means ± SD) (*p < 0.05). (D) Kaplan–Meier survival analysis of MOSEC/luc tumor-bearing mice treated according to the various treatment regimens. For gross morphological analysis of the tumor-bearing mice treated with the various regimens, see Supplementary Fig.1 at www.liebertonline.com/hum.
Treatment with the combination of CpG-ODN and LL-37 can enhance proliferation of NK cells and macrophages in the peritoneal cavity
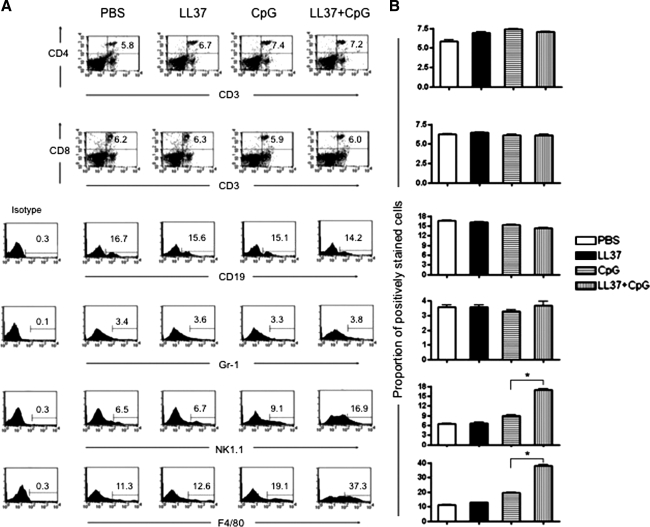
We then characterized the immune cell subsets that were present in the peritoneal cavity after stimulation with CpG-ODN and LL-37 either alone or in combination. Groups of naive C57BL/6 mice (five per group) were treated either with PBS, LL-37 alone, CpG-ODN alone, or CpG-ODN plus LL-37 as described in Fig. 3. Two days after the last treatment, cells from the peritoneal cavity were harvested and stained with surface markers for CD4+ cells, CD8+ cells, B cells, neutrophils, NK cells, and macrophages. The number of the various peritoneal cell types was determined by flow cytometric analysis. As shown in Fig. 4, there was no significant difference in the numbers of CD4+ and CD8+ T cells, B lymphocytes, or neutrophils after stimulation with the various reagents. However, there was a significant increase in the number of NK (NK1.1+) cells and macrophages (F4/80+) after stimulation with CpG-ODN plus LL-37 compared with stimulation with CpG or LL-37 alone (p < 0.05). Thus, our data indicate that treatment with the combination of CpG-ODN and LL-37 can enhance proliferation of NK cells and macrophages in the peritoneal cavity.
FIG. 4.
Flow cytometric analysis to determine numbers of the various cell types in the peritoneal cavity after LL-37 plus CpG-ODN treatment. Groups of naive C57BL/6 mice (five per group) were treated either with PBS, LL-37 alone, CpG-ODN alone, or CpG-ODN plus LL-37 as described in Fig. 3. Two days after the last treatment, cells from the peritoneal cavity were harvested, counted, washed once in FACScan buffer, and stained with surface markers for innate and adaptive effectors including PE-conjugated anti-CD4 (L3T4), PE-conjugated anti-CD8 (53-6.7), FITC-conjugated anti-GR-1 (RB6-8C5), PE-conjugated anti-CD19 (1D3), PE-conjugated anti-NK1.1 (PK136), and PE–Cy5-conjugated anti-F4/80 (BM8). Numbers of the various peritoneal cell types were determined by flow cytometric analysis. (A) Representative flow cytometric analysis demonstrating numbers of the various peritoneal cell types after treatment with LL-37 plus CpG-ODN. (B) Bar graph depicting numbers of the various peritoneal cell types after treatment with LL-37 plus CpG-ODN. *p < 0.05 (means ± SD). Data shown are representative of two experiments performed.
Peritoneal NK cells derived from mice treated with the combination of CpG-ODN with LL-37 generated the best cytotoxic activity against MOSEC/luc cells
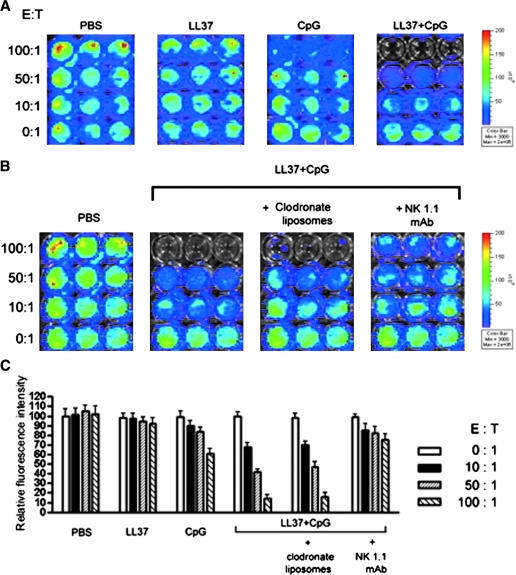
To determine whether peritoneal cells derived from mice treated with the combination of CpG-ODN with LL-37 are capable of killing MOSEC/luc tumor cells, we performed an in vitro cytotoxicity assay using luminescence imaging. Groups of mice (three per group) were treated with PBS, LL-37 alone, CpG-ODN alone, or CpG-ODN plus LL-37. Two days after the last treatment, cells from the peritoneal cavity were harvested and added to MOSEC/luc cells at various effector-to-target ratios and the plates were imaged for bioluminescence activity. As shown in Fig. 5A, the lowest luciferase activity was observed in the wells incubated with cells derived from mice treated with CpG-ODN plus LL-37 as compared with the wells incubated with cells from mice treated with LL-37 alone or CpG-ODN alone.
FIG. 5.
In vitro cytotoxicity assay using luminescence imaging. (A) Representative luminescence images depicting the cytotoxicity of MOSEC/luc tumor cells. Groups of mice (three per group) were treated with PBS, LL-37 alone, CpG-ODN alone, or CpG-ODN plus LL-37 on days 0, 4, and 8. Two days after the last treatment (day 10), cells from the peritoneal cavity were harvested, washed, and counted. MOSEC/luc cells (at 60–80% confluence) were seeded into a round-bottom 96-well microplate at 5 × 103 per well in complete medium. After 2 hr, counted cells from the peritoneal cavity were then added to each well in triplicate at titrated effector-to-target ratios (0:1, 10:1, 50:1, and 100:1). Sixteen hours after incubation at 37°C with 5% CO2, the plates were imaged for bioluminescence activity. (B) Representative luminescence images depicting the cytotoxicity of MOSEC/luc tumor cells after depletion of macrophages or NK cells. Groups of mice (three per group) were administered CpG-ODN plus LL-37 on days 0, 4, and 8. Mice were depleted of peritoneal macrophages, using clodronate liposomes, or of NK cells, using NK1.1 antibody, on days 0 and 4. Undepleted mice were used as negative controls. Two days after the last treatment (day 10), cells from the peritoneal cavity were added to MOSEC/luc cells followed by luminescence imaging as described previously. (C) Bar graph depicting the relative fluorescence intensities of the MOSEC/luc tumor cells in the various treatment groups. Data shown are representative of two experiments performed.
Because treatment with the combination of CpG-ODN with LL-37 led to increased numbers of NK cells and macrophages in the peritoneal cavity, we sought to determine which cell subset (or subsets) play a crucial role in the killing of MOSEC/luc tumor cells. Mice were treated with CpG-ODN plus LL-37 and depleted of either peritoneal macrophages, using clodronate liposomes, or NK cells, using NK1.1 antibody. Undepleted mice were used as negative controls. Two days after the last treatment, cells from the peritoneal cavity were harvested and added to MOSEC/luc cells followed by luminescence imaging as described previously. As shown in Fig. 5B, the wells incubated with cells derived from mice depleted of NK cells demonstrated the highest luciferase activity compared with wells incubated with cells derived from mice depleted of macrophages, or from undepleted mice. A graphical representation of the relative fluorescence intensity is depicted in Fig. 5C. Taken together, our data indicate that peritoneal NK cells derived from mice treated with CpG-ODN in combination with LL-37 play a critical role in the killing of MOSEC/luc tumor cells.
LL-37 can enhance the expression of CD69 and IFN-γ on peritoneal NK cells stimulated with CpG-ODN
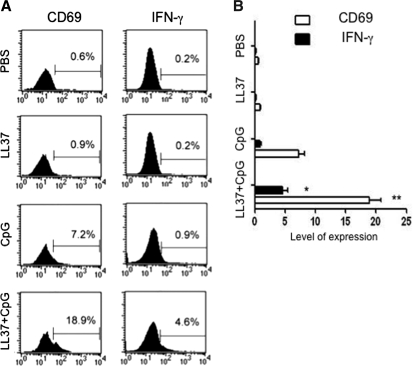
Cytokines such as IFN-γ have been shown to be important for antitumor or antiviral effects generated by NK cells (Long et al., 2008; Shey and Ballas, 2008). Furthermore, CpG-ODN has been shown to be able to stimulate CD69 and IFN-γ expression in NK cells (Cowdery et al., 1996; Hartmann et al., 2000; Marshall et al., 2006). Therefore, we sought to determine whether treatment with the combination of CpG-ODN with LL-37 could enhance the expression of CD69 and IFN-γ in NK cells derived from the peritoneal cavity. Cells from the peritoneal cavity harvested from naive mice were incubated with CpG-ODN and/or LL-37. Gated NK cells were characterized for the expression of CD69 and IFN-γ, using flow cytometric analysis. As shown in Fig. 6, the expression of both CD69 and IFN-γ was significantly higher in NK cells treated with CpG-ODN combined with LL-37 compared with cells treated with CpG-ODN alone (*p < 0.05; **p < 0.01). Thus, our data indicate that treatment with LL-37 can further enhance the expression of CD69 and IFN-γ in peritoneal NK cells stimulated with CpG-ODN.
FIG. 6.
Flow cytometric analysis demonstrating the expression of CD69 and IFN-γ on peritoneal NK cells stimulated with LL-37 plus CpG-ODN. Cells from the peritoneal cavity were harvested from naive mice, and seeded into round-bottom 24-well plates at a density of 1 × 106/well and stimulated with either PBS, LL-37 alone (50 μg/ml), CpG-ODN alone (10 μg/ml), or CpG-ODN plus LL-37. Stimulated cells were then retrieved after 16 hr of incubation and stained for CD69 and IFN-γ. NK cells were gated and analyzed by flow cytometric analysis. (A) Representative flow cytometric data demonstrating the expression of CD69 and IFN-γ on NK cells stimulated with the various reagents. (B) Bar graph depicting the level of expression of CD69 and IFN-γ on NK cells stimulated with the various reagents. *p < 0.05; **p <0.01. Data shown are representative of two experiments performed.
Peritoneal NK cells play a critical role in the antitumor effects generated by treatment of MOSEC/luc tumors in vivo with the combination of CpG-ODN with LL-37
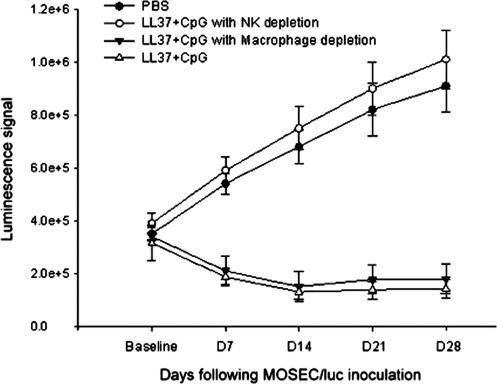
To confirm the role of peritoneal NK cells in the treatment of MOSEC/luc tumors with the combination of CpG-ODN with LL-37, we performed an in vivo antibody depletion experiment. Groups of mice (five per group) were inoculated with MOSEC/luc cells and treated with CpG-ODN plus LL-37. Mice treated with PBS were used as controls. Mice were depleted of either macrophages or NK cells as described in Materials and Methods. Undepleted mice were used as a control. Tumor growth was monitored with the IVIS imaging system series 200 every 7 days. As shown in Fig. 7, a significantly higher luminescence signal was observed in treated mice depleted of NK cells compared with treated mice depleted of macrophages or without depletion (p < 0.05). Thus, our data indicate that peritoneal NK cells play a critical role in the antitumor effects on MOSEC/luc tumors in vivo generated by treatment with the combination of CpG-ODN with LL-37.
FIG. 7.
In vivo antibody depletion experiment. Groups of C57BL/6 mice (five per group) were inoculated with MOSEC/luc (2 × 105 per mouse) and treated with PBS or CpG-ODN plus LL-37 on days 4, 8, and 12 as depicted in Fig. 3. Mice were either depleted of peritoneal macrophages, using clodronate liposomes, or of NK cells, using anti-mouse NK1.1 monoclonal antibody (PK136), 1 day before and 3 days after the first treatment and thereafter once per week until the end of follow-up as described in Materials and Methods. Undepleted mice were used as a negative control. Mice were imaged with the IVIS imaging system series 200 every 7 days. Bioluminescence signals were acquired for 1 min. Shown is a line graph depicting the quantification of luminescence activity in the tumors of tumor-challenged mice treated with PBS or with CpG-ODN plus LL-37 and depleted of peritoneal macrophages or NK cells (means ± SD).
Discussion
Our study demonstrated that peritoneal NK cells play a critical role in the antitumor effects against MOSEC/luc tumors generated by the combinational treatment of CpG-ODN with LL-37. We also showed that the expression of CD69 and IFN-γ on peritoneal NK cells stimulated with CpG-ODN can be enhanced by treatment with the LL-37 peptide, thus leading to further activation of NK cells in the peritoneal cavity. Previous studies have shown that CpG-ODN is highly potent in activating NK cells (for a review see Ballas, 2007). Because NK cells do not express TLR9, it is assumed that CpG-ODN activate dendritic cells (DCs), which in turn activate NK cells. In fact, CpG-ODN have been employed in clinical trials on melanoma patients and have been shown to activate plasmacytoid and myeloid dendritic cells, which in turn activate NK cells (Molenkamp et al., 2007). The importance of the role played by NK cells in antitumor immune responses highlights opportunities for controlling tumors by manipulating the NK cell arm of innate immunity (Orange and Ballas, 2006; Terme et al., 2008).
Our study showed that treatment with the combination of CpG-ODN and LL-37 could enhance proliferation of NK cells and macrophages in the peritoneal cavity (Fig. 4). Although some studies suggest that CpG-ODN may indirectly augment NK cell activity by inducing the secretion of interleukin (IL)-12 in macrophages (Ballas et al., 1996; Cowdery et al., 1996), peritoneal macrophages were not found to play an essential role in the antitumor effects generated by CpG-ODN in combination with LL-37 as demonstrated by depletion experiments. Thus, the role of peritoneal macrophages in the observed antitumor effects caused by CpG-ODN remains to be further characterized.
The encouraging results from the current study serve as an important foundation for future clinical translation, particularly in patients with ovarian cancer, which tends to lead to ascites and cachexia in patients. It has been shown that cachexia syndrome may be caused by cytokines either produced by the tumor or released by the immune cells in response to the tumor and tumor products (for a review see Seruga et al., 2008). Thus, the reduction of tumor load by immunotherapeutic strategies may potentially lead to the improvement of cachexia syndrome, resulting in better survival and quality of life for patients
For clinical translation, it is important to address concerns regarding the potential toxicity associated with administration of the specific drugs or reagents into the body. We have previously developed a Hsp70-secreting ovarian tumor cell-based vaccine for the control of lethal ovarian cancer (Chang et al., 2007). In this respect, the current study is highly translatable because the LL-37 peptide is a natural compound and is expressed in humans (Durr et al., 2006). In addition, CpG-ODN is commercially available and has been used in several clinical trials with no harmful side effects (Link et al., 2006; McHutchison et al., 2007). Thus, the current strategy employing CpG-ODN and LL-37 reagents has significant potential for future clinical translation.
In summary, our study has demonstrated that the LL-37 peptide can potentiate delivery of CpG-ODN into peritoneal immune effectors, leading to potent tumor cytotoxic effects in treated mice. In fact, CpG-ODN is currently being employed in several clinical trials in several cancer models with promising results. Moreover, TLR3, TLR7, TLR8, and TLR9 are expressed in the same intracellular compartments, mainly in the endosomes and the endoplasmic reticulum, and therefore our strategy may be applied to enhance ligand function to these Toll-like receptors. Our results potentially serve as an important foundation for future clinical translation. Furthermore, similar strategies may potentially be applied to other cancer systems.
Supplementary Material
Acknowledgments
The authors thank Dr. T.-C. Wu for helpful discussions and critical review of the manuscript. This work was supported by ovarian cancer grants from the Alliance for Cancer Gene Therapy (ACGT), the NCDGG (1U19 CA113341-01), and the American Cancer Society (ACS).
References
- Aderem A. Ulevitch R.J. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Akira S. Takeda K. Toll-like receptor signalling. Nat. Rev. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Akira S. Takeda K. Kaisho T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Ballas Z.K. Modulation of NK cell activity by CpG oligodeoxynucleotides. Immunologic Res. 2007;39:15–21. doi: 10.1007/s12026-007-0066-3. [DOI] [PubMed] [Google Scholar]
- Ballas Z.K. Rasmussen W.L. Krieg A.M. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 1996;157:1840–1845. [PubMed] [Google Scholar]
- Broche F. Tellado J.M. Defense mechanisms of the peritoneal cavity. Curr. Opin. Crit. Care. 2001;7:105–116. doi: 10.1097/00075198-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Chang C.L. Tsai Y.C. He L. Wu T.C. Hung C.F. Cancer immunotherapy using irradiated tumor cells secreting heat shock protein 70. Cancer Res. 2007;67:10047–10057. doi: 10.1158/0008-5472.CAN-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.H. Wang T.L. Hung C.F. Yang Y. Young R.A. Pardoll D.M. Wu T.C. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–1042. [PubMed] [Google Scholar]
- Cowdery J.S. Chace J.H. Yi A.K. Krieg A.M. Bacterial DNA induces NK cells to produce IFN-γ in vivo and increases the toxicity of lipopolysaccharides. J. Immunol. 1996;156:4570–4575. [PubMed] [Google Scholar]
- Deng J.C. Zeng X. Newstead M. Moore T.A. Tsai W.C. Thannickal V.J. Standiford T.J. STAT4 is a critical mediator of early innate immune responses against pulmonary Klebsiella infection. J. Immunol. 2004;173:4075–4083. doi: 10.4049/jimmunol.173.6.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durr U.H. Sudheendra U.S. Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta. 2006;1758:1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Funda D. Holub M. Sykora V. Development of the cellular response in the mouse omentum after intraperitoneal immunization. APMIS. 1993;101:939–945. doi: 10.1111/j.1699-0463.1993.tb00205.x. [DOI] [PubMed] [Google Scholar]
- Fung-Kee-Fung M. Oliver T. Elit L. Oza A. Hirte H.W. Bryson P. Optimal chemotherapy treatment for women with recurrent ovarian cancer. Curr. Oncol. 2007;14:195–208. doi: 10.3747/co.2007.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber S.A. Rybalko V.Y. Bigelow C.E. Lugade A.A. Foster T.H. Frelinger J.G. Lord E.M. Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth. Am. J. Pathol. 2006;169:1739–1752. doi: 10.2353/ajpath.2006.051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guermonprez P. Valladeau J. Zitvogel L. Thery C. Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- Hartmann G. Weeratna R.D. Ballas Z.K. Payette P. Blackwell S. Suparto I. Rasmussen W.L. Waldschmidt M. Sajuthi D. Purcell R.H. Davis H.L. Krieg A.M. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J. Immunol. 2000;164:1617–1624. doi: 10.4049/jimmunol.164.3.1617. [DOI] [PubMed] [Google Scholar]
- Hung C.F. Calizo R. Tsai Y.C. He L. Wu T.C. A DNA vaccine encoding a single-chain trimer of HLA-A2 linked to human mesothelin peptide generates anti-tumor effects against human mesothelin-expressing tumors. Vaccine. 2007;25:127–135. doi: 10.1016/j.vaccine.2006.06.087. [DOI] [PubMed] [Google Scholar]
- Ioannou X.P. Griebel P. Hecker R. Babiuk L.A. van Drunen Littel-van den Hurk S. The immunogenicity and protective efficacy of bovine herpesvirus 1 glycoprotein D plus Emulsigen are increased by formulation with CpG oligodeoxynucleotides. J. Virol. 2002;76:9002–9010. doi: 10.1128/JVI.76.18.9002-9010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A. Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Jemal A. Siegel R. Ward E. Murray T. Xu J. Thun M.J. Cancer statistics, 2007. CA Cancer J. Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Krieg A.M. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- Krieg A.M. Development of TLR9 agonists for cancer therapy. J. Clin. Invest. 2007;117:1184–1194. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicka U. Olszewski W.L. Tarnowski W. Bielecki K. Ziolkowska A. Wierzbicki Z. Normal human immune peritoneal cells: Subpopulations and functional characteristics. Scand. J. Immunol. 1996;44:157–163. doi: 10.1046/j.1365-3083.1996.d01-297.x. [DOI] [PubMed] [Google Scholar]
- Lande R. Gregorio J. Facchinetti V. Chatterjee B. Wang Y.H. Homey B. Cao W. Wang Y.H. Su B. Nestle F.O. Zal T. Mellman I. Schroder J.M. Liu Y.J. Gilliet M. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- Link B.K. Ballas Z.K. Weisdorf D. Wooldridge J.E. Bossler A.D. Shannon M. Rasmussen W.L. Krieg A.M. Weiner G.J. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J. Immunother. 2006;29:558–568. doi: 10.1097/01.cji.0000211304.60126.8f. [DOI] [PubMed] [Google Scholar]
- Long B.R. Michaelsson J. Loo C.P. Ballan W.M. Vu B.A. Hecht F.M. Lanier L.L. Chapman J.M. Nixon D.F. Elevated frequency of γ interferon-producing NK cells in healthy adults vaccinated against influenza virus. Clin. Vaccine Immunol. 2008;15:120–130. doi: 10.1128/CVI.00357-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markman M. Intraperitoneal chemotherapy as primary treatment of advanced ovarian cancer: Efficacy, toxicity, and future directions. Rev. Recent Clin. Trials. 2007;2:169–173. doi: 10.2174/157488707781662698. [DOI] [PubMed] [Google Scholar]
- Marshall J.D. Heeke D.S. Abbate C. Yee P. van Nest G. Induction of interferon-γ from natural killer cells by immunostimulatory CpG DNA is mediated through plasmacytoid-dendritic-cell-produced interferon-α and tumour necrosis factor-α. Immunology. 2006;117:38–46. doi: 10.1111/j.1365-2567.2005.02261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHutchison J.G. Bacon B.R. Gordon S.C. Lawitz E. Shiffman M. Afdhal N.H. Jacobson I.M. Muir A. Al-Adhami M. Morris M.L. Lekstrom-Himes J.A. Efler S.M. Davis H.L. Phase 1B, randomized, double-blind, dose-escalation trial of CPG 10101 in patients with chronic hepatitis C virus. Hepatology. 2007;46:1341–1349. doi: 10.1002/hep.21773. [DOI] [PubMed] [Google Scholar]
- Molenkamp B.G. van Leeuwen P.A. Meijer S. Sluijter B.J. Wijnands P.G. Baars A. van den Eertwegh A.J. Scheper R.J. de Gruijl T.D. Intradermal CpG-B activates both plasmacytoid and myeloid dendritic cells in the sentinel lymph node of melanoma patients. Clin. Cancer Res. 2007;13:2961–2969. doi: 10.1158/1078-0432.CCR-07-0050. [DOI] [PubMed] [Google Scholar]
- Nizet V. Ohtake T. Lauth X. Trowbridge J. Rudisill J. Dorschner R.A. Pestonjamasp V. Piraino J. Huttner K. Gallo R.L. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Orange J.S. Ballas Z.K. Natural killer cells in human health and disease. Clin. Immunol. 2006;118:1–10. doi: 10.1016/j.clim.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Rees D.G. Gates A.J. Green M. Eastaugh L. Lukaszewski R.A. Griffin K.F. Krieg A.M. Titball R.W. CpG-DNA protects against a lethal orthopoxvirus infection in a murine model. Antiviral Res. 2005;65:87–95. doi: 10.1016/j.antiviral.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Roby K.F. Taylor C.C. Sweetwood J.P. Cheng Y. Pace J.L. Tawfik O. Persons D.L. Smith P.G. Terranova P.F. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A. Yang J.C. Restifo N.P. Cancer immunotherapy: Moving beyond current vaccines. Nat. Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seruga B. Zhang H. Bernstein L.J. Tannock I.F. Cytokines and their relationship to the symptoms and outcome of cancer. Nat. Rev. Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- Shey M.R. Ballas Z.K. Assessment of natural killer (NK) and NKT cells in murine spleens and livers. Methods Mol. Biol. 2008;447:259–276. doi: 10.1007/978-1-59745-242-7_18. [DOI] [PubMed] [Google Scholar]
- Speiser D.E. Lienard D. Rufer N. Rubio-Godoy V. Rimoldi D. Lejeune F. Krieg A.M. Cerottini J.C. Romero P. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J. Clin. Invest. 2005;115:739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terme M. Ullrich E. Delahaye N.F. Chaput N. Zitvogel L. Natural killer cell-directed therapies: Moving from unexpected results to successful strategies. Nat. Immunol. 2008;9:486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- van Rooijen N. Bakker J. Sanders A. Transient suppression of macrophage functions by liposome-encapsulated drugs. Trends Biotechnol. 1997;15:178–185. doi: 10.1016/s0167-7799(97)01019-6. [DOI] [PubMed] [Google Scholar]
- Weigel B.J. Rodeberg D.A. Krieg A.M. Blazar B.R. CpG oligodeoxynucleotides potentiate the antitumor effects of chemotherapy or tumor resection in an orthotopic murine model of rhabdomyosarcoma. Clin. Cancer Res. 2003;9:3105–3114. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.