Abstract
The use of adeno-associated viral (AAV) vectors for gene replacement therapy is currently being explored in several clinical indications. However, reports have suggested that input capsid proteins from AAV-2 vector particles may result in the stimulation of cytotoxic T lymphocyte (CTL) responses that can result in a loss of transduced cells. To explore the impact of anti-AAV CTLs on AAV-mediated transgene expression, both immunocompetent C57BL/6 mice and B cell-deficient μMT mice were immunized against the AAV2 capsid protein (Cap) and were injected intravenously with an AAV-2 vector encoding α-galactosidase (α-Gal). C57BL/6 mice, which developed both CTL and neutralizing antibody responses against Cap, failed to show any detectable α-Gal expression. In contrast, serum α-Gal levels comparable to those of naive mice were observed in μMT mice despite the presence of robust CTL activity against Cap, indicating that preexisting Cap-specific CTLs did not have any effect on the magnitude and duration of transgene expression. The same strategy was used to assess the impact of CTLs against the α-Gal transgene product on AAV-mediated gene delivery and persistence of transgene expression. Preimmunization of μMT mice with an Ad/α-Gal vector induced a robust CTL response to α-Gal. When these mice were injected with AAV2/α-Gal vector, initial levels of α-Gal expression were reduced by more than 1 log and became undetectable by 2 weeks postinjection. Overall, our results indicate that CTLs against the transgene product as opposed to AAV capsid protein are more likely to interfere with AAV transgene expression.
Introduction
The diversity and design of gene therapy vectors have advanced greatly in their design and utility since their initial introduction for clinical use (Meyer and Wagner, 2006; Alton et al., 2007a,b). Nevertheless, for monogenetic diseases such as hemophilia or lysosomal storage diseases, the goal of gene therapy remains the same: to deliver a therapeutic protein to patients who either do not correctly express the wild-type protein or express it at subtherapeutic levels. Research has focused on the use of adeno-associated viral (AAV) vectors for several reasons including their ability to transduce nondividing cells and potential to mediate long-term transgene expression (Chao and Walsh, 2004; Wu et al., 2006). Because AAV vectors do not contain any viral genes, they are generally thought to be less immunogenic and less toxic than other gene therapy vectors such as adenoviral vectors, which retain some viral gene expression and can induce immune responses to virus-associated proteins (Yang et al., 1994; Jooss and Chirmule, 2003) as well as induce the production of several proinflammatory cytokines (Bessis et al., 2004). The lack of viral gene expression by AAV vectors suggests that any immune response generated after AAV administration would be directed against the transgene product or the input capsid protein from the initial injection of vector particles.
It has been demonstrated by several groups that neutralizing antibodies (NAbs) to AAV can significantly affect viral transduction (Murphy et al., 2008; Scallan et al., 2008). Because NAbs are serotype specific and more than 100 serotypes or variants of AAV have been described to date, it is possible that by using AAV vectors of different serotypes, the issue of NAbs can be circumvented (Halbert et al., 2000). In addition to anti-viral antibody responses, humoral responses against the transgene product have also been observed after intramuscular administration of AAV expressing foreign proteins such as ovalbumin (OVA) (Brockstedt et al., 1999) or therapeutic proteins such as factor IX (FIX) (Ge et al., 2001). By altering the route of administration, it was possible to circumvent antibody responses to FIX in mice; OVA-specific antibodies were still generated irrespective of the route of injection. It is clear that not only the nature of the transgene itself, but also the route of administration, will affect the induction of an immune response to the transgene product. Although the impact of humoral responses on AAV transduction and subsequent gene expression has been well characterized, the role of T cell responses to AAV and the transgene product is less well understood.
In a hemophilia clinical trial, administration of an AAV vector encoding the FIX protein resulted in an initial increase in circulating levels of FIX that declined over a short period of time (Manno et al., 2006). The decline was accompanied by an increase in the level of serum transaminase levels and the appearance of a T cell response to the AAV capsid protein in one patient. Manno and colleagues (2006) suggested that the host immune response to AAV capsid protein may significantly affect long-term transgene expression resulting in destruction of the transduced hepatocytes by capsid-specific cytolytic T cell responses. However, reports suggest that AAV capsid-specific T cell responses may have little effect on transgene expression in the mouse (H. Li et al., 2007; C. Li et al., 2007; Wang et al., 2007). To further investigate this issue, we have compared the impact of cytotoxic T lymphocytes (CTLs) against AAV capsid protein versus the encoded transgene product on AAV-mediated transgene expression in immunocompetent C57BL/6 mice and μMT mice, which lack the ability to mount a humoral response. The transgene used in these studies encodes the α-galactosidase (α-Gal) protein, which is the therapeutic protein currently used to treat patients with Fabry disease. Fabry disease is a rare lysosomal storage disorder resulting in the accumulation of the glycosphingolipid globotriaosylceramide in tissues because of a deficiency in the α-Gal enzyme as a result of mutations, gene rearrangement, and deletions in the α-Gal gene (Ashley et al., 2001). Enzyme replacement therapy has proven to be a successful treatment for this disease, making it a possible candidate disease for gene therapy (Desnick, 2004). However, similar to factor IX therapy, immunogenicity may be a potential problem as some patients may not be tolerized against the protein.
Using a plasmid or adenoviral vector expression system to generate strong CTL responses to the capsid protein or the α-Gal transgene product, we demonstrate that, in the absence of NAb responses in μMT mice, preexisting capsid-specific CTL responses have no effect on the strength or duration of α-Gal expression. In contrast, preexisting α-Gal-specific CTL responses significantly affected long-term transgene expression, resulting in a 2-log decrease in initial expression that dropped to undetectable levels by 2 weeks after AAV administration. The drop in expression was also accompanied by an increase in serum transaminase levels. These results suggest that CTL responses against the transgene product, as opposed to AAV capsid protein, are the major factor limiting transgene expression from AAV vectors.
Materials and Methods
Cell lines
The C57BL/6 histocompatible (H-2b) SVB6 fibroblast cell line was used in CTL assays and was a gift from L. Gooding (Emory University, Atlanta, GA). HeLa cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (Lonza Walkersville, Walkersville, MD), penicillin (100 units/ml), streptomycin (100 μg/ml), and 2 mM glutamine. Cells were maintained at 37°C in a 5% CO2 atmosphere and confirmed to be free of mycoplasma by routine testing.
Generation of viral vectors
AAV2/α-Gal contained serotype 2 inverted terminal repeats and the human α-Gal cDNA under the control of the DC190 liver-restricted promoter (Ziegler et al., 2004; Barbon et al., 2005; McEachern et al., 2006). Recombinant vectors were produced by triple-plasmid transfection of 293 cells and were column purified as reported (O'Riordan et al., 2000). The final titer of AAV2/α-Gal was determined by TaqMan polymerase chain reaction (PCR) of the bovine growth hormone polyadenylation signal sequence. Quality control of each lot of recombinant included analysis for bioburden (presence of gram-positive bacteria) and endotoxin testing (<0.6 EU/ml). The Ad5/Cap virus expressing the capsid protein under the control of the cytomegalovirus (CMV) promoter was a kind gift from N. Muzyczka (University of Florida, Gainesville, FL).
Immunization of mice to generate Cap-specific cytotoxic T cell responses
Groups of three to five C57BL/6 or BALB/c mice were immunized with peptide, plasmid, or recombinant adenoviral vectors to induce capsid-specific CTL responses. AAV2 capsid peptides were identified by the BIMAS (BioInformatics and Molecular Analysis Section, Center for Information Technology, National Institutes of Health, Bethesda, MD) peptide prediction algorithm. For peptide analysis, mice were immunized with 100 μg of peptide emulsified in Freund's incomplete adjuvant and injected intradermally into mice. To generate T cell-specific responses using plasmid or recombinant adenoviral vectors, mice were immunized intramuscularly with either 100 μg of pCAP-plasmid (plasmid expressing AAV2 Cap driven by the CMV promoter) or 1 × 1010 particles of Ad5/Cap virus. Two weeks postimmunization, mice were killed and spleens were collected for analysis as detailed subsequently.
Long-term transgene expression experiments
Six- to 8-week-old female C57BL/6 or B cell-deficient μMT mice were purchased from Charles River Laboratories (Wilmington, MA). To generate antigen-specific T cell responses, mice were immunized intradermally with 1 × 1010 particles of an adenoviral vector expressing either the α-Gal or AAV2 capsid protein or with plasmid as previously described. Two weeks after immunization, a subset of mice was killed and spleens were collected to confirm the induction of a CTL response. The remaining animals were injected intravenously with 1 × 1011 particles of a recombinant AAV2/α-Gal vector under the control of a liver-specific promoter. At various time points postinjection, serum was collected and analyzed for the presence of circulating α-Gal protein. In some studies, long-term transgene expression was examined in the presence and absence (no preimmunization) of specific cytolytic T cell responses. All animal experiments were conducted in accordance with the guidelines established by the Institutional Animal Care and Use Committee at Genzyme (Framingham, MA).
Cytotoxic T lymphocyte assay
Evaluation of CTL activity was performed 2 weeks after immunization of mice with plasmid or adenoviral vectors. Naive mice were used as a negative control. Spleen cells from naive or immunized mice were stimulated with mitomycin C-inactivated syngeneic SVB6 cells infected with Ad vector encoding either α-Gal or the AAV2 capsid protein. Cells were cultured in 24-well plates containing 5 × 106 spleen cells and 6 × 104 stimulator fibroblasts in 2 ml of RPMI 1640 medium with 10% fetal calf serum. Cytolytic activity was assayed after 6 days of culture. Target cells consisted of SVB6 fibroblasts infected for 48 hr with an Ad vector expressing either the α-Gal protein or the capsid protein, or with an Ad vector lacking a transgene (Ad/empty vector [EV]) as a control. Target cells were treated with recombinant mouse interferon (IFN)-γ (100 U/ml; R&D Systems, Madison, WI) for the last 24 hr to enhance MHC class I presentation, labeled with chromium-51 (PerkinElmer Life and Analytical Sciences, Boston, MA) overnight (25 μCi/1 × 105 cells), and plated in round-bottom 96-well plates at 5 × 103 cells per well. Effector cells were added at various effector-to-target (E:T) cell ratios in triplicate in a total volume of 200 μl. After a 5-hr incubation, 25 μl of cell-free supernatant was collected from each well and counted in a Wallac MicroBeta TriLux scintillation counter (PerkinElmer Life and Analytical Sciences). The amount of 51Cr spontaneously released was determined by incubating target cells in medium alone. Spontaneous release from target cells was typically less than 20%. The total amount of 51Cr incorporated was determined by adding 1% Triton X-100 in distilled water, and the percentage lysis was calculated as follows: [(sample cpm − spontaneous cpm)/(total cpm −spontaneous cpm)] × 100.
Analysis of circulating α-Gal protein
Levels of circulating α-Gal protein in individual mice were determined using a sandwich enzyme-linked immunosorbent assay (ELISA). For this assay, 96-well plates were coated with a rabbit polyclonal anti-α-Gal antibody for 2 hr at 37°C. After washing with phosphate-buffered saline (PBS)–Tween, blocking buffer (Tris-buffered saline with Tween [TBS-T] and 5% milk) was added and plates were incubated overnight at 4°C. Serum samples were serially diluted 2-fold, plated in duplicate, and incubated at 37°C for 1 hr. Washed plates were incubated with a biotinylated goat anti-α-Gal monoclonal antibody for 1 hr at 37°C followed by the addition of streptavidin–horseradish peroxidase (HRP) for 90 min. SIGMAFAST substrate (Sigma-Aldrich, St. Louis, MO) was added and assay plates were analyzed with a VMax plate reader (Molecular Devices, Sunnyvale, CA) at 490 nm. The amount of α-Gal protein contained in the serum was derived by comparison with a standard curve with known concentrations of recombinant α-Gal protein.
Assessment of AAV2-neutralizing antibodies
For the AAV2 neutralization assay, mouse serum was tested for its ability to inhibit infection of HeLa cells by an AAV2 vector encoding β-galactosidase (β-Gal), resulting in decreased β-Gal transgene expression. Briefly, HeLa cells were plated into 96-well tissue culture plates at a density of 2 × 104 cells per well and allowed to adhere for 2 hr. The adhered cells were incubated with an Ad2 wild-type helper virus for 4 hr at 37°C, 5% CO2. During this incubation period, mouse serum samples were serially diluted 2-fold in a separate 96-well plate. AAV2/β-Gal vector was added to each well containing serum, and incubated for 1 hr at 37°C, 5% CO2. At the end of both incubation periods, the neutralizing sera–AAV samples were added to the wild-type Ad2-infected HeLa cells, and the plates were incubated for approximately 3 days at 37°C, 5% CO2. The medium was removed from all wells of the assay plate, the cells were lysed, and the supernatants were tested with a Tropix Galacto-Star kit for β-Gal expression according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). The neutralizing serum titer was defined as the dilution of serum that reduced β-Gal expression of the positive control (naive serum sample) by: 50%.
Statistical analysis
Statistical analysis of transgene expression data was performed with GraphPad Prism version 3.0a for the Macintosh (GraphPad Software, San Diego CA). Differences were considered to be statistically significant when p < 0.05.
Results
Generation of AAV2 capsid-specific cytolytic T cell responses in C57BL/6 mice
To study the impact of preexisting CTLs on AAV transgene expression, we first sought to induce AAV-specific CTLs using peptides for immunization. Previously, other investigators have used several methods to identify potentially immunogenic peptides from the AAV2 capsid protein sequence including capsid peptide libraries or computer prediction algorithms (Sabatino et al., 2005; Chen et al., 2006). Using these computer prediction algorithms, we were able to identify potential CD8+ T cell epitopes contained within the capsid protein sequence predicted to bind to MHC class I molecules with high affinity for both the C57BL/6 (H-2Kb) and BALB/c (H-2Kd) MHC class I molecules. In some cases, immunization with these peptides generated a CD8+ T cell response that resulted in IFN-γ production on restimulation of splenocytes with the immunizing peptide (Table 1). However, no direct cytolytic activity was observed from these T cells (Table 1).
Table 1.
Assessment of Immunogenicity of Computer-Predicted AAV2 Capsid Peptides
| |
|
|
|
Immunogenicc |
Recognized as target |
||
|---|---|---|---|---|---|---|---|
| Peptidea | H2 locus | Start positionb | Strain | ELISPOT | CTL | ELISPOTd | CTLe |
| KYLGPFNGL | H2-Kd (9-mer) | 50 | BALB/c | Yes | No | Not tested | Not tested |
| QYGSVSTNL | H2-Kd (9-mer) | 574 | BALB/c | No | No | Not tested | Not tested |
| VFQAKKRVL | H2-Kd (9-mer) | 117 | BALB/c | No | No | Not tested | Not tested |
| VFTDSEYQL | H2-Kd (9-mer) | 341 | BALB/c | No | No | Not tested | Not tested |
| KFFPQSGVL | H2-Kd (9-mer) | 531 | BALB/c | No | No | Not tested | Not tested |
| FFPQSGVLI | H2-Kd (9-mer) | 532 | BALB/c | No | No | Not tested | Not tested |
| QYSTGQVSV | H2-Kd (9-mer) | 671 | BALB/c | No | No | Not tested | Not tested |
| VPQYGYLTL | H2-Kb (9-mer) | 371 | C57BL/6 | No | No | Yes | No |
| LVLPGYKYL | H2-Kb (9-mer) | 44 | C57BL/6 | No | No | Yes | No |
| FMVPQYGYL | H2-Kb (9-mer) | 369 | C57BL/6 | No | No | Yes | No |
| DSLVNPARA | H2-Db (9-mer) | 513 | C57BL/6 | No | No | Yes | Yes |
| GEPVNEADA | H2-Db (9-mer) | 61 | C57BL/6 | No | No | Yes | No |
| LTLNNGSQA | H2-Db (9-mer) | 377 | C57BL/6 | No | No | Yes | No |
Peptide prediction was done with the BIMAS program.
Sequence start position relative to VP1.
Following direct peptide immunization.
For ELISPOT Ad5/CAP-generated CTLs were incubated with the indicated peptide and IFN-γ release was analyzed.
Peptide was pulsed onto target cells and incubated with Ad5/CAP-generated CTLs.
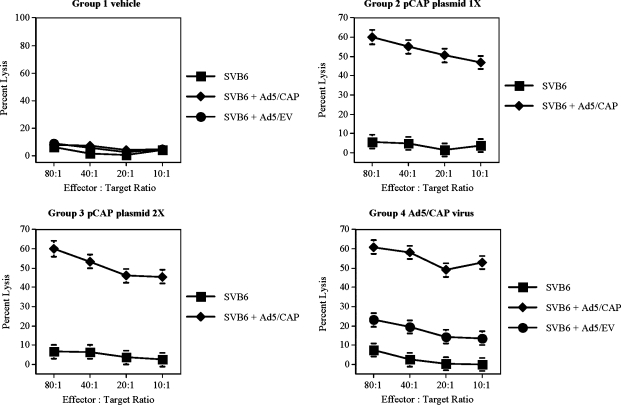
As an alternative method for immunization, recombinant adenovirus and plasmid expression vectors were generated that expressed high levels of the full-length AAV2 capsid protein (Cap) under the control of a CMV promoter. To generate capsid-specific cytolytic T cell responses, mice were immunized either intramuscularly with the pCap plasmid or intradermally with the recombinant Ad5/Cap vector. Ten days after immunization, spleens were collected and analyzed for the presence of a capsid-specific cytolytic T cell response. As shown in Fig. 1, immunization with a single dose of the pCap plasmid resulted in a strong capsid-specific CTL response that was present in all mice as evidenced by the ability of effector cells to lyse syngeneic targets expressing the AAV2 capsid protein. The strength of this response was not enhanced after a second dose of plasmid. Similarly, injection of the Ad5/Cap vector also induced a potent Cap-specific CTL response that resulted in a significant degree of target lysis. However, a slight CTL response was also observed against the adenoviral proteins themselves (Ad5/EV-transduced targets), demonstrating one of the drawbacks to Ad-based gene therapy, that is, the ability to induce T cell responses to both the transgene product and Ad proteins. Interestingly, immunization with the Ad5/Cap virus generated a response that resulted in IFN-γ secretion after restimulation with each of the predicted H-2Kb-binding peptides, but T cells from Ad5/Cap-immunized mice lysed targets pulsed with only one of the predicted peptides (Table 1). Taken together, these data suggest that the strength of the stimulus and the context in which it is delivered (peptide vs. viral vector) play a role in generating cytolytic T cell responses. In addition, these data confirm published reports indicating that discordant results can be obtained using different immunoassays (Whiteside et al., 2003), implying that not every CD8+ T cell that secretes IFN-γ in response to peptide stimulation has the ability to lyse cells expressing the target antigen.
FIG. 1.
Generation of AAV2 capsid-specific CTL responses. To generate capsid-specific CTL responses, mice were immunized either intramuscularly with a plasmid or intradermally with an adenoviral vector expressing the AAV2 capsid protein under the control of a CMV promoter. Two weeks later, spleens were collected to analyze the ability of T cells to lyse targets in a chromium release assay. Targets consisted of uninfected SVB6 cells (squares), SVB6 cells infected with the Ad5/Cap virus (diamonds), and SVB6 cells infected with Ad5/EV control virus (circles). Representative graphs are shown from three independent experiments and data are expressed as means ± standard deviation at each effector-to-target ratio.
Neutralizing antibodies, but not preexisting CTLs against AAV2 capsid protein, inhibit transgene expression
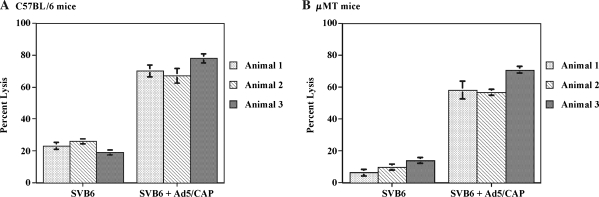
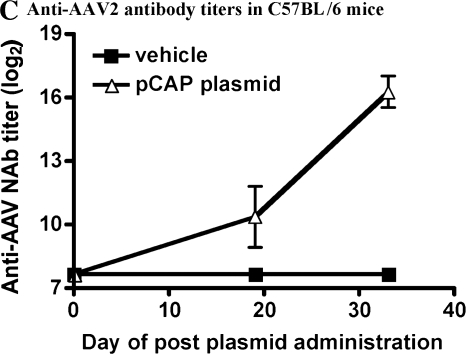
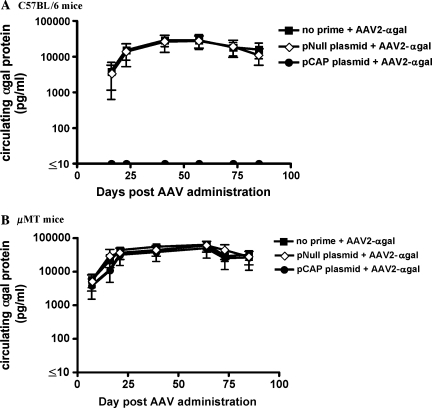
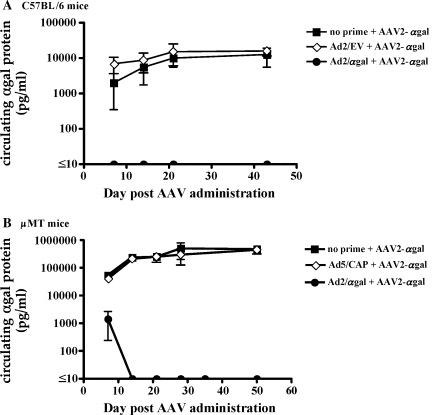
It has been previously demonstrated that infection of cells with AAV vector can be severely inhibited by the presence of neutralizing antibodies (Sun et al., 2002, 2003). In addition, it has been suggested that uncoating of the vector within the cell may result in the presentation of capsid peptides on the cell surface in the context of MHC class I molecules (Manno et al., 2006), rendering infected cells susceptible to lysis by capsid-specific CTLs. Therefore, both T and B cell responses could potentially affect the overall strength and duration of transgene expression after the administration of AAV vector. To explore the contribution of humoral and CTL responses, experiments were conducted in immunocompetent C57BL/6 mice and μMT mice, which do not possess functional B cells. The μMT mouse harbors a disruption of the μM locus resulting in an arrest during B cell maturation (Kitamura et al., 1991). These mice can mount normal T cell responses but do not have the ability to generate antibodies (Perricone et al., 2004). The μMT mouse allows for assessment of the impact of preexisting CTLs on AAV transduction and subsequent transgene expression separate from any contribution from neutralizing antibodies. Administration of the pCap plasmid to either C57BL/6 mice (Fig. 2A) or μMT mice (Fig. 2B) resulted in the generation of capsid-specific CTLs that lysed target cells expressing the capsid antigen to a similar extent. In addition, immunized C57BL/6 mice also generated capsid-specific neutralizing antibodies (Fig. 2C); this response was not present in μMT mice. After induction of these anti-Cap immune responses, mice were injected intravenously with an AAV2 vector expressing the α-galactosidase (α-Gal) protein to determine the strength and duration of transgene expression. Control mice primed with an empty plasmid followed by the AAV2/α-Gal vector, or mice injected with the AAV2/α-Gal vector alone, expressed similarly high, sustained levels of α-Gal protein during the course of the experiment (Fig. 3A). In contrast, C57BL/6 mice that were treated with the pCap plasmid (and developed a capsid-specific CTL response as well as neutralizing antibodies) did not demonstrate measurable levels of α-Gal in the serum at any of the time points analyzed, indicating that either the neutralizing anti-Cap antibody response, the anti-Cap CTL response, or a combination of the two limited the transduction efficiency of the vector or transgene expression. Similar expression studies were carried out in the μMT mouse. As shown in Fig. 3B, after the injection of AAV2/α-Gal vector, there was no difference in the level of α-Gal expression in mice injected with AAV2/α-Gal vector alone, mice primed with empty plasmid, or mice primed with pCap plasmid. Although μMT mice primed with the pCap plasmid developed a strong capsid-specific CTL response, high levels of transgene expression were still observed, comparable to those seen in mice that did not possess capsid-specific cytolytic T cells. These data suggest that AAV2-specific CTL responses do not limit the level of transgene expression, a finding consistent with data from C. Li and colleagues (2007) and Wang and colleagues (2007). The results also confirm that neutralizing antibodies can play a significant role in inhibiting transduction and subsequent transgene expression by AAV2 vector in C57BL/6 mice.
FIG. 2.
Immune responses in mice after administration of pCap plasmid. C57BL/6 and μMT mice were injected intramuscularly with 100 μg of the pCap plasmid. Two weeks later both T and B cell responses were analyzed. CTL responses were evaluated from individual C57BL/6 (A) or μMT (B) mice in a chromium release assay. Targets consisted of uninfected SVB6 cells or SVB6 cells infected with the Ad5/Cap virus. Data are expressed as means ± standard deviation at an 80:1 effector-to-target cell ratio. Serum samples were analyzed for the presence of anti-AAV2 neutralizing antibodies (C) by ELISA as described in Materials and Methods.
FIG. 3.
α-Gal expression in mice with preexisting Cap-specific immune responses. C57BL/6 mice (A) and μMT mice (B) were unprimed (squares), primed with a control pNull plasmid (diamonds), or primed with pCap plasmid (circles) to generate capsid-specific immune responses. Two weeks later mice were injected intravenously with 1 × 1011 particles of AAV2/α-Gal vector. At various time points throughout the course of the experiment serum was collected to analyze the circulating levels of α-Gal protein by ELISA, as described in Materials and Methods.
Presence of preexisting CTLs to α-Gal, but not capsid protein, severely limits α-Gal expression in μMT mice
Because capsid-specific cytolytic T cells did not appear to affect transgene expression, we examined whether preexisting CTLs to the α-Gal transgene product would have an impact on the strength and duration of expression after an IV delivery of AAV-2/α-Gal vector. C57BL/6 mice were preimmunized with an Ad vector expressing the α-Gal protein driven by a CMV promoter to generate both antibody and CTL responses to the α-Gal transgene. Two weeks later, mice were shown to have both a CTL response (90% specific lysis at an E:T ratio of 80:1), as well as antibodies to the α-Gal transgene (average titer of 6400) and were injected with the AAV/α-Gal vector. Mice that received no priming or received the Ad2/EV control vector as a priming agent displayed similarly high levels of α-Gal expression (Fig. 4A). In contrast, no circulating α-Gal protein could be detected in the group of preimmunized C57BL/6 mice that developed both antibody and CTL responses to α-Gal, suggesting that either the CTLs played a role in limiting transgene expression or the presence of an antibody response to α-Gal limited our ability to quantitatively measure it in serum. To circumvent any involvement of neutralizing antibodies to the transgene product, a similar experiment was performed in the μMT mice. μMT mice were immunized with either the Ad5/Cap vector or the Ad2/α-Gal vector to determine the individual effects of cytolytic T cell responses to Cap and α-Gal on transgene expression. Mice immunized with the Ad5/Cap vector generated a capsid-specific CTL response (54% specific lysis at an E:T cell ratio of 80:1), whereas mice immunized with the Ad2/α-Gal vector generated an α-Gal-specific CTL response (47% specific lysis at an E:T cell ratio of 80:1). As shown in Fig. 4B, the presence of capsid-specific CTLs alone did not impair the level of transgene expression compared with the expression levels observed in unprimed mice, confirming the results shown in Fig. 3. However, in the group of μMT mice with CTLs but no antibodies to α-Gal, there was a significant decrease in the level of transgene expression; a 2-log difference at the onset of expression that quickly declined to background levels. These data indicate that CTLs to the α-Gal transgene product, and not the AAV capsid protein, severely limit transgene expression.
FIG. 4.
α-Gal expression is inhibited in the presence of preexisting α-Gal-specific immune responses. C57BL/6 mice (A) were unprimed (squares), primed with a control Ad2/EV virus (diamonds), or primed with Ad2/α-Gal vector (circles) to generate α-Gal-specific immune responses. μMT mice (B) were unprimed (squares), primed with a control Ad5/Cap virus (diamonds), or primed with Ad2/α-Gal vector (circles) to generate capsid or α-Gal-specific immune responses. Ten days later both groups of mice were injected intravenously with 1 × 1011 particles of AAV2/α-Gal vector. At various time points throughout the course of the experiment serum was collected to analyze the circulating levels of α-Gal protein by ELISA, as described in Materials and Methods.
Decrease in α-Gal transgene expression is accompanied by increase in serum transaminase levels
Results from a clinical trial using AAV as a delivery vehicle for factor IX therapy implicate immune responses to the AAV capsid protein as a complicating issue (Manno et al., 2006). A decrease in circulating factor IX levels was observed in some patients and was accompanied by elevations in serum transaminase levels, suggesting liver toxicity. Although the authors demonstrated that CD8+ T cell responses could be detected against the capsid protein, T cell responses against the factor IX protein were not examined. In light of the finding that preexisting CTLs against α-Gal and not the capsid protein play a significant role in limiting transgene expression, serum from μMT mice with preexisting CTL responses (Fig. 4B) was analyzed to determine whether the decrease in expression was accompanied by increases in serum transaminase levels. As shown in Table 2, a direct correlation exists between the decrease in transgene expression in the Ad/α-Gal-primed group and the increase in serum transaminase levels. Mice that were not primed with antigen or primed with the Ad/Cap vector to induce a capsid-specific CTL response did not display an increase in either alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level. In contrast, mice that were primed with Ad/α-Gal displayed significantly higher levels of both ALT and AST in serum at the time when transgene expression was reduced (days 7 to 14). Transaminase levels returned to vehicle control levels by day 28, at which point circulating α-Gal could no longer be detected.
Table 2.
Serum Transaminase Levels in Experimental Groups
| |
Alanine aminotransferase (ALT) |
Aspartate aminotransferase (AST) |
||||||
|---|---|---|---|---|---|---|---|---|
| Priming agent | Day 7 | Day 14 | Day 21 | Day 28 | Day 7 | Day 14 | Day 21 | Day 28 |
| Vehicle | 21.2 ± 9.6 | 24.0 ± 7.1 | 12.0 ± 2.4 | 19.8 ± 1.8 | 24.4 ± 6.1 | 34.2 ± 2.8 | 18.8 ± 6.5 | 28.2 ± 11.1 |
| Ad/CAP | 17.2 ± 2.2 | 25.8 ± 4.7 | 12.2 ± 1.6 | 21.2 ± 3.8 | 24.8 ± 5.5 | 34.0 ± 9.4 | 19.0 ± 5.8 | 37.6 ± 7.6 |
| Ad/αgal | 101.8 ± 21.5a | 88.6 ± 16.9a | 28.8 ± 6.4a | 25.8 ± 6.3 | 170.0 ± 41.9a | 143.4 ± 34.8a | 30.4 ± 14.5 | 44.8 ± 15.0 |
p ≤ 0.001 compared with vehicle group or Ad/CAP-primed group.
Discussion
AAV vectors have long been regarded as having little potential to induce a CTL response because they do not contain any viral genes. However, it has been suggested that injection of AAV2 vectors may actually result in the generation of de novo CTL responses elicited by the input particles or in the activation of a memory T cell response due to prior AAV exposure (Manno et al., 2006). In a clinical trial, patients treated with an AAV-factor IX vector were found to express factor IX only transiently and the decline in expression correlated with a rise in serum transaminase levels and the appearance of capsid-specific T cells. On the basis of these observations, it was hypothesized that the loss of expression was due to the induction of an AAV-specific CTL response leading to the destruction of transduced liver cells. In view of these observations, it becomes important to better understand the role that the host immune response plays in affecting transgene expression after the administration of AAV vectors. Although much attention has been focused on the possible involvement of AAV-specific CTLs, it is important to consider that host immune responses can be directed against not only the viral vector itself but also the transgene being expressed and can consist of both T and B cell components.
In this study, we have explored the relative contribution of B and T cell responses as well as the impact of responses directed against the AAV capsid and the transgene product on AAV-mediated expression. Our results confirm that neutralizing antibodies against AAV2 can prevent transduction and subsequent transgene expression by an AAV2/α-Gal vector. Analysis of CTL responses indicated that a CTL response against the α-Gal transgene product is capable of limiting transgene expression whereas CTLs against AAV capsid have little impact on the magnitude or duration of expression.
Adenoviral and plasmid expression systems were used to create immune responses against the Cap protein of AAV2. In immune-competent C57BL/6 mice primed with plasmid encoding Cap, both antibody and CTL responses developed against the AAV2 capsid protein and no transgene expression was detected, making it difficult to determine whether T cells, B cells, or a combination of the two was responsible for affecting AAV transduction and subsequent transgene expression. The presence of neutralizing antibodies to AAV has been previously reported to negatively influence AAV transduction, resulting in low levels of transgene expression (Scallan et al., 2008). To assess the role of Cap-specific CTLs in the absence of neutralizing antibodies, the experiment was repeated in B cell-deficient μMT mice, which developed a strong CTL response to the AAV capsid protein but no antibodies. Under these conditions, the Cap-specific CTL response in μMT mice failed to affect the strength or duration of transgene expression. This would suggest that in immunocompetent mice, Cap-specific CTLs did not affect transgene expression but that neutralizing antibodies effectively prevented transduction with AAV vector. These results are consistent with the reports of C. Li and colleagues and Wang and colleagues, who demonstrated that factor IX expression remains constant in the presence of capsid-specific CTLs. Therefore, although CTLs can be raised against the AAV Cap protein and may be detected in the host, they are unlikely to be the main contributing factor in the loss of transgene expression.
Comparatively little attention has been paid to the potential role of CTLs against the transgene product in limiting expression from AAV vectors. The use of AAV for gene therapy delivery of a protein that is either truncated or not expressed in a patient may result in the generation of a therapy-specific immune response. In these cases, T cell responses induced by the transgene may significantly impact the overall therapeutic benefit of the gene therapy being used. For proof-of-concept experiments, we have chosen to use the α-Gal transgene as a way to mimic the delivery of a therapeutic protein against which the patient is not immunologically tolerized. Our results demonstrate that transgene-specific CTLs have a direct effect on the level and duration of expression from an AAV vector. This was clearly demonstrated by experiments in which B cell-deficient μMT mice were preimmunized with Ad2/α-Gal vector to generate CTLs, but no antibodies, against α-Gal. When subsequently injected with AAV2/α-Gal vector, these mice exhibited a more than 2-log decrease in initial levels of transgene expression compared with unprimed mice or mice that were preimmunized with Ad5/Cap to generate CTLs to the capsid protein. Within 2 weeks, circulating levels of α-Gal protein were undetectable, indicating that transgene expression was severely compromised by the presence of a CTL response against the transgene product whereas Cap-specific CTLs had no impact. Similar to results observed in the hemophilia clinical trial, the decrease in circulating levels of therapeutic protein was accompanied by an elevation in serum transaminase levels.
It remains unclear why preformed CTLs against the transgene product are capable of terminating transgene expression whereas CTLs against Cap, when present at equivalent levels of lytic activity (as measured in vitro), have no measurable impact on transgene expression. One possible explanation may reside in the degree of antigen presentation required for sensitization of target cells to the lytic activity of specific CTLs. After AAV vector administration, there is abundant transgene expression that should result in robust presentation on the cell surface in association with MHC class I. However, the Cap protein is not expressed by the vector and transduced hepatocytes must process and present capsid protein from the initial input number of viral particles by a nonclassical method of MHC class I presentation. It is conceivable that the amount of processed capsid peptide presented on the cell surface is not sufficient to render the cells susceptible to lysis by capsid-specific CTLs. It has also been hypothesized that murine hepatocytes may process and present antigen less efficiently than human hepatocytes, explaining observed differences between mouse experiments and the hemophilia clinical trial described previously. On the basis of our results, α-Gal-specific CTLs appear capable of directly affecting transduced cells and abolish transgene expression, suggesting that the processing and presentation pathway is fully functional in transduced murine hepatocytes.
Results from our studies have potential implications for the clinical application of AAV-based gene therapy. Although preexisting CTLs against AAV appear unlikely to represent a significant impediment to transgene expression, the induction of CTLs against the transgene product potentially represents a hurdle in the clinic. For many genetic diseases such as hemophilia, the goal of gene therapy is to introduce a functional protein into patients who either do not express the protein, express a truncated version, or express nontherapeutic levels of the protein. During the course of T cell development, these patients may not become tolerized to the wild-type protein because of this alteration in or lack of expression. Therefore, the likelihood exists that T cell responses to these proteins may affect long-term expression from a gene therapy vector. In such instances, the therapeutic protein expressed by the vector may be recognized as a nonself antigen, resulting in the induction of a destructive CTL response. To circumvent the potential effect of CTL responses against the transgene product, it may be necessary to develop strategies to tolerize patients against the therapeutic protein before AAV vector administration or to establish transient immunosuppression regimens that can be delivered at the time of vector administration to minimize the generation of CTL responses.
Author Disclosure Statement
The authors are employees of Genzyme Corporation and own stock in the company.
References
- Alton E. Ferrari S. Griesenbach U. Progress and prospects: Gene therapy clinical trials (part 1) Gene Ther. 2007a;14:1439–1447. doi: 10.1038/sj.gt.3303001. [DOI] [PubMed] [Google Scholar]
- Alton E. Ferrari S. Griesenbach U. Progress and prospects: Gene therapy clinical trials (part 2) Gene Ther. 2007b;14:1555–1563. doi: 10.1038/sj.gt.3303001. [DOI] [PubMed] [Google Scholar]
- Ashley G.A. Shabbeer J. Yasuda M. Eng C.M. Desnick R.J. Fabry disease: Twenty novel α-galactosidase A mutations causing the classical phenotype. J. Hum. Genet. 2001;46:192–196. doi: 10.1007/s100380170088. [DOI] [PubMed] [Google Scholar]
- Barbon C.M. Ziegler R.J. Li C. Armentano D. Cherry M. Desnick R.J. Schuchman E.H. Cheng S.H. AAV8 mediated hepatic expression of acid sphingomeylinase corrects the metabolic defect in the visceral organs of a mouse model of Neimann-Pick disease. Mol. Ther. 2005;12:431–440. doi: 10.1016/j.ymthe.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Bessis N. Garcia Cozar F.J. Biossier M.-C. Immune responses to gene therapy vectors: Influence on vector function and effector mechanisms. Gene Ther. 2004;11:S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- Brockstedt D.G. Podsakoff G.M. Fong L. Kurtzman G. Mueller-Ruchholtz W. Engleman E.G. Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin. Immun. 1999;92:67–75. doi: 10.1006/clim.1999.4724. [DOI] [PubMed] [Google Scholar]
- Chao H. Walsh C.E. AAV vectors for hemophilia B gene therapy. Mt. Sinai J. Med. 2004;71:305–313. [PubMed] [Google Scholar]
- Chen J. Wu Q. Yang PA. Hsu H.-C. Mountz J.D. Determination of specific CD4 and CD8 T cell epitopes after AAV2 and AAV8-hF.IX gene therapy. Mol. Ther. 2006;13:260–269. doi: 10.1016/j.ymthe.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Desnick R.J. Enzyme replacement and enhancement therapies for lysosomal diseases. J. Inherit. Metab. Dis. 2004;27:385–410. doi: 10.1023/B:BOLI.0000031101.12838.c6. [DOI] [PubMed] [Google Scholar]
- Ge Y. Powell S. van Roey M. McArthur J.G. Factors influencing the development of an anti-factor IX (FIX) immune response following administration of adeno-associated virus-FIX. Blood. 2001;97:3733–3737. doi: 10.1182/blood.v97.12.3733. [DOI] [PubMed] [Google Scholar]
- Halbert C.L. Rutledge E.A. Allen J.M. Russell D.W. Miller D. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J. Virol. 2000;74:1524–1532. doi: 10.1128/jvi.74.3.1524-1532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jooss K. Chirmule N. Immunity to adenovirus and adeno-associated viral vectors: Implications for gene therapy. Gene Ther. 2003;10:955–963. doi: 10.1038/sj.gt.3302037. [DOI] [PubMed] [Google Scholar]
- Kitamura D. Roes J. Kuhn R. Rajewsky K. A B-cell deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Li C. Hirsch M. Asokan A. Zeithaml B. Ma H. Kafri T. Samulski J. Adeno-associated virus type 2 (AAV2) Capsid-specific cytotoxic T lymphocytes eliminate only vector-transduced cells coexpressing the AAV2 capsid in vivo. J. Virol. 2007;81:7540–7547. doi: 10.1128/JVI.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Murphy S.L. Giles-Davis W. Edmonson S. Xiang Z. Li Y. Lasaro M.O. High K.A. Ertl H.C. Pre-existing AAV capsid-specific CD8+ T cells are unable to eliminate AAV-transduced hepatocytes. Mol. Ther. 2007;4:792–800. doi: 10.1038/sj.mt.6300090. [DOI] [PubMed] [Google Scholar]
- Manno C.S. Arruda V.R. Pierce G.F. Glader B. Ragni M. Rasko J. Ozelo M.C. Hoots K. Blatt P. Konkele B. Dake M. Kaye R. Razavi M. Zajko A. Zehnder J. Nakai H. Chew A. Leonard D. Wright J.F. Lessard R.R. Sommer J.M. Tigges M. Sabatino D. Luk A. Jiang H. Mingozzi F. Couto L. Ertl H.C. High K.A. Kay M.A. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- McEachern K.A. Nietupski J.B. Chuang W.L. Armentano D. Johnson J. Hutto E. Grabowski G.A. Cheng S.H. Marshall J. AAV8-mediated expression of glucocerebrosidase ameliorates the storage pathology in the visceral organs of a mouse model of Gaucher disease. J. Gene Med. 2006;8:719–729. doi: 10.1002/jgm.901. [DOI] [PubMed] [Google Scholar]
- Meyer M. Wagner E. Recent developments in the application of plasmid DNA-based vectors and small interfering RNA therapeutics for cancer. Hum. Gene Ther. 2006;17:1062–1076. doi: 10.1089/hum.2006.17.1062. [DOI] [PubMed] [Google Scholar]
- Murphy S.L. Li H. Zhou S. Schlachterman A. High K. Prolonged susceptibility to antibody-mediated neutralization for adeno-associated vectors targeted to the liver. Mol. Ther. 2008;16:138–145. doi: 10.1038/sj.mt.6300334. [DOI] [PubMed] [Google Scholar]
- O'Riordan C.R. Lachapelle A.L. Vincent K.A. Wadsworth S.C. Scaleable chromatographic purification process for recombinant adeno-associated virus (rAAV) J. Gene Med. 2000;2:444–454. doi: 10.1002/1521-2254(200011/12)2:6<444::AID-JGM132>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Perricone M.A. Smith K.A. Claussen K.A. Plog M.S. Hempel D.M. Roberts B.L. St. George J.A. Kaplan J.K. Enhanced efficacy of melanoma vaccines in the absence of B lymphocytes. J. Immunother. 2004;4:273–281. doi: 10.1097/00002371-200407000-00003. [DOI] [PubMed] [Google Scholar]
- Sabatino D.E. Mingozzi F. Hui D.J. Chen H. Colosi P. Ertl H.C.J. High K.A. Identification of mouse AAV capsid-specific CD8+ T cell epitopes. Mol. Ther. 2005;6:1023–1033. doi: 10.1016/j.ymthe.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Scallan C.D. Jiang H. Liu T. Patarroyo-White S. Sommer J.M. Zhou S. Couto L.B. Pierce G.F. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- Sun J.Y. Chatterjee S. Wong K.K. Immunogenic issues concerning recombinant adeno-associated virus vectors for gene therapy. Curr. Gene Ther. 2002;2:485–500. doi: 10.2174/1566523023347616. [DOI] [PubMed] [Google Scholar]
- Sun J.Y. Anand-Jawa V. Chatterjee S. Wong K.K. Immune responses to adeno-associated virus and its recombinant vectors. Gene Ther. 2003;10:964–976. doi: 10.1038/sj.gt.3302039. [DOI] [PubMed] [Google Scholar]
- Wang L. Figueredo J. Calcedo R. Lin J. Wilson J.M. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum. Gene Ther. 2007;18:185–194. doi: 10.1089/hum.2007.001. [DOI] [PubMed] [Google Scholar]
- Whiteside T.L. Zhao Y. Tsukishiro T. Elder E.M. Gooding W. Baar J. Enzyme-linked immunospot, cytokine flow cytometry, and tetramers in the detection of T-cell responses to a dendritic cell-based multipeptide vaccine in patients with melanoma. Clin. Cancer Res. 2003;9:641–649. [PubMed] [Google Scholar]
- Wu Z. Asokan A. Samulski R.J. Adeno-associated virus serotypes: Vector toolkit for human gene therapy. Mol. Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Yang Y. Nunes F.A. Berencsi K. Furth E.E. Gönczöl E. Wilson J.M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4407–4441. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler R.J. Lonning S.M. Armentano D. Li C. Souza D.W. Cherry M. Ford C. Barbon C.M. Desnick R.J. Gao G. Wilson J.M. Peluso R. Godwin S. Carter B.J. Gregory R.J. Wadsworth S.C. Cheng S.H. AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of α-galactosidase A and the induction of immune tolerance in Fabry mice. Mol. Ther. 2004;9:231–240. doi: 10.1016/j.ymthe.2003.11.015. [DOI] [PubMed] [Google Scholar]