Abstract
The intradermal administration of DNA vaccines by tattooing is a promising delivery technique for genetic immunization, with proven high immunogenicity in mice and in nonhuman primates. However, the parameters that result in optimal expression of DNA vaccines that are applied by this strategy to human skin are currently unknown. To address this issue we set up an ex vivo human skin model in which DNA vaccine-induced expression of reporter proteins could be monitored longitudinally. Using this model we demonstrate the following: First, the vast majority of cells that express DNA vaccine-encoded antigen in human skin are formed by epidermal keratinocytes, with only a small fraction (about 1%) of antigen-positive epidermal Langerhans cells. Second, using full randomization of DNA tattoo variables we show that an increase in DNA concentration, needle depth, and tattoo time all significantly increase antigen expression (p < 0.001), with DNA concentration forming the most critical variable influencing the level of antigen expression. Finally, in spite of the marked immunogenicity of this vaccination method in animal models, transfection efficiency of the technique is shown to be extremely low, estimated at approximately 2 to 2000 out of 1 × 1010 copies of plasmid applied. This finding, coupled with the observed dependency of antigen expression on DNA concentration, suggests that the development of strategies that can enhance in vivo transfection efficacy would be highly valuable. Collectively, this study shows that an ex vivo human skin model can be used to determine the factors that control vaccine-induced antigen expression and define the optimal parameters for the evaluation of DNA tattoo or other dermal delivery techniques in phase 1 clinical trials.
Introduction
Skin has become increasingly used as a successful delivery route for DNA vaccines (Mitragotri, 2005). The excellent immunogenicity of dermal DNA vaccination is probably related to the high prevalence of antigen-presenting cells (APCs) in the skin, in the form of Langerhans cells (LCs) in the epidermis and dendritic cells in the dermis (Kanitakis, 2002; Mathers and Larregina, 2006). Several techniques have been developed for the intradermal administration and cellular uptake of naked DNA, such as gene gun and particle injection systems (Klinman et al., 1998; Mitragotri, 2005; Steitz et al., 2006); jet injectors (Mitragotri, 2005; Bahloul et al., 2006); electroporators (Maruyama et al., 2001; Heller et al., 2007; Hooper et al., 2007; Hirao et al., 2008); and a technique, developed at our institute, that has been called “DNA tattooing” (Bins et al., 2005; Pokorna et al., 2008). DNA tattooing delivers naked plasmid DNA into the skin through thousands of punctures made with a multiple-needle tattoo device. We have demonstrated that DNA tattooing results in local transfection and expression of the encoded antigen by cells in murine skin. More importantly, the efficacy of DNA tattooing in inducing strong vaccine-specific immune responses has been established in murine models (Bins et al., 2005) and the superiority of DNA tattooing over intramuscular DNA vaccination has been demonstrated in nonhuman primates (Verstrepen et al., 2008).
A major difficulty when translating novel dermal delivery techniques, such as DNA tattooing, toward clinical application is that they have been developed and optimized in nonhuman skin. Because mouse and macaque skin have a higher density of hair follicles than human skin and a different thickness when compared with human skin (Godin and Touitou, 2007), direct translation of vaccination protocols to clinical application is difficult. To prepare for the use of DNA tattoo vaccination in a phase 1 clinical trial, we have therefore developed an ex vivo human skin model that allows the measurement of vaccine-induced gene expression in real time. Having established this ex vivo human skin model, we have used the model to define optimized conditions for DNA tattoo vaccination of human skin.
Materials and Methods
Plasmids
DNA vaccines were generated by the insertion of reporter genes into the minimal pVAX1 plasmid backbone (Invitrogen, Carlsbad, CA). pVAX:Luc was generated by insertion of the gene encoding firefly luciferase into the EcoRI/NotI site of pVAX1. pVAX:GFP was generated by inserting green fluorescent protein (GFP)-encoding DNA into the BamHI/NotI site of pVAX1. pVAX:LacZ, encoding β-galactosidase, was purchased from Invitrogen. Histone-2B-GFP (H2B-GFP) fusion protein-encoding DNA cloned into the pN1 vector (Clontech, Palo Alto, CA) was described previously (Kanda et al., 1998). pVAX:Luc was produced in one large batch for all experiments according to a uniform process described previously (Quaak et al., 2008). All other plasmids were purified with an EndoFree plasmid kit (Qiagen, Hilden, Germany). All plasmids were dissolved in water for injections (Braun, Melsungen, Germany). The purity and concentration of plasmid DNA were assessed by agarose gel electrophoresis and ultraviolet spectroscopy, respectively.
Tattooing and injection of human skin
Healthy human abdominal skin from female patients (41–63 years of age) was obtained from the plastic surgery department, according to institutional guidelines. Subcutaneous fat was directly removed by blunt dissection. Skin was transported on ice and used within 2 hr of surgical removal.
Before DNA tattooing, skin was cleaned with sterile phosphate-buffered saline (PBS) and pinched onto a polypropylene board with drawing pins. Next, a black marker was used to apply a chessboard pattern on the skin to define the various areas for tattooing. DNA tattooing was performed by application of 10 μl of DNA solution onto the skin in a custom-fabricated mold to keep the area of tattooing consistent (diameter, 8 mm; surface, 50 mm2). The droplet of DNA was subsequently administered onto skin with an Aella (now called Permanent Make Up [PMU]) or Cheyenne tattoo machine (both machines and needles from MT.DERM, Berlin, Germany). For all tattoos, nine-needle cartridges and an oscillating frequency of 100 Hz were used. During the experiments, the tattoo depth, tattoo duration, DNA concentration, and the two tattoo machines were varied on the basis of a randomization protocol (see Table 1). Needle amplitude was adjustable with an accuracy of 0.1 mm, using a custom-built device that contained a stroboscope and microscope (MT.DERM). For intradermal injections, 50 μl of DNA solution at a concentration of 1 mg/ml was injected intraepidermally with a 29-gauge, 12 mm needle in a side-by-side comparison with a tattoo of 10 μl of 1 mg/ml DNA solution at a 1.5 mm needle depth for 20 seconds with the Aella machine (n = 3, performed two times in separate experiments).
Table 1.
Fixed Effects (Tattoo Variations) Tested in Linear Effect Model Skin Tattooing
| Effect | Parameters |
|---|---|
| DNA concentration (mg/ml) | 0.2, 1, 5 |
| Tattoo duration (sec) | 5, 10, 20 |
| Tattoo depth (mm) | 0.5, 1.0, 1.5 |
| Tattoo machine | Aella, Cheyenne |
After tattooing or injection, skin samples were kept at 5% CO2, 37°C in complete keratinocyte serum-free medium (SFM) containing 1% penicillin–streptomycin and amphotericin B (0.25 μg/ml) (all from Invitrogen). During this incubation, skin was cultured at the air–medium interface with the epidermis exposed to the air to mimic the natural situation.
For histology and flow cytometry experiments tattooed areas of interest were removed from the intact skin with a 6-mm biopsy punch and transferred into 48-well plates.
Histology
Four-micrometer cryostat cross-sections of skin tattooed with β-galactosidase encoding plasmid (pVAX:LacZ) were prepared. Cryostat sections were fixed in acetone for 10 min and washed for 10 min in PBS. Sections were stained for 10 min with X-Gal staining solution (Roche Applied Science, Indianapolis, IN) to visualize β-galactosidase expression. Subsequently, sections were stained with hematoxylin and eosin according to standard procedures.
Flow cytometric analysis of DNA vaccine-induced antigen expression
Directly after tattooing with pVAX:GFP, skin samples were incubated for 1 hr in 10 mg/ml dispase II (Sigma-Aldrich, St. Louis, MO) in keratinocyte medium at 37°C, at which point the epidermis was mechanically peeled from skin samples. The obtained epidermal sheet and dermis were cultured overnight in complete keratinocyte medium to allow accumulation of vaccination-induced GFP expression. Eighteen hours later, epidermal sheets were digested at 37°C in complete keratinocyte medium containing 0.05% trypsin and DNase I (300 U/ml) (Roche Applied Science). After 15 min, the epidermis was disrupted with a glass pipette and 10% fetal calf serum (FCS) was added to the medium. Dermal samples were digested with collagenase type IV (50 mg/ml; Sigma-Aldrich) at 37°C for 3 hr, after which the cells were filtered through 70 μm (pore size) nylon gauze to remove debris. Filtered epidermal cell suspensions and dermal cell suspensions were washed with PBA (1 × PBS, 0.5% bovine serum albumin [BSA], and 0.02% sodium azide) before antibody staining. The antibodies used were phycoerythrin-conjugated mouse anti-human CD1a (Immunotech, Prague, Czech Republic), allophycocyanin-conjugated mouse anti-human CD1a (Immunotech), and mouse anti-human cytokeratin (an equal mixture of clones LP34 and MNF116, both from Dako, Glostrup, Denmark), labeled with Alexa Fluor 647 (Invitrogen) according to the manufacturer's protocol. Before cytokeratin staining, epidermal cell suspensions were permeabilized with a BD Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA) according to the manufacturer's protocol. Cell suspensions were analyzed and sorted with a FACSCalibur or a FACSAria (BD Biosciences) and Summit analysis software (Dako). In the case of anti-CD1a staining, live cells were selected on the basis of propidium iodide exclusion.
Confocal laser scanning microscopy
Skin samples were tattooed with an H2B-GFP-encoding construct as described previously. The next day, epidermal cell suspensions were prepared and GFP-positive epidermal keratinocytes and Langerhans cells were isolated by fluorescence-activated cell sorting on the basis of CD1a expression and subsequently analyzed by confocal laser scanning microscopy (Leica SP2) in PBA.
Calculation of transfection efficiency
The transfection efficiency with DNA tattooing was calculated by determining the number of GFP-expressing cells per tattoo area, using flow cytometry. The amount of administered molecules of plasmid DNA was calculated on the basis of the molecular mass of the construct (2.26 × 103 kDa, 3724 bp) and the dose used per tattoo. Transfection efficiency was calculated as follows:
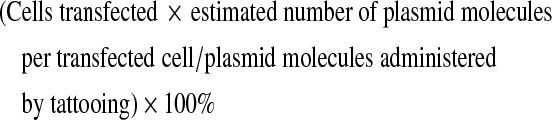 |
Imaging of luciferase expression
Luciferase expression was measured in intact skin samples 3, 18, 24, 48, and 72 hr after tattooing. The substrate luciferin (Xenogen/Caliper Life Sciences, Hopkinton, MA) was added to the medium to a final concentration of 45 μg/ml. During this procedure extra medium was added to the box in which skin was incubated, to completely cover the epidermis of skin samples with fluid to guarantee full accessibility of luciferin to the tattooed areas. Thirty minutes after the addition of luciferin, luminescence produced by active luciferase was acquired during 30 sec with an IVIS system 100 charge-coupled device (CCD) camera (Xenogen/Caliper Life Sciences).
Signal intensity was quantified as the sum of all detected light within the tattoo area of interest. During each measurement, background luminescence was measured to allow correction during data analysis. After each measurement, medium was refreshed to remove residual luciferin.
Linear mixed effects model
To study the effect of various tattoo parameters on the level of antigen (i.e., luciferase) expression, the natural log transform of the area under the curve (AUC) over the 72-hr period was analyzed. To account for possible within-skin correlation of the repeated measurements of antigen expression (at various parameter levels), a linear mixed effects model was constructed. Fixed effects included the four variables of primary interest: the DNA concentration, the duration of tattooing, the depth of tattoo, and the type of tattoo machine (see Table 1). Patient age, background luminescence (measured in nontattooed regions), the location of the tattoo in the piece of skin (edge vs. center), and the time from surgery to tattooing were also included as fixed effects to adjust for any possible confounding influences. Patient identifier was implemented as the random grouping variable. Pair-wise interactions were examined between all fixed effects. Backward stepwise selection was performed, removing terms at the 0.05 significance level. Conventional residual analysis was performed to assess model fit. Data analysis was performed with S-PLUS version 6.2 Pro (Insightful/TIBCO Software, Seattle, WA).
Results
Histology of DNA tattoo-treated ex vivo human skin
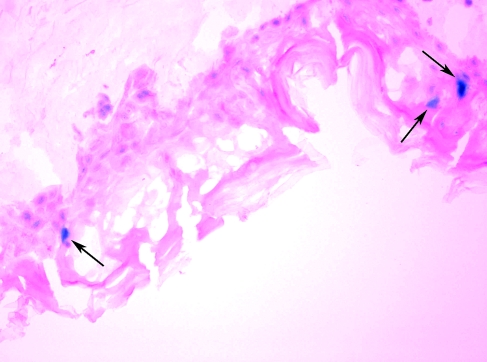
In view of the documented value of DNA tattoo in murine and nonhuman primate models (Bins et al., 2005; Pokorna et al., 2008; Verstrepen et al., 2008), we set out to further develop this technique toward clinical application. As the morphology of animal skin differs substantially from that of human skin, an ex vivo human skin model was deemed essential to allow translation of this new DNA vaccination technique toward clinical testing. In a first set of experiments we aimed to determine whether DNA tattooing results in transfection of human skin cells and to assess the effect of DNA tattoo application on general skin structure and transfection. To this purpose, a β-galactosidase-encoding plasmid was introduced into ex vivo human skin by DNA tattooing. Application of DNA to human skin by tattooing with a needle at a depth of 1.0 mm resulted in substantial disruption of the epidermal layer of human skin, but only a slight disturbance of the underlying dermal layer (Fig. 1). Furthermore, consistent with the data obtained in mouse models (Bins et al., 2005), DNA tattooing resulted in sporadic transfection of cells in the epidermis (Fig. 1). Transfection of cells in the dermal layer was not observed.
FIG. 1.
Expression of LacZ in a cryosection (original magnification, × 20) of human skin after application of LacZ DNA by tattooing. Eighteen hours after tattooing, cross-sections of skin were prepared and stained with X-Gal solution to generate a blue precipitate in transfected cells (indicated by arrows). The skin was tattooed with pVAX:LacZ at 5 mg/ml for 20 sec with an Aella tattoo machine (needle depth, 1.0 mm).
Visualization of vaccine-induced antigen expression in human skin
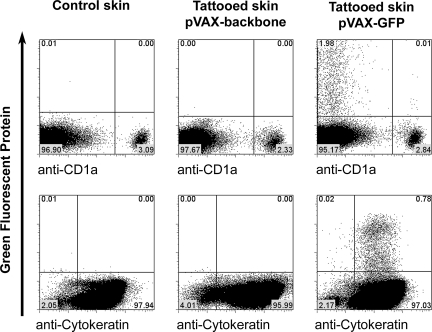
To determine which specific cell type(s) expressed the vaccine-encoded antigen after DNA tattoo vaccination, skin samples were tattooed with a GFP-encoding plasmid and cell suspensions of these samples were analyzed by flow cytometry, using specific markers for epidermal keratinocytes and LCs.
Of the viable cells (75.3 ± 6.3% of total cells, based on propidium iodide [PI] exclusion, mean ± SD of three separate experiments) recovered from epidermal preparations, approximately 2% expressed the vaccine-encoded GFP (1.80 ±1.35%, mean ± SD of three different patients, all measured in triplicate). The vast majority (>98%) of these epidermal GFP+ cells appeared to be keratinocytes, as based on expression of cytokeratin (Fig. 2). The fact that antigen expression is almost exclusively restricted to keratinocytes was confirmed by the observation that of all GFP+ cells, only 1% (1.29 ± 0.53%, mean ± SD, three different patients, all measured in triplicate) expressed the Langerhans cell marker CD1a. In the dermal layer of the skin only a small number of GFP+ cells could be detected (approximately 2.5% of all GFP+ cells in the skin sample were detected in the dermis, measured at a tattoo depth of 1.5 mm; data not shown).
FIG. 2.
Flow cytometric analysis of epidermal cell suspension of tattooed skin. Control skin or skin tattooed at 1.5 mm for 20 sec per 50 mm2 with pVAX:GFP (5 mg/ml) or empty pVAX backbone (5 mg/ml) was stained with anti-CD1a antibody or with anti-cytokeratin antibody.
Collectively, these data indicate that transfection on DNA tattooing is almost exclusively restricted to keratinocytes within the epidermal layer. Transfection of CD1a+ LCs is also observed but is relatively rare, and essentially proportional to the relative frequency of LCs and keratinocytes in human skin (a ratio of 2:98).
Quantification of the number of cells expressing the vaccine-encoded antigen allowed us to determine the “biological availability” of DNA vaccines, administered by the tattoo technique. For this calculation, we assumed that the bulk of the GFP-expressing cells was directly transfected with the plasmid, as there is no evidence in the literature that keratinocytes (which constitute >98% of the GFP-positive cells) are able to cross-present antigens. The total number of GFP-expressing cells per tattoo area of 50 mm2 was 2848 ± 762 (mean ± SD, three different patients, all measured in triplicate), using a DNA concentration of 5 mg/ml (20-sec application at a needle amplitude of 1.5 mm, using the Aella tattoo machine). Prior work has shown that on tattooing mouse skin, using a mixture of two different fluorescent reporter plasmids, coexpression of two reporter genes in a single cell occurs in some but not all cells (Bins et al., 2007a). On the basis of this observation and on the fact that the log difference in GFP expression is about 3-fold (see Fig. 2), we consider it reasonable to assume that a single transfected cell can take up approximately 1–1000 DNA molecules, indicating that the 2.8 × 103 antigen-expressing cells were transfected with a total of 2.8 × 103−2.8 × 106 DNA molecules. As the number of administered molecules of plasmid DNA per tattoo was calculated to be 1.33 × 1013, this observation indicates that the in vivo transfection efficiency is between 2 × 10–8 and 2 × 10–5% (i.e., 2 to 2000 out of 10,000,000,000 applied plasmid copies are taken up and translated into protein). These data indicate that transfection of epidermal cells by DNA tattooing represents a highly inefficient process, when compared with the currently available in vitro cell transfection methods. As an example, on transfection of cells in in vitro systems, using cationic liposomes, approximately 8% of administered DNA molecules have been shown to become expressed, a 4 × 105– 4 × 108-fold higher efficiency than that observed here on intradermal DNA vaccination (Tseng et al., 1997).
Longitudinal measurements of gene expression
To explore the possibility of monitoring antigen expression in a longitudinal fashion, human skin was tattooed with firefly luciferase reporter plasmid (pVAX:Luc) and expression was measured at several time points in intact skin by optical imaging.
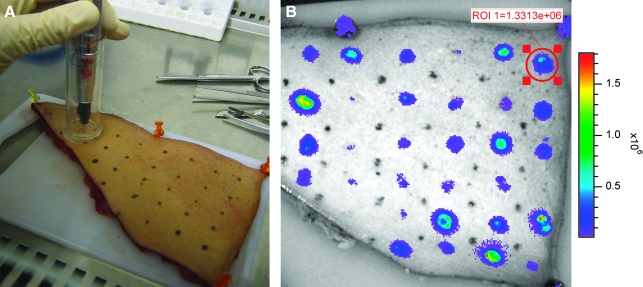
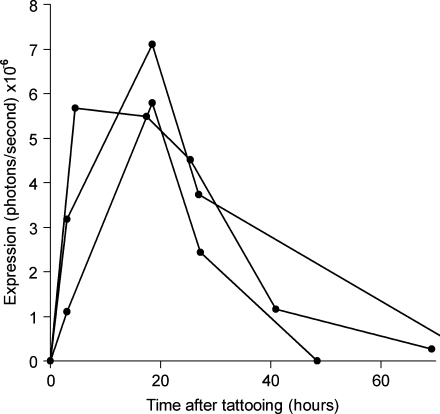
Luciferase expression could readily be detected with a light-sensitive camera and expression was restricted to the areas of tattooing (Fig. 3B). Expression was observed at significant levels as early as 2 hr after tattooing, indicating that DNA transfection, translation, and expression of the protein take place rapidly after DNA tattooing. Luciferase expression peaked between 2 and 18 hr after tattooing and remained detectable for approximately 2–3 days (Fig. 4). On the basis of the preceding data it seems plausible that the longitudinal measurement of gene expression may be used as a preclinical model to assess different methods of intradermal genetic vaccination (Mitragotri, 2005; Giudice and Campbell, 2006). As a first step toward such a comparison, we evaluated the capacity of intradermal tattoo and intradermal DNA injection to induce luciferase expression in intact skin. Remarkably, on intradermal injection, luciferase expression levels were not above background levels (whereas a tattoo with the same solution gives an expression level that is at least 10- to 20-fold higher as background), indicating that within ex vivo human skin, the expression on DNA tattooing is at least 10-fold higher than that obtained on classical intradermal injection.
FIG. 3.
Tattooing procedure of human skin (A) and typical expression of luciferase (B), visualized with a light-sensitive camera, 18 hr after tattooing. Each area of 50 mm2 was tattooed with a different tattoo setting. Note the marked variation in luciferase signal obtained under different vaccination conditions.
FIG. 4.
Typical longitudinal luciferase expression kinetics in intact ex vivo human skin on DNA tattooing. Lines represent expression in three different tattooed areas of 50 mm2, on three different skin explants. The skin was tattooed with pVAX:Luc at 5 mg/ml for 20 sec, using the Aella tattoo machine at a depth of 1.5 mm. Note that the kinetics of luciferase expression are comparable in skin samples derived from different donors.
Optimization of DNA tattooing
Having established the feasibility of performing longitudinal measurements of DNA vaccine-induced antigen expression in human skin, we aimed to optimize variables that we considered likely to influence the efficiency of DNA vaccination. To this purpose, a total of 428 skin areas with a constant surface of 50 mm2 were tattooed with a luciferase-encoding plasmid, using 10 samples of healthy abdominal skin. The tattoo variables tested for were as follows: DNA concentration, duration of tattooing, needle depth, and tattoo machine (see Table 1).
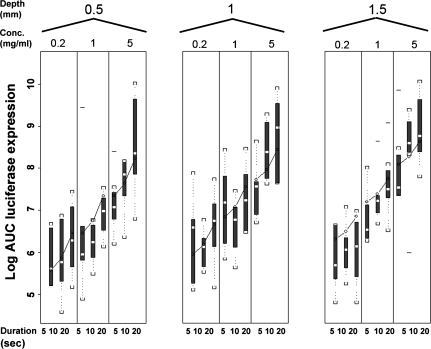
The relationship between log AUC and the various fixed effects tested with the Aella machine is shown in Fig. 5 and data obtained with the Cheyenne machine showed a similar profile (data not shown). All the tested fixed effects (Table 1), except the two different tattoo machines, had a significant effect (p < 0.001) on antigen expression. Specifically, within the tested range of values an increase in DNA concentration, needle depth, and tattoo time resulted in an increase in antigen expression.
FIG. 5.
Results of the randomization study, demonstrating the relationship between the log area under the curve (AUC) and the pVAX:Luc DNA concentration, needle depth, and tattoo duration for the Aella tattoo machine. Log AUC is visualized as a box plot (showing the lowest observation, the lowest 25% of data, the median, the highest 25% of data, and highest observation). Lines visualize the predicted AUCs obtained from the model equation.
Patient age and tattoo location (edge or center) were found not to be significantly related to gene expression AUC. Interactions that were significant were between tattoo machine and pretattoo time (p = 0.01), between tattoo machine and tattoo depth (p = 0.0007), between the log of DNA concentration and pretattoo time (p = 0.002), and between tattoo duration and tattoo depth (p = 0.03). However, none of these interactions had an influence on the tattoo settings that resulted in maximal antigen expression. For both tattoo machines the relationship between the AUC of antigen expression and the variations is expressed by the equation
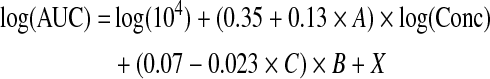 |
where X is different for the different tattoo machines (Aella, X = 6.9 − 0.76 × A + 0.84 × C; Cheyenne, X = 7.1 − 0.47 × A +0.41 × C) and where A is pretattoo time (hr), B is tattoo duration (sec), and C is tattoo depth (mm).
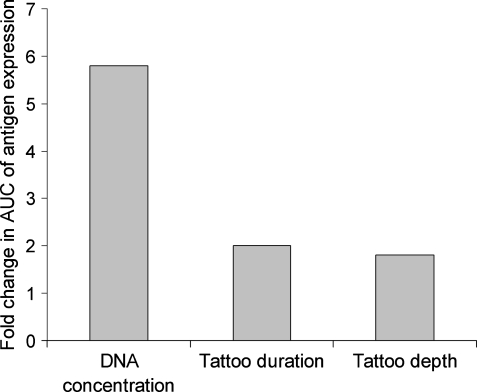
When varying each term separately (and fixing the others at their median values), the AUC changes by a factor of 5.8 over the range of DNA concentrations tested (0.2 to 5 mg/ml), by a factor of 2.0 over the range of tattoo durations (5 to 20 sec), by a factor of 1.8 and 1.2 over the range of tattoo depths (0.5 to 1.5 mm) for tattoo machines Aella and Cheyenne, respectively, and by a factor of 1.2 for Cheyenne versus Aella. These data demonstrate that, of the variables tested, the concentration of the DNA solution that is applied is by far the major determinant of antigen expression in human skin (see Fig. 6). The lines in Fig. 5 visualize the predicted AUC of antigen expression obtained from the model. This figure shows that the obtained equation fits the experimental data.
FIG. 6.
Results from the linear mixed effect model, demonstrating the influence of changing each tattoo variation over its full range (with fixing of the others at median value) on change in AUC of antigen expression for the Aella tattoo machine.
Discussion
Several dermal delivery techniques are currently moving from preclinical to clinical evaluation, and consequently there is a strong need to optimize these methods in a clinically relevant model. To allow such evaluation, we developed an ex vivo human skin model and used this model to characterize and optimize a DNA tattooing strategy for clinical application.
As a first step toward developing this model we analyzed vaccination-induced antigen expression by flow cytometry and histology. Flow cytometric analysis of tattooed human skin showed that only a small fraction of the epidermal cells, approximately 2%, is transfected. This low frequency of transfection was confirmed in an analysis of treated skin sections by histology that also showed an infrequent transfection of cells in this cell layer. This rather inefficient transfection of cells in the epidermis is comparable to that observed with other intradermal delivery techniques, such as the gene gun (Mitragotri, 2005), and to a prior analysis of DNA tattoo-induced transfection in murine skin (Bins et al., 2005). We assume that the large amount of DNA that is not taken up by skin cells is rapidly degraded in the skin by endonucleases, similar to observations in murine skin (Barry et al., 1999).
We subsequently demonstrated that vaccination-induced antigen expression can be quantified by longitudinal monitoring of luciferase activity in human skin samples. Tattooing of firefly luciferase encoding DNA in intact skin showed fast and reproducible expression of the antigen, which we could measure longitudinally and was retained for 2–3 days. This kinetic profile is comparable to the in vivo antigen kinetics observed on DNA tattooing of mice (Bins et al., 2005). This rather transient nature of vaccine-induced antigen expression in skin may possibly be explained by the high turnover of the epidermis (Gelfant, 1982) but could also be due to gene silencing. Importantly, it has previously been shown that fresh skin in culture does not lose its viability in the first 30 hr of culturing, with a viability decrease of 50% after 60 hr in medium at 37°C (Castagnoli et al., 2003; Messager et al., 2003). Therefore measurement of ex vivo antigen expression over a maximal time span of 3 days in this study was deemed appropriate.
As the level and duration of vaccination-induced antigen expression are correlated with the magnitude of vaccine-specific cytotoxic T lymphocyte (CTL) response in mice on tattoo immunization (Bins et al., 2005), we have used the ex vivo human skin model to determine the relationship between different vaccination parameters and antigen expression. From these experiments, analyzed in a linear mixed effects model, we conclude that the effect of DNA concentration forms the most important factor influencing the AUC of antigen expression in human skin (Fig. 6). We did not reach saturation for DNA concentration in these experiments. This observation is in line with data obtained in mouse models, in which intramuscular or intradermal injections of naked DNA were used (Wolff et al., 1990). In contrast, it was reported that transfection on intradermal injection of messenger RNA is saturated at a concentration of 0.05 mg/ml. This suggests a different uptake mechanism between intradermal mRNA injection and the tattooing of double-stranded DNA (Probst et al., 2007).
Using the ex vivo human skin model, we also showed that the effects of tattoo time and tattoo depth on antigen expression are significant, but less dominant. The fact that transfection of skin cells is almost exclusively observed in the epidermal layer of the skin may explain why an increase in needle depth has only a small effect on antigen expression. The epidermis of the abdominal skin samples used in this study was between 200 and 300 μm in depth. Thus, the minimal needle depth of 0.5 mm that was used here should be enough to reach the complete epidermal layer when considered a fixed object. However, it is important to stress that injection depth is probably lower than the needle depth, because of the flexibility and resistance characteristics of the skin.
To what extent can data on vaccination-induced antigen expression obtained in the ex vivo human skin model that we have developed here be expected to translate to vaccination-induced antigen expression and vaccine-induced immune responses in human subjects? A first issue of relevance here is whether the observed luciferase signal that is primarily derived from keratinocytes forms a good measure of vaccine efficacy. Specifically, can expression in keratinocytes be expected to correlate with immunogenicity?
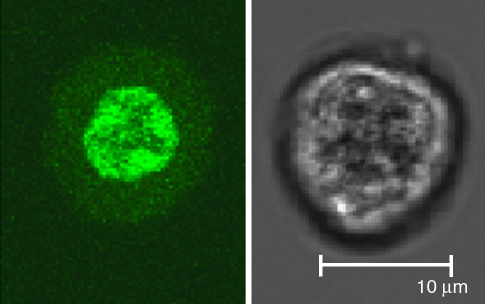
Murine studies using a plasmid carrying a K14 promoter, which is active only in keratinocytes, suggest that cross-presentation is an important route in the induction of cytotoxic T cell responses by DNA tattooing (Bins et al., 2007b). These findings were in line with a study showing that exclusive transfection of dendritic cells (DCs) (using a CD11c promoter) by gene gun immunization cannot trigger strong CD4+ and CD8+ cell responses in mice (Lauterbach et al., 2006), suggesting that antigen expression in non-APC cell types may be required for the generation of strong cellular immunity. Thus, antigen expression within keratinocytes is likely to be in large part responsible for the immunogenicity of intradermal DNA vaccines and the measurement of antigen expression in human skin samples is therefore likely to have predictive value. Interestingly, it has previously been shown that phagocytosed GFP is quenched in the acidic environment of the endosomes (Tsien, 1998). As the small population of GFP-positive LCs that is observed in human skin samples does show a lower GFP intensity than the GFP-positive keratinocyte population (Fig. 2), it may be speculated that the GFP in these LCs may possibly be derived from phagocytosed material. To further unravel the source of the GFP signal observed in LCs, we tattooed skin with a construct encoding a histone–GFP fusion protein. Direct transfection of cells with this construct results in expression of GFP exclusively in the nucleus of the cells, where GFP taken up by cross-presentation should be present in the endosomal pathway and possibly also in the cytosol/nucleus. By sorting GFP+CD1a− cells and GFP+CD1a+ cells and analyzing these separated populations by confocal laser microscopy, we aimed to reveal the subcellular localization of the GFP expressed in these cells. As shown in Fig. 7, GFP expression in CD1a-negative keratinocytes is clearly restricted to the nucleus, demonstrating their direct transfection. Unfortunately, because of their low numbers and fragility during the sorting procedure, we were not able to successfully visualize GFP-positive Langerhans cells by confocal laser scanning microscopy. Therefore, the source of their GFP signal remains unknown at present.
FIG. 7.
Localization of GFP in keratinocytes on tattooing at 1.5 mm for 20 sec per 50 mm2 with a 5 mg/ml concentration of H2B-GFP-encoding DNA, visualized by confocal laser scanning microscopy. GFP expression (left) is clearly localized to the nucleus, confirming that this cell type was directly transfected on DNA tattooing.
On accepting that the antigen expression observed in ex vivo skin samples has predictive value for in vivo immunogenicity, it is also of importance to consider the effect of the variables that have been tested here. With regard to the influence of DNA concentration on antigen expression, it may be expected that the influence of this parameter on antigen expression will be similar in vitro and in vivo, as an increase in DNA concentration is not expected to alter the location of antigen expression or cell type involved. In addition, high antigen expression due to increased DNA concentration is likely to translate directly into increased antigen presentation and immunogenicity. Also, with respect to the influence of DNA tattoo time on antigen expression, the in vitro skin model is likely to translate well as the site of antigen expression and cell types involved are unlikely to be influenced by this parameter. It does, however, seem possible that the effect of DNA tattoo time on immunogenicity is greater than predicted by the monitoring of ex vivo antigen expression, as the increased tissue damage that occurs on prolonged vaccination may have an adjuvant effect. Finally, it may be argued that the effect of tattoo depth on antigen expression measured here may perhaps have the smallest predictive value for in vivo antigen expression and immunogenicity, as the flexibility of skin in patients may well be greater than that of the fixed ex vivo skin samples used here.
On the basis of the analyses performed in this study we will initiate a phase 1 clinical trial, in which we will administer a DNA vaccine at a concentration of 5 mg/ml for 20 sec per 50 mm2 of skin surface, at a needle depth of 1.5 mm, the settings that showed the maximal level of antigen expression in this study. In this trial, a DNA vaccine encoding the HLA-A2-restricted MART-1 (melanoma antigen recognized by T cells-1) epitope will be administered to patients with metastatic melanoma. This will be a dose escalation study, in which an increased dose of the DNA vaccine will be achieved by a simple increase in the skin area used for DNA tattoo application, ranging from 4 cm2 up to 32 cm2.
In conclusion, we here demonstrate that ex vivo human skin is an adequate model for the characterization and optimization of intradermal DNA vaccines. Furthermore, we have shown that at fixed volumes, DNA concentration is the most important parameter influencing vaccination-induced antigen expression. In ongoing experiments, the skin model developed in this study is being used to determine the value of nonviral DNA carriers and other dermal delivery techniques for their ability to improve dermal antigen delivery. It seems reasonable to assume that the preclinical testing of such DNA vaccine formulations in this ex vivo human skin model will form an efficient strategy to select promising vaccination strategies for subsequent testing in clinical trials.
Acknowledgments
The authors thank Johan Westerga, Inge Heins, and Chantal Lamers-de Ruiter for help with histology experiments, and Silvia Ariotti for help with confocal laser scanning microscopy.
Author Disclosure Statement
No competing financial interests exist.
References
- Bahloul C. Taieb D. Diouani M. F. Ahmed S.B. Chtourou Y. B'Chir B.I. Kharmachi H. Dellagi K. Field trials of a very potent rabies DNA vaccine which induced long lasting virus neutralizing antibodies and protection in dogs in experimental conditions. Vaccine. 2006;24:1063–1072. doi: 10.1016/j.vaccine.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Barry M.E. Pinto-Gonzalez D. Orson F.M. McKenzie G.J. Petry G.R. Barry M.A. Role of endogenous endonucleases and tissue site in transfection and CpG-mediated immune activation after naked DNA injection. Hum. Gene Ther. 1999;10:2461–2480. doi: 10.1089/10430349950016816. [DOI] [PubMed] [Google Scholar]
- Bins A.D. Jorritsma A. Wolkers M.C. Hung C.F. Wu T.C. Schumacher T.N. Haanen J.B. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat. Med. 2005;11:899–904. doi: 10.1038/nm1264. [DOI] [PubMed] [Google Scholar]
- Bins A.D. van Rheenen J. Jalink K. Halstead J.R. Divecha N. Spencer D.M. Haanen J.B. Schumacher T.N. Intravital imaging of fluorescent markers and FRET probes by DNA tattooing. BMC Biotechnol. 2007a;7:2. doi: 10.1186/1472-6750-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bins A.D. Wolkers M.C. van den Boom M.D. Haanen J.B. Schumacher T.N. In vivo antigen stability affects DNA vaccine immunogenicity. J. Immunol. 2007b;179:2126–2133. doi: 10.4049/jimmunol.179.4.2126. [DOI] [PubMed] [Google Scholar]
- Castagnoli C. Alotto D. Cambieri I. Casimiri R. Aluffi M. Stella M. Alasia S.T. Magliacani G. Evaluation of donor skin viability: Fresh and cryopreserved skin using tetrazolium salt assay. Burns. 2003;29:759–767. doi: 10.1016/j.burns.2003.01.001. [DOI] [PubMed] [Google Scholar]
- Gelfant S. “Of mice and men”: The cell cycle in human epidermis in vivo. J. Invest. Dermatol. 1982;78:296–299. doi: 10.1111/1523-1747.ep12507367. [DOI] [PubMed] [Google Scholar]
- Giudice E.L. Campbell J.D. Needle-free vaccine delivery. Adv. Drug Deliv. Rev. 2006;58:68–89. doi: 10.1016/j.addr.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Godin B. Touitou E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv.Drug Deliv.Rev. 2007;59:1152–1161. doi: 10.1016/j.addr.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Heller L.C. Jaroszeski M.J. Coppola D. McCray A.N. Hickey J. Heller R. Optimization of cutaneous electrically mediated plasmid DNA delivery using novel electrode. Gene Ther. 2007;14:275–280. doi: 10.1038/sj.gt.3302867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao L.A. Wu L. Khan A.S. Satishchandran A. Draghia-Akli R. Weiner D.B. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine. 2008;26:440–448. doi: 10.1016/j.vaccine.2007.10.041. [DOI] [PubMed] [Google Scholar]
- Hooper J.W. Golden J.W. Ferro A.M. King A.D. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25:1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T. Sullivan K.F. Wahl G.M. Histone–GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 1998;8:377–385. doi: 10.1016/s0960-9822(98)70156-3. [DOI] [PubMed] [Google Scholar]
- Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002;12:390–399. [PubMed] [Google Scholar]
- Klinman D.M. Sechler J.M. Conover J. Gu M. Rosenberg A.S. Contribution of cells at the site of DNA vaccination to the generation of antigen-specific immunity and memory. J. Immunol. 1998;160:2388–2392. [PubMed] [Google Scholar]
- Lauterbach H. Gruber A. Ried C. Cheminay C. Brocker T. Insufficient APC capacities of dendritic cells in gene gun-mediated DNA vaccination. J. Immunol. 2006;176:4600–4607. doi: 10.4049/jimmunol.176.8.4600. [DOI] [PubMed] [Google Scholar]
- Maruyama H. Ataka K. Higuchi N. Sakamoto F. Gejyo F. Miyazaki J. Skin-targeted gene transfer using in vivo electroporation. Gene Ther. 2001;8:1808–1812. doi: 10.1038/sj.gt.3301604. [DOI] [PubMed] [Google Scholar]
- Mathers A.R. Larregina A.T. Professional antigen-presenting cells of the skin. Immunol. Res. 2006;36:127–136. doi: 10.1385/IR:36:1:127. [DOI] [PubMed] [Google Scholar]
- Messager S. Hann A.C. Goddard P.A. Dettmar P.W. Maillard J.Y. Assessment of skin viability: Is it necessary to use different methodologies? Skin Res. Technol. 2003;9:321–330. doi: 10.1034/j.1600-0846.2003.00039.x. [DOI] [PubMed] [Google Scholar]
- Mitragotri S. Immunization without needles. Nat. Rev. Immunol. 2005;5:905–916. doi: 10.1038/nri1728. [DOI] [PubMed] [Google Scholar]
- Pokorna D. Rubio I. Muller M. DNA-vaccination via tattooing induces stronger humoral and cellular immune responses than intramuscular delivery supported by molecular adjuvants. Genet. Vaccines Ther. 2008;6:4. doi: 10.1186/1479-0556-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst J. Weide B. Scheel B. Pichler B.J. Hoerr I. Rammensee H.G. Pascolo S. Spontaneous cellular uptake of exogenous messenger RNA in vivo is nucleic acid-specific, saturable and ion dependent. Gene Ther. 2007;14:1175–1180. doi: 10.1038/sj.gt.3302964. [DOI] [PubMed] [Google Scholar]
- Quaak S.G. van den Berg J.H. Toebes M. Schumacher T.N. Haanen J.B. Beijnen J.H. Nuijen B. GMP production of pDERMATT for vaccination against melanoma in a phase I clinical trial. Eur. J. Pharm. Biopharm. 2008;70:429–438. doi: 10.1016/j.ejpb.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Steitz J. Britten C.M. Wolfel T. Tuting T. Effective induction of anti-melanoma immunity following genetic vaccination with synthetic mRNA coding for the fusion protein EGFP.TRP2. Cancer Immunol. Immunother. 2006;55:246–253. doi: 10.1007/s00262-005-0042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng W.C. Haselton F.R. Giorgio T.D. Transfection by cationic liposomes using simultaneous single cell measurements of plasmid delivery and transgene expression. J. Biol. Chem. 1997;272:25641–25647. doi: 10.1074/jbc.272.41.25641. [DOI] [PubMed] [Google Scholar]
- Tsien R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- Verstrepen B.E. Bins A.D. Rollier C.S. Mooij P. Koopman G. Sheppard N.C. Sattentau Q. Wagner R. Wolf H. Schumacher T.N. Heeney J.L. Haanen J.B. Improved HIV-1 specific T-cell responses by short-interval DNA tattooing as compared with intramuscular immunization in non-human primates. Vaccine. 2008;26:3346–3351. doi: 10.1016/j.vaccine.2008.03.091. [DOI] [PubMed] [Google Scholar]
- Wolff J.A. Malone R.W. Williams P. Chong W. Acsadi G. Jani A. Felgner P.L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]