Abstract
We injected lentiviral vectors into the eyes of live nonhuman primates to assess potential for glaucoma gene therapy. Anterior chambers of five cynomolgus monkeys were injected with green fluorescent protein (GFP)-encoding feline immunodeficiency viral vectors. The monkeys were monitored for in vivo transgene expression and clinical parameters. Their eyes were harvested 2–15 months postinjection for tissue analyses. All seven eyes injected with 1.0–2.0 × 108 transducing units (TU) showed substantial GFP fluorescence in the trabecular meshwork (TM), which was observable even by goniophotographic monitoring for up to 15 months. Only the lowest dose (0.03 × 108 TU) failed to result in TM fluorescence detectable in vivo, and five of the eight vector-injected eyes continued to display substantial GFP expression when enucleated eyes were examined at 2, 7, or 15 months postinjection. Some transduced cells were also detected in the iris and ciliary body. Mild, transient postinjection inflammatory responses exceeding that induced by a control saline injection were observed, but vectors did not raise intraocular pressure and were well tolerated. The results demonstrate the first lentiviral vector transduction of the nonhuman primate aqueous humor outflow pathway and support application of the system to human glaucoma gene therapy.
Introduction
Primary open angle glaucoma (POAG), the most common form of glaucoma, is a leading cause of irreversible blindness (Quigley and Broman, 2006). Increased intraocular pressure (IOP) is the major risk factor for POAG development and progression, and its reduction is the primary treatment modality (Boland and Quigley, 2007). Chronic IOP elevation results from impeded outflow of aqueous humor. This fluid is produced continuously by the ciliary body and exits the eye from the anterior chamber, primarily via the trabecular outflow tract (trabecular meshwork or TM) and to a lesser extent through a pressure-independent uveoscleral pathway. The TM generates much of the resistance to normotensive aqueous outflow (Grant, 1963; Rohen, 1983). The normal result is maintenance of IOP in a narrow range (10–21 mmHg), which is essential for optical clarity and viability of the intraocular tissues.
Most small-molecule glaucoma therapeutics exert their effects in the TM or uveoscleral outflow tracts, or ciliary body tissues (Weinreb et al., 2002). Prostaglandin analogs enhance outflow primarily via the uveoscleral pathway. The trabecular pathway can be targeted indirectly via increased ciliary muscle tone with cholinergic agents and directly with β-adrenergic agonists, but the latter two are now rarely used because of local and systemic side effects. Aqueous humor formation can be inhibited with β-adrenergic antagonists, carbonic anhydrase inhibitors, and α2-adrenergic agonists.
Numerous features of POAG have interested researchers in the prospect of gene therapy as a long-term treatment (Borras et al., 2002; Martin and Quigley, 2004; Ferrer, 2006). Disease outcomes can be severely disabling, risk is chronic (lifelong), and current therapies are unable to prevent progression in all patients. Current glaucoma treatment strategies have drawbacks. Pharmacologic therapies require that patients self-administer topical medications that may have side effects for a condition that is often asymptomatic until late in its course, often contributing to poor therapeutic adherence (Hattenhauer et al., 1998; Schwartz, 2006). Surgical treatments may initially bypass compliance issues but carry risks such as infection, cataracts, and hypotony and many surgical patients eventually must resume topical therapy.
In addition, translational feasibility of gene therapy is favored by the confined anatomy, low number of target cells (4–8 × 105 TM cells in the human eye; Grierson and Howes, 1987), and relative immunoprivilege of the anterior chamber (AC). Although a number of genes have been associated with less common forms of glaucoma (Stone et al., 1997; Rezaie et al., 2002), these findings have not yet been translated into workable gene therapy strategies for POAG. However, various candidate transgene systems are in development to target the regulatory processes of the aqueous outflow pathway tissues.
Any gene therapy approach to glaucoma must address the chronicity of this disease and the mitotically quiescent state of TM cells. Lentiviral vectors possess promise for this and other ocular applications because they integrate permanently into target cell DNA and, unlike gammaretroviral vectors, do so in terminally differentiated, postmitotic cells (Saenz and Poeschla, 2004). Several lentiviruses have been engineered into effective lentiviral vectors (Naldini et al., 1996; Olsen, 1998; Poeschla et al., 1998; Mitrophanous et al., 1999) and the three main types (human immunodeficiency virus type 1 [HIV-1], feline immunodeficiency virus [FIV], and equine infectious anemia virus [EIAV]) have been studied in the AC, using various models. HIV-1 and FIV vectors were effective in short-term ex vivo studies in cultured human donor eyes (Loewen et al., 2001, 2002). EIAV vectors were shown to transduce mouse TM (Balaggan et al., 2006). The most extensive prior animal work was carried out in a domestic cat model, in which FIV vectors produced sustained transgene expression for more than 2 years, with expression largely localized to the TM (Loewen et al., 2004a; Khare et al., 2007). Although the feline AC is much closer to human size, structure, and physiology than that of rodents, the extent to which any gene therapy vector is efficacious and tolerated can vary greatly with the transition from lower mammals to primates. Here, we assess the efficacy and tolerability of FIV vectors in the primate AC.
Materials and Methods
FIV vectors
The FIV-based lentiviral vector system employed in these monkey experiments has been iteratively modified since its inception (Poeschla et al., 1998). As reviewed elsewhere (Loewen and Poeschla, 2005; Saenz et al., 2006; Barraza and Poeschla, 2008), these modifications have minimized viral sequences, improved separation of cis- and trans-acting functions, and enhanced efficiency of gene transfer. Vector preparations were produced as described, using packaging construct pFP93 and transfer vector pGINSIN (Loewen et al., 2003a,b, 2004a,b; Saenz et al., 2006; Khare et al., 2007). The green fluorescent protein (GFP) allele is the enhanced (eGFP) version. Vectors were pseudotyped with vesicular stomatitis virus glycoprotein G (VSV-G). Briefly, pFP93 (253.5 μg), transfer vector pGINSIN (253.5 μg), and VSV-G expression plasmid pMD.G (84.5 μg) were transiently transfected into 293T cells in 1-liter culture devices (Cell Factories; Nunc, Naperville, IL) by the calcium phosphate coprecipitation method. Medium was changed 12 hr later and supernatant was harvested 48 hr after replacement. Particles were concentrated by double ultracentrifugation and resuspended in phosphate-buffered saline (PBS). Titers were determined by flow cytometric analysis of GFP expression in transduced Crandell feline kidney (CrFK) cells as described (Saenz et al., 2006) and GFP-transducing capacity of banked vector was reverified before monkey injection.
Animals and lentiviral vector delivery
Animals were handled in accordance with the Institutional Animal Care and Use Committee and the Association for Research in Vision and Ophthalmology (ARVO, Rockville, MD) Statement for the Use of Animals in Ophthalmic and Visual Research (see http://www.arvo.org/eweb/dynamicpage.aspx?site=arvo2&webcode=AnimalsResearch; accessed January 2009). Experiments were conducted in five ocular normotensive adult cynomolgus monkeys (Macaca fascicularis), of either sex, 6 to 16 years of age, weighing 4.2 to 8.7 kg. Animals had been housed and used for routine intraocular pressure (IOP) and slit-lamp examinations for several years before this study. All animals were viral vector naive. Each had undergone at least one to three AC perfusions to determine outflow facility in the 1–4 years before the study; some received facility-influencing small-molecule drugs as well at that time but none received any viral vector. One eye (627, left eye [OS]) had received two AC perfusions and one intravitreal injection before initiation of this study. The one eye (591 OS) that had received TM laser treatment (and later vitrectomy) was not injected in this study. Before vector administration, monkeys were anesthetized (ketamine-HCl, 10 mg/kg, supplemented with ketamine-HCl at 5 mg/kg) for ocular examination and IOP measurements with a miniaturized Goldmann tonometer (Kaufman and Davis, 1980). On the day of vector injection, monkeys were again anesthetized with the same ketamine regimen plus medetomidine (15–75 μg/kg) and a 20- to 30-μl bolus of vector preparation was injected transcorneally into the AC. Vector solutions were injected with a 30-gauge needle connected to a micrometer syringe with polyethylene tubing. Under microscopic visualization, the needle was threaded through the cornea for several millimeters, and then the tip was angled into the anterior chamber. The needle was left in place for 2 min after injection of the solutions to allow for hysteresis due to the tensile properties of the tubing to subside. No fluid leakage was observed during needle withdrawal. Monkeys were regularly examined with a slit-lamp biomicroscope for corneal and lenticular clarity and for evidence of AC and vitreal cavity cells and flare. Eight eyes in total from the five monkeys were employed and injected with 0.03, 0.3, or 1.0 transducing units (TU) (four eyes), or with 2.0 × 108 TU (two eyes). One eye was injected with saline, and one eye was not injected. One animal was reinjected at 4 months, and another at 5 months (Table 1).
Table 1.
Dose Assignments, Timing, and Intraocular Pressure Changes in Cynomolgus Monkeys
| |
|
|
Reinjected |
|
IOP (baseline and changes, mmHg) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal | Eye | Dose (108TU) | Date | Dose | Time of sacrifice | Baseline | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Sac |
| 591 | OD | 1.0 | 15 | 16 | −5 | −2 | −1 | −1 | −2 | |||
| OS | No inj. | |||||||||||
| 627 | OD | 1.0 | 2 | 19 | −4 | |||||||
| OS | 2.0 | 19.5 | −10 | |||||||||
| 654 | OD | 2.0 | 7 | 15 | −12 | −3 | −1 | −2 | −1 | |||
| OS | 1.0 | 15 | −11 | −2 | −1 | −3 | −2 | |||||
| 666 | OD | 1.0 | 5 months | 1.0 | 7 | 13 | −10 | −1 | 0 | −1 | −1 | |
| OS | Saline | 5 months | Saline | 14 | ||||||||
| 682 | OD | 0.03 | 4 months | 1.0 | 9 | 18 | −2 | −4 | −2 | 0 | 0 | |
| OS | 0.3 | 19 | −11 | −8 | −1 | 0 | 2 | |||||
Abbreviations: inj., injection; IOP, intraocular pressure; OD, right eye; OS, left eye; Sac, IOP at sacrifice.
Noninvasive goniophotographic monitoring of transgene expression in vivo
GFP expression was monitored serially and noninvasively as previously described (Borras et al., 2001). Briefly, after installation of 0.5% proparacaine hydrochloride, a Swan-Jacob gonioscopy lens was placed on the corneal surface of anesthetized, supine-positioned monkeys to view the iridocorneal angle and TM tissue. A Topcon 50EX retinal camera (Latham and Phillips Ophthalmic Products [LPO], Grove City, OH) fitted with a Nikon D1x digital single-lens reflex (SLR) camera body (Nikon Instruments, Melville, NY) was positioned to photograph the anterior chamber angle. Standard clinical fluorescein exciter and barrier filters were used. The same Nikon camera body was also mounted to a slit-lamp biomicroscope and photos were taken with a three-mirrored gonioscopy lens with a 10-mm contact diameter (OG3M-10; Ocular Instruments, Bellevue, WA) applied to the eye without coupling fluid. A customized Nikon microscope (Nikon Instruments) with a 12-bit, monochromatic, cooled-CCD (charge-coupled device) camera (Retiga-2000RV; QImaging, Surrey, BC, Canada) and a specific GFP filter set was also used to collect images at one time point.
Outflow facility measurements
Outflow facility was determined by two-level constant pressure perfusion (∼15 and 25 mmHg) of the anterior chamber with Bárány's solution, correcting for internal apparatus resistance, as described previously (Bárány, 1964). Briefly, the anterior chamber of the monkey was cannulated with a branched needle, with one branch connected to an external reservoir by tubing (inflow line) and the other to a pressure transducer. During perfusion, Bárány's solution flowed from the reservoir into the eye through the inflow line, and the volume of fluid flow was determined with a strain gauge connected to the reservoir. Outflow facility was calculated as described previously (Tian and Kaufman, 2005).
Enucleation, tissue processing, histology, and immunofluorescence
Animals were killed with intravenous Euthasol (1 ml/4.5 kg; each milliliter contains 390 mg of pentobarbital and 50 mg of phenytoin). Eyes were enucleated, placed in a small amount of saline packed in ice, transported from the University of Wisconsin (Madison, WI) to the Mayo Clinic College of Medicine (Rochester, MN), and dissected within 5 hr of enucleation. Eyes were bisected and AC hemispheres were segmented into six wedges per eye. Three wedges were flat-mounted and imaged under fluorescence light microscopy. Gene expression levels were graded from 0 to 4 (Loewen et al., 2004a; Khare et al., 2007). Remaining wedges were used for histology and immunofluorescence. Anterior segment tissues including cornea, TM, iris, and ciliary body were fixed in 4% paraformaldehyde, infiltrated with paraffin, and cut in 6-μm sections. Some tissue samples were stained with hematoxylin and eosin (H&E). Other tissue samples were deparaffinated and antigen retrieval was enhanced by boiling in 0.05% citraconic anhydride (pH 7.4). Rabbit anti-GFP (NB 600-303, diluted 1:500; Novus Biologicals, Littleton, CO) and Alexa Fluor 594-conjugated goat anti-rabbit IgG (A11037, diluted 1:100; Invitrogen, Carlsbad, CA) were used as primary and secondary antibodies, respectively. Specimens were photographed with a digital camera mounted on a BX60F5 light-field/fluorescence microscope (Olympus America, Melville, NY). H&E-stained tissue section slides from all injected and control eyes were placed in random order and then analyzed and scored by an ophthalmic pathologist (J.D.C.) masked to all prior history and injection assignments.
Results
Clinical findings
The primary end points of this study were extent and duration of transgene expression in primate eyes injected with an FIV-based lentiviral vector. We also monitored IOP, clinical tolerance, and AC inflammation. All animals were viral vector naive. We used a GFP-encoding vector at doses (Table 1) comparable to those in previous studies in domestic cats (Loewen et al., 2004a; Khare et al., 2007). The vector was well tolerated in monkeys. Most eyes demonstrated mild inflammation 1 week postinjection, characterized by low levels of cells (generally 1 + /4, occasionally 2 + /4) and flare (1 + /4) in the AC. This generally had completely resolved by 3–4 weeks postinjection, with a mean recovery time to trace cells/flare of 16.67 ± 3.1 days (range, 11–28) and a mean time to complete AC clarity of 27.3 ± 4.9 days (range, 6–50). Of note, injection of saline alone also triggered AC inflammation, with recovery to trace cells achieved on day 4 and clarity on day 12 (monkey 666, OS). In the two monkeys who were reinjected with 1.0 × 108 TU at 4 and 5 months, respectively (monkeys 666 and 682), recovery times to trace cells/flare were similar (7 and 27 days), but full AC clarity was delayed longer than after the first injection (50 and 61 days). IOP, which was also measured weekly, initially decreased in vector-injected eyes averaging ∼8 mmHg (∼50%), but returned to baseline levels by 3 weeks (Table 1).
Gene expression assessed in vivo
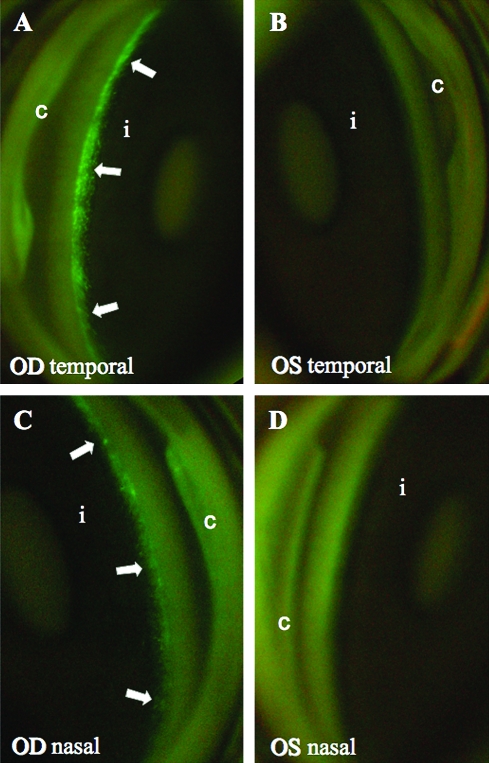
Gene expression was evaluated in two main ways: standard tissue examinations postmortem and, during life, by serial slit-lamp examinations and goniophotography under white and fluorescent light sources. Although the noninvasive gonioscopic method naturally has lower sensitivity than fluorescence microscopy of enucleated eye tissue, GFP expression was nevertheless ample and readily visualized in vivo as a green fluorescent ring in the location corresponding to the TM (Fig. 1 and Table 2). Although predominantly seen in the TM, GFP expression was also observed in the iris and anterior ciliary body/uveal meshwork (Fig. 2). No fluorescence was observed in control eyes (Fig. 1 and Table 2).
FIG. 1.
GFP expression in cynomolgus monkey anterior segment. Images were taken with a fundus camera and Swan-Jacob gonioprism. (A and B) Temporal quadrant; (C and D) nasal quadrant. Arrows indicate fluorescence in the region of the TM in animal 666, 12 days after injection. i, iris; c, cornea. The right eye (OD) received 1 × 108 TU of vector and the left eye (OS) received saline. Slit-lamp examination at this time showed rare cells, no flare. Photomicrograph in (A) is reproduced with permission from Liu and coworkers (2007).
Table 2.
Transgene Expression Assessed in Vivo and in Enucleated Eyes
| |
|
|
In vivo (0–4) |
Postmortem (0–4) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Animal | Eye | Dose (108TU) | Week 1 | Week 2 | Week 4 | Week 6 | At sacrifice | Montha | Gradeb |
| 591 | OD | 1.0 | 3.0 | 2.5 | 2.0 | 2.0 | 1.0 | 15 | 3.0 |
| OS | No inj. | 0 | 0 | 0 | 0 | 0 | 15 | 0 | |
| 627 | OD | 1.0 | 2.0 | 0 | 0 | 0 | 0 | 2 | 2.0 |
| OS | 2.0 | 2.0 | 0 | 0 | 0 | 0 | 2 | 1.0 | |
| 654 | OD | 2.0 | 3.0 | 3.0 | 2.5 | 2.0 | 2.0 | 7 | 3.0 |
| OS | 1.0 | 3.0 | 3.5 | 3.0 | 2.0 | 1.0 | 7 | 3.0 | |
| 666 | OD | 1.0 | 3.0 | 3.0 | 3.0 | 0c | 0 | 7 | 0 |
| OS | Saline | 0 | 0 | 0 | 0 | 0 | 7 | 0 | |
| 682 | OD | 0.03 | 0 | 0 | 0 | 0 | 0 | 9 | 0 |
| OS | 0.3 | 2.0 | 1.0 | 1.0 | 1.0 | 0 | 9 | 0 | |
Time of animal sacrifice after vector injection.
Grades assessed from 0–4 by direct UV microscopy of TM flat mount at necropsy.
Gonioscopic detection lost after perfusion experiment.
FIG. 2.
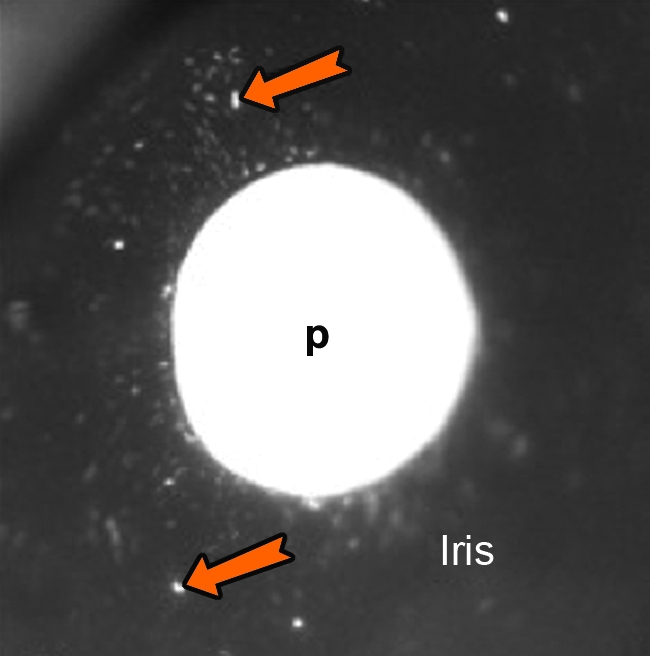
Transduced cells in the iris. Image taken with microscope system, 392 days postinjection. Orange arrows indicate areas of fluorescence. p, pupil.
At 1 week postinjection, all but one FIV vector-injected eye demonstrated gonioscopically visible GFP expression in the trabecular band (Table 2). GFP expression was graded from 0 to 4, using the same scale as in previous feline studies (Loewen et al., 2004a). Grade 0 was defined as no visible fluorescence, grade 1 as single fluorescent spots, grade 2 as numerous nonconfluent fluorescent spots with some confluent areas, grade 3 as extensive, mostly confluent, mid-level transduction, and grade 4 as extensive, high-level, and completely confluent fluorescence. All six eyes receiving 1–2 × 108 TU were graded as 2+ or 3+ initially (Table 2). Only the monkey receiving 100-fold less (i.e., 3.0 × 106 TU, the lowest dose in the study) demonstrated no detectable GFP expression in vivo. The eye receiving 0.3 × 108 TU was graded 2+. Visible expression grade peaked between 1 and 2 weeks (Table 2 and Fig. 3).
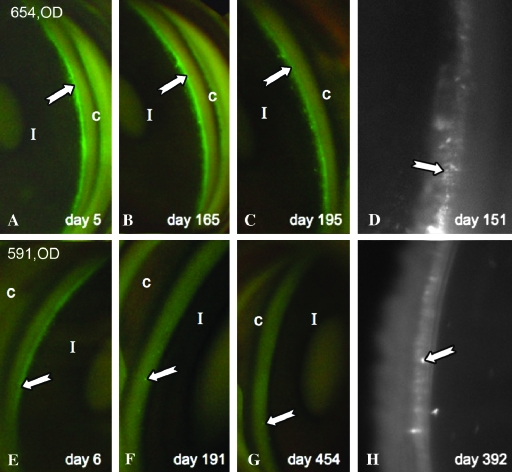
FIG. 3.
Noninvasively detectable expression over time. (A–C) and (E–G) were taken with a fundus camera. (D) and (H) were taken with a customized Nikon microscope system with GFP-specific filters and higher magnification. All photos were taken with a Swan-Jacob gonioprism. Arrows indicate fluorescence.
Five eyes had stable, long-term expression (Table 2). In three, this could be detected in vivo goniophotographically until sacrifice, that is, for durations from 214 days (654, OS and OD) to 455 days (591, OD). Expression was no longer visible noninvasively in some eyes at various time points, but the relative insensitivity of gonioscopic detection was also apparent because loss of in vivo expression occurred in eyes where postmortem detection revealed sustained GFP at much later time points (e.g., animal 627; Table 2). One eye (666, OD) lost expression subsequent to a perfusion outflow facility experiment on day 41.
Outflow facility
Three eyes from two animals underwent perfusion outflow facility measurements (Table 3). Monkey 666 had baseline measurements taken 58 days preinjection and subsequent measurements on days 41 and 108 postinjection. Facility in the vector-injected eye decreased by 52 and 48% whereas the saline-injected eye increased by 52 and 23%, relative to baseline, on days 41 and 108, respectively. Monkey 654 had baseline measurements taken 58 days preinjection and a subsequent measurement in the eye that received 2 × 108 TU on day 159 postinjection. Facility in the vector-injected eye decreased by 19%, relative to baseline. In addition, the vector-injected eye of monkey 666 had a longer period of inflammation postperfusion than the control eye and expression was no longer visible. Monkey 654 recovered normally and retained visible transgene expression.
Table 3.
Outflow Facility
| Animal | Days | Eye | Dose (TU) | Outflow facility (μl/min/mmHg) | Change in outflow facility from baseline | Slit-lamp biomicroscopy postperfusion | |
|---|---|---|---|---|---|---|---|
| 654 | Baseline | OD | 0.425 | ||||
| 58 days pre | OS | 0.587 | |||||
| Day 159 post | OD | 2.00 × 108 | 0.345 | 19% decrease | Clear day 6 | ||
| 666 | Baseline | OD | 0.493 | ||||
| 58 days pre | OS | 0.553 | |||||
| Day 41 post | OD | 1.00 × 108 | 0.237 | 52% decrease | Trace cells day 14 | Clear day 26 | |
| Day 41 post | OS | Saline | 0.847 | 53% increase | Clear day 6 | ||
| Day 108 post | OD | 1.00 × 108 | 0.256 | 48% decrease | Rare cell day 9, 1+ cells day 29 | Clear day 50 | |
| Day 108 post | OS | Saline | 0.681 | 23% increase | Rare cell day 9 | Clear day 29 | |
Transgene expression assessed directly in enucleated eye tissue
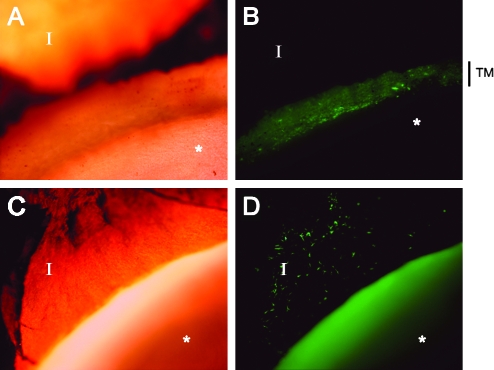
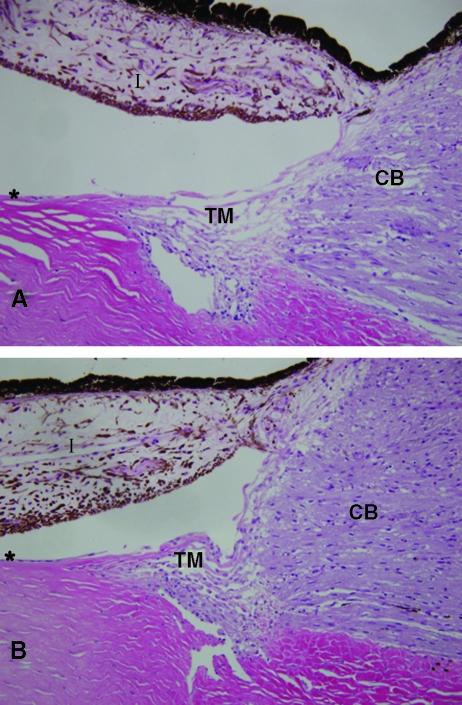
Anterior segments were isolated postmortem (2–15 months postinjection) and dissected into six wedges per eye. Three wedges were imaged as unfixed flat mounts for GFP expression (Fig. 4 and Table 2) and three were fixed for sectioning and slide mounting (Figs. 5 and 6). Animals injected with 1–2 × 108 TU demonstrated high-grade, persistent transgene expression in the TM and to a lesser extent in the iris and ciliary body. Trabecular meshwork anatomies and cellularity were normal. Mild chronic iridocyclitis was noted in the left eye of animal 627 (2.0 × 108 TU). Histologically, the right eye of animal 654, which also received 2.0 × 108 TU, appeared completely normal. It is unclear whether the observed iridocyclitis in animal 627 was related to the vector injection because this was the one eye that had received an intravitreal injection before this study. A mild lymphoplasmacytic infiltrate was observed in only one section of the right eye of animal 666. Other sections of this eye appeared completely normal. Anterior chamber tissue of all other eyes appeared anatomically normal and no signs of acute or ongoing inflammation were observed (Fig. 6).
FIG. 4.
GFP expression in cynomolgus TM and iris immediately postmortem. (A and B) TM, outflow tract (original magnification, ×40; animal 654, OD). (C and D) Iris. Anterior chamber (AC) wedge with iris folded back to expose anterior surface (original magnification, ×40; animal 654, OS). The homogeneously green area in the foreground in (D) is autofluorescence. TM, trabecular meshwork; I, iris; asterisk (*), corneal endothelium.
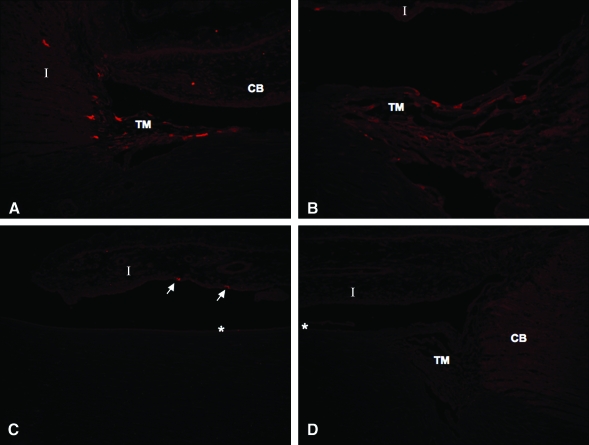
FIG. 5.
Immunofluorescence labeling of AC tissue for GFP in eyes transduced with 1 × 108 TU or more. (A) GFP expression was observed in the outflow tract and, to a lesser extent, in the ciliary body (original magnification, ×40; animal 654, OD). (B) Trabecular outflow tract tissue (original magnification, ×200; animal 654, OD). (C) Labeled cells detected on anterior surface of iris (arrows) (animal 654, OD). (D) Labeling control (saline-injected eye; animal 666, OS). TM, trabecular meshwork; CB, ciliary body; I, iris; asterisk (*), corneal endothelium.
FIG. 6.
Histology. H&E-stained AC tissues are shown. (A) Eye injected with 2 × 108 TU of vector (animal 654, OD). (B) Eye injected with saline (animal 666, OS). Note normal anterior segment structure and absence of inflammatory infiltrates in both eyes. TM, trabecular meshwork; CB, ciliary body; I, iris; asterisk (*), corneal endothelium.
Discussion
Anterior chamber-focused gene therapy approaches to glaucoma may require long-term transgene expression in outflow tract tissues. Here we have shown that this can be achieved in a nonhuman primate with a single transcorneal injection of an FIV vector. Substantial transgene expression was observed, primarily in the TM but also in some neighboring cells. This was achievable at relatively low vector doses (i.e., a total of 108 TU), and for a sustained duration (i.e., at least 455 days). Thus, in addition to gene delivery efficacy these results also demonstrate a scale of vector production and use per eye that enhances the feasibility of clinical translation.
The TM was highly favored for transduction, although GFP-expressing cells could also be seen in the iris and ciliary body. The postmortem flat mounts and the individual transduced cells detected by immunofluorescence in 6-μm sections of TM correlate well with the in vivo imaging of the whole tissue by goniophotography. The normal flow of aqueous likely carries most of the vector to the TM after transcorneal injection and the vectors may also exhibit cell-specific preferential transduction that favors the TM (Loewen et al., 2004a). This targeted transduction opens the door to delivery of therapeutic transgenes that can regulate aqueous humor outflow. On the basis of cell and organ culture and live animal data, the structures and biochemical/enzymatic pathways involved in cellular and tissue contractility/relaxation, cell shape maintenance, cell–cell and cell–extracellular matrix (ECM) interactions (Honjo et al., 2001; Rao et al., 2001; Carr and Noisakran, 2002; Tian and Kaufman, 2005; Gabelt et al., 2006; Liu et al., 2007), and the Wnt signaling pathway (Wang et al., 2008) are examples of potential TM targets. Other gene therapy strategies with potential to lower IOP by targeting the TM or neighboring tissue include regulating matrix proteins in both conventional and uveoscleral pathways and expressing prostaglandin biosynthesis and response pathway components. Transfer of matrix metalloproteinases to the TM of rat and human donor eyes with an adenoviral vector has been demonstrated (Kee et al., 2001).
The issue of species-specific restriction of retroviruses deserves brief comment. By extending our prior FIV vectors studies from cats to the cynomolgus monkey, an Old World primate, the present work indicates that there are no significant intrinsic immunity barriers to experimental use of these vectors in the nonhuman primate AC. As discussed below, human gene therapy use is even less likely to be impaired by postentry restriction. Like HIV-1 vectors (Stremlau et al., 2004), FIV vectors are known to be restricted postentry in some cultured Old World (rhesus macaque) monkey cells by the restriction factor tripartite motif (TRIM)-5α (Saenz et al., 2005; Diaz-Griffero et al., 2007). In a number of New World (owl monkey) and Old World (rhesus and pigtail macaque) monkey cells, FIV is also restricted by related TRIMcyp proteins, in which the capsid-binding element is cyclophilin A (Diaz-Griffero et al., 2006, 2007; Lin and Emerman, 2006; Virgen et al., 2008; Wilson et al., 2008). The effects of cynomolgus TRIM proteins on FIV have not been determined. Nevertheless, it is probable that cynomolgus macaque TRIMcyp does restrict FIV because the closely related TRIMcyp proteins of pigtail and rhesus macaques do so (Virgen et al., 2008; Wilson et al., 2008). Comparative expression levels in various body tissues are not established for any of these proteins in any species, but they are believed to restrict retroviruses at low intracellular levels.
Even if TRIM protein restriction of FIV can occur in cynomolgus monkey AC cells, we likely easily overcame it in this ocular gene therapy setting by focal deposition of vector particles, which readily saturates target cell TRIM protein restriction (Hatziioannou et al., 2003), as previously discussed for FIV vectors (Loewen and Poeschla, 2005; Saenz et al., 2005). Furthermore, there is no evidence of a TRIMcyp protein in humans. Of most relevance to human gene therapy, human TRIM-5α displays substantially weaker postentry restricting activity against FIV than does Old World monkey TRIM5-α (Saenz et al., 2005; Diaz-Griffero et al., 2007). Consistent with this, we have also seen good FIV vector efficacy in short-term cultures of human donor eyes (Loewen et al., 2001, 2002). Considering our present results in the cynomolgus macaque along with the human donor eye data, we suggest that postentry restriction by human TRIM-5α is unlikely to present any impediment to the use of FIV vectors in AC-focused human gene therapy. Moreover, in common gene therapy settings, in which vector is focally deposited, the existence of even mild, locally saturable restriction by human TRIM-5α might be a net advantage, providing a potential block to systemic propagation of any replication-competent retrovirus species that have theoretical potential to arise after gene therapy.
We conclude that an easily achievable dose of FIV vector results in sustained transgene expression in the primate eye. All but the lowest dose eye (0.03 × 108 CrFK TU) had goniophotographically visible expression after injection. Three eyes retained in vivo expression at all in vivo and postmortem examinations, two lost in vivo expression but had expression visible postmortem by direct ultraviolet microscopy of TM flat mounts, and two lost all evidence of transgene expression. In the six eyes receiving doses: 1 × 108 TU, only in one eye (666, OD) did goniophotographic expression appear to be lost in vivo and remain so postmortem, and the in vivo loss was directly subsequent to an invasive perfusion experiment. In the other eyes, goniophotographic expression persisted in vivo or was found postmortem. Note that actual outflow tract cell-transducing units are without doubt much lower than 108 because the input vector transducing unit values are determined with CrFK cells, a highly transducible fibroblast cell line.
Long-term, H&E-stained sections of transduced AC tissue generally appeared histologically normal with no vector-attributable alteration of AC tissue when comparing saline-injected with vector-injected eyes. In general, then, the vectors were well tolerated and inflammatory responses were short-lived and mild. The transient inflammatory signs were accompanied by IOP reductions, all of which subsided permanently by 4 weeks postinjection. The two attempts to re-treat previously injected eyes were unsuccessful, possibly because of recall immune responses. It may be of interest to investigate whether short-term topical or systemic antiinflammatory treatments are beneficial in this regard. In addition to such immunosuppressive measures around the time of vector administration, there are potential ways to forestall such immune responses entirely with vector-incorporated strategies such as tissue-specific microRNA target sequences (Brown et al., 2006, 2007).
Both the cat and the cynomolgus (or rhesus) monkey models should be useful for future studies. The efficacy of glaucoma therapeutics has historically been well predicted using these two normotensive animal models, which provide good simulations of human ocular outflow anatomy and physiology (Zhan et al., 1992; Sagara et al., 1999; Wang et al., 1999, 2007; Stjernschantz, 2001; Gabelt et al., 2003; Rasmussen and Kaufman, 2005; Toris et al., 2006). Although challenges remain, especially the identification of a therapeutic transgene(s), the potential clinical benefits of gene therapy for glaucoma are appealing.
Acknowledgments
The authors thank John Peterson for assistance with goniophotography. This work was supported by RO1 EY14411 and R01 AI47536 to E.M.P.; RO1 EY002698 and RPB, OPREF to P.L.K., as well as by P30 EY016665 (Department of Ophthalmology and Visual Sciences, UWSMPH).
Author Disclosure Statement
For all authors, no competing financial interests exist.
References
- Balaggan K.S. Binley K. Esapa M. Iqball S. Askham Z. Kan O. Tschernutter M. Bainbridge J.W. Naylor S. Ali R.R. Stable and efficient intraocular gene transfer using pseudotyped EIAV lentiviral vectors. J. Gene Med. 2006;8:275–285. doi: 10.1002/jgm.845. [DOI] [PubMed] [Google Scholar]
- Bárány E. Simultaneous measurements of changing intraocular pressure and outflow facility in the vervet monkey by constant pressure infusion. Invest. Ophthalmol. 1964;3:135–143. [PubMed] [Google Scholar]
- Barraza R. Poeschla E. Human gene therapy vectors derived from feline lentiviruses. Vet. Immunol. Immunopathol. 2008;123:23–31. doi: 10.1016/j.vetimm.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland M.V. Quigley H.A. Risk factors and open-angle glaucoma: Classification and application. J. Glaucoma. 2007;16:406–418. doi: 10.1097/IJG.0b013e31806540a1. [DOI] [PubMed] [Google Scholar]
- Borras T. Gabelt B. Klintworth K. Peterson J. Kaufman P. Non-invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo. J. Gene Med. 2001;3:437–449. doi: 10.1002/jgm.210. [DOI] [PubMed] [Google Scholar]
- Borras T. Brandt C.R. Nickells R. Ritch R. Gene therapy for glaucoma: Treating a multifaceted, chronic disease. Invest. Ophthalmol. Vis. Sci. 2002;43:2513–2518. [PubMed] [Google Scholar]
- Brown B.D. Venneri M.A. Zingale A. Sergi Sergi L. Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat. Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- Brown B.D. Cantore A. Annoni A. Sergi L.S. Lombardo A. Della Valle P. D'Angelo A. Naldini L. A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood. 2007;110:4144–4152. doi: 10.1182/blood-2007-03-078493. [DOI] [PubMed] [Google Scholar]
- Carr D.J. Noisakran S. The antiviral efficacy of the murine α-1 interferon transgene against ocular herpes simplex virus type 1 requires the presence of CD4+, α/β T-cell receptor-positive T lymphocytes with the capacity to produce gamma interferon. J. Virol. 2002;76:9398–9406. doi: 10.1128/JVI.76.18.9398-9406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Griffero F. Vandegraaff N. Li Y. McGee-Estrada K. Stremlau M. Welikala S. Si Z. Engelman A. Sodroski J. Requirements for capsid-binding and an effector function in TRIMCyp-mediated restriction of HIV-1. Virology. 2006;351:404–419. doi: 10.1016/j.virol.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Diaz-Griffero F. Kar A. Lee M. Stremlau M. Poeschla E. Sodroski J. Comparative requirements for the restriction of retrovirus infection by TRIM5α and TRIMCyp. Virology. 2007;369:400–410. doi: 10.1016/j.virol.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer E. Trabecular meshwork as a new target for the treatment of glaucoma. Drug News Perspect. 2006;19:151–158. doi: 10.1358/dnp.2006.19.3.985929. [DOI] [PubMed] [Google Scholar]
- Gabelt B.T. Gottanka J. Lutjen-Drecoll E. Kaufman P.L. Aqueous humor dynamics and trabecular meshwork and anterior ciliary muscle morphologic changes with age in rhesus monkeys. Invest. Ophthalmol. Vis. Sci. 2003;44:2118–2125. doi: 10.1167/iovs.02-0569. [DOI] [PubMed] [Google Scholar]
- Gabelt B.T. Hu Y. Vittitow J.L. Rasmussen C.R. Grosheva I. Bershadsky A.D. Geiger B. Borras T. Kaufman P.L. Caldesmon transgene expression disrupts focal adhesions in HTM cells and increases outflow facility in organ-cultured human and monkey anterior segments. Exp. Eye Res. 2006;82:935–944. doi: 10.1016/j.exer.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Grant W.M. Experimental aqueous perfusion in enucleated human eyes. Arch. Ophthalmol. 1963;69:783–801. doi: 10.1001/archopht.1963.00960040789022. [DOI] [PubMed] [Google Scholar]
- Grierson I. Howes R.C. Age-related depletion of the cell population in the human trabecular meshwork. Eye. 1987;1:204–210. doi: 10.1038/eye.1987.38. [DOI] [PubMed] [Google Scholar]
- Hattenhauer M.G. Johnson D.H. Ing H.H. Herman D.C. Hodge D.O. Yawn B.P. Butterfield L.C. Gray D.T. The probability of blindness from open-angle glaucoma. Ophthalmology. 1998;105:2099–2104. doi: 10.1016/S0161-6420(98)91133-2. [DOI] [PubMed] [Google Scholar]
- Hatziioannou T. Cowan S. Goff S.P. Bieniasz P.D. Towers G.J. Restriction of multiple divergent retroviruses by Lv1 and Ref1. EMBO J. 2003;22:385–394. doi: 10.1093/emboj/cdg042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo M. Tanihara H. Inatani M. Kido N. Sawamura T. Yue B.Y. Narumiya S. Honda Y. Effects of Rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest. Ophthalmol. Vis. Sci. 2001;42:137–144. [PubMed] [Google Scholar]
- Kaufman P.L. Davis G.E. “Minified” Goldmann applanating prism for tonometry in monkeys and humans. Arch. Ophthalmol. 1980;98:542–546. doi: 10.1001/archopht.1980.01020030538022. [DOI] [PubMed] [Google Scholar]
- Kee C. Sohn S. Hwang J.M. Stromelysin gene transfer into cultured human trabecular cells and rat trabecular meshwork in vivo. Invest. Ophthalmol. Vis. Sci. 2001;42:2856–2860. [PubMed] [Google Scholar]
- Khare P. Loewen N. Teo W. Barraza R.A. Saenz D.T. Johnson D.H. Poeschla E.M. Durable, safe, multi-gene lentiviral vector expression in feline trabecular meshwork. Mol. Ther. 2007;16:97–106. doi: 10.1038/sj.mt.6300318. [DOI] [PubMed] [Google Scholar]
- Lin T.Y. Emerman M. Cyclophilin A interacts with diverse lentiviral capsids. Retrovirology. 2006;3:70. doi: 10.1186/1742-4690-3-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Brandt C.R. Rasmussen C.A. Kaufman P.L. Ocular drug delivery: Molecules, cells, and genes. Can. J. Ophthalmol. 2007;42:447–454. [PubMed] [Google Scholar]
- Loewen N. Poeschla E.M. Lentiviral vectors. Adv. Biochem. Eng. Biotechnol. 2005;99:169–191. doi: 10.1007/10_007. [DOI] [PubMed] [Google Scholar]
- Loewen N. Fautsch M. Peretz M. Bahler C. Cameron J.D. Johnson D.H. Poeschla E.M. Genetic modification of human trabecular meshwork with lentiviral vectors. Hum. Gene Ther. 2001;12:2109–2119. doi: 10.1089/10430340152677449. [DOI] [PubMed] [Google Scholar]
- Loewen N. Bahler C. Teo W. Whitwam T. Peretz M. Xu R. Fautsch M. Johnson D.H. Poeschla E.M. Preservation of aqueous outflow facility after second-generation FIV vector-mediated expression of marker genes in anterior segments of human eyes. Invest. Ophthalmol. Vis. Sci. 2002;43:3686–3690. [PubMed] [Google Scholar]
- Loewen N. Barraza R. Whitwam T. Saenz D.T. Kemler I. Poeschla E. FIV vectors. Methods Mol. Biol. 2003a;229:251–271. doi: 10.1385/1-59259-393-3:251. [DOI] [PubMed] [Google Scholar]
- Loewen N. Leske D. Chen Y. Teo W. Saenz D.T. Peretz M. Holmes J. Poeschla E.M. Comparison of wild-type and class I integrase mutant-FIV vectors in retina demonstrates sustained expression of integrated transgenes in retinal pigment epithelium. J. Gene Med. 2003b;5:1009–1017. doi: 10.1002/jgm.447. [DOI] [PubMed] [Google Scholar]
- Loewen N. Fautsch M.P. Teo W.L. Bahler C.K. Johnson D.H. Poeschla E.M. Long-term, targeted genetic modification of the aqueous humor outflow tract coupled with noninvasive imaging of gene expression in vivo. Invest. Ophthalmol. Vis. Sci. 2004a;45:3091–3098. doi: 10.1167/iovs.04-0366. [DOI] [PubMed] [Google Scholar]
- Loewen N. Leske D.A. Cameron J.D. Chen Y. Whitwam T. Simari R.D. Teo W.L. Fautsch M.P. Poeschla E.M. Holmes J.M. Long-term retinal transgene expression with FIV versus adenoviral vectors. Mol. Vis. 2004b;10:272–280. [PubMed] [Google Scholar]
- Martin K.R. Quigley H.A. Gene therapy for optic nerve disease. Eye. 2004;18:1049–1055. doi: 10.1038/sj.eye.6701579. [DOI] [PubMed] [Google Scholar]
- Mitrophanous K. Yoon S. Rohll J. Patil D. Wilkes F. Kim V. Kingsman S. Kingsman A. Mazarakis N. Stable gene transfer to the nervous system using a non-primate lentiviral vector. Gene Ther. 1999;6:1808–1818. doi: 10.1038/sj.gt.3301023. [DOI] [PubMed] [Google Scholar]
- Naldini L. Bloemer U. Gallay P. Ory D. Mulligan R. Gage F.H. Verma I.M. Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Olsen J.C. Gene transfer vectors derived from equine infectious anemia virus. Gene Ther. 1998;5:1481–1487. doi: 10.1038/sj.gt.3300768. [DOI] [PubMed] [Google Scholar]
- Poeschla E. Wong-Staal F. Looney D. Efficient transduction of nondividing cells by feline immunodeficiency virus lentiviral vectors. Nat. Med. 1998;4:354–357. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- Quigley H.A. Broman A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P.V. Deng P.F. Kumar J. Epstein D.L. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest. Ophthalmol. Vis. Sci. 2001;42:1029–1037. [PubMed] [Google Scholar]
- Rasmussen C.A. Kaufman P.L. Primate glaucoma models. J. Glaucoma. 2005;14:311–314. doi: 10.1097/01.ijg.0000169409.01635.bc. [DOI] [PubMed] [Google Scholar]
- Rezaie T. Child A. Hitchings R. Brice G. Miller L. Coca-Prados M. Heon E. Krupin T. Ritch R. Kreutzer D. Crick R.P. Sarfarazi M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- Rohen J.W. Why is intraocular pressure elevated in chronic simple glaucoma? Anatomical considerations. Ophthalmology. 1983;90:758–765. doi: 10.1016/s0161-6420(83)34492-4. [DOI] [PubMed] [Google Scholar]
- Saenz D.T. Poeschla E.M. FIV: From lentivirus to lentivector. J. Gene Med. 2004;6((Suppl. 1)):S95–S104. doi: 10.1002/jgm.500. [DOI] [PubMed] [Google Scholar]
- Saenz D.T. Teo W. Olsen J.C. Poeschla E. Restriction of feline immunodeficiency virus by Ref1, LV1 and primate TRIM5α proteins. J. Virol. 2005;79:15175–15188. doi: 10.1128/JVI.79.24.15175-15188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz D.T. Barraza R. Loewen N. Teo W. Poeschla E. Production and use of feline immunodeficiency virus (FIV)-based lentiviral vectors. In: Rossi J., editor; Friedman T., editor. In Gene Transfer: A Cold Spring Harbor Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2006. pp. 57–74. [Google Scholar]
- Sagara T. Gaton D.D. Lindsey J.D. Gabelt B.T. Kaufman P.L. Weinreb R.N. Topical prostaglandin F2α treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathway. Arch. Ophthalmol. 1999;117:794–801. doi: 10.1001/archopht.117.6.794. [DOI] [PubMed] [Google Scholar]
- Schwartz G. Adherence and persistence in glaucoma. In: Grehn F., editor; Stamper R., editor. In Glaucoma. Springer-Verlag; Berlin: 2006. pp. 91–105. [Google Scholar]
- Stjernschantz J.W. From PGF2α-isopropyl ester to latanoprost: A review of the development of xalatan. The Proctor Lecture. Invest. Ophthalmol. Vis. Sci. 2001;42:1134–1145. [PubMed] [Google Scholar]
- Stone E.M. Fingert J.H. Alward W.L.M. Nguyen T.D. Polansky J.R. Sunden S.L.F. Nishimura D. Clark A.F. Nystuen A. Nichols B.E. Mackey D.A. Ritch R. Kalenak J.W. Craven E.R. Sheffield V.C. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- Stremlau M. Owens C.M. Perron M.J. Kiessling M. Autissier P. Sodroski J. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Tian B. Kaufman P.L. Effects of the Rho kinase inhibitor Y-27632 and the phosphatase inhibitor calyculin A on outflow facility in monkeys. Exp. Eye Res. 2005;80:215–225. doi: 10.1016/j.exer.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Toris C.B. Zhan G.L. Feilmeier M.R. Camras C.B. McLaughlin M.A. Effects of a prostaglandin DP receptor agonist, AL-6598, on aqueous humor dynamics in a nonhuman primate model of glaucoma. J. Ocul. Pharmacol. Ther. 2006;22:86–92. doi: 10.1089/jop.2006.22.86. [DOI] [PubMed] [Google Scholar]
- Virgen C.A. Kratovac Z. Bieniasz P.D. Hatziioannou T. Independent genesis of chimeric TRIM5–cyclophilin proteins in two primate species. Proc. Natl. Acad. Sci. U.S.A. 2008;105:3563–3568. doi: 10.1073/pnas.0709258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.F. Gagliuso D.J. Mittag T.W. Podos S.M. Effect of 15-keto latanoprost on intraocular pressure and aqueous humor dynamics in monkey eyes. Invest. Ophthalmol. Vis. Sci. 2007;48:4143–4147. doi: 10.1167/iovs.07-0035. [DOI] [PubMed] [Google Scholar]
- Wang W.H. McNatt L.G. Pang I.H. Millar J.C. Hellberg P.E. Hellberg M.H. Steely H.T. Rubin J.S. Fingert J.H. Sheffield V.C. Stone E.M. Clark A.F. Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. J. Clin. Invest. 2008;118:1056–1064. doi: 10.1172/JCI33871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.L. Toris C.B. Zhan G. Yablonski M.E. Effects of topical epinephrine on aqueous humor dynamics in the cat. Exp. Eye Res. 1999;68:439–445. doi: 10.1006/exer.1998.0623. [DOI] [PubMed] [Google Scholar]
- Weinreb R.N. Toris C.B. Gabelt B.T. Lindsey J.D. Kaufman P.L. Effects of prostaglandins on the aqueous humor outflow pathways. Surv. Ophthalmol. 2002;47((Suppl. 1)):S53–S64. doi: 10.1016/s0039-6257(02)00306-5. [DOI] [PubMed] [Google Scholar]
- Wilson S.J. Webb B.L. Ylinen L.M. Verschoor E. Heeney J.L. Towers G.J. Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc. Natl. Acad. Sci. U.S.A. 2008;105:3557–3562. doi: 10.1073/pnas.0709003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan G.L. Miranda O.C. Bito L.Z. Steroid glaucoma: Corticosteroid-induced ocular hypertension in cats. Exp. Eye Res. 1992;54:211–218. doi: 10.1016/s0014-4835(05)80210-6. [DOI] [PubMed] [Google Scholar]