Abstract
Interstitial cystitis/painful bladder syndrome (IC/PBS) is a major challenge to treat. We studied the effect of targeted and localized expression of enkephalin in afferent nerves that innervate the bladder by gene transfer using replication-defective herpes simplex virus (HSV) vectors in a rat model of bladder hyperactivity and pain. Replication-deficient HSV vectors encoding preproenkephalin, which is a precursor for Met- and Leu-enkephalin, or control vector encoding the lacZ reporter gene, were injected into the bladder wall of female rats. After viral vector injection, quantitative polymerase chain reaction showed high preproenkephalin transgene levels in bladder and dorsal root ganglia innervating the bladder in enkephalin vector-treated animals. Functionally, enkephalin vector-treated animals showed reductions in bladder hyperactivity and nociceptive behavior induced by intravesical application of capsaicin; however, vector-mediated expression of enkephalin did not alter normal voiding. This antinociceptive effect of enkephalin gene therapy was antagonized by naloxone hydrochloride administration. Together, our results with HSV vectors encoding preproenkephalin demonstrated physiological improvement in visceral pain induced by bladder irritation. Thus, gene therapy may represent a potentially useful treatment modality for bladder hypersensitive disorders such as IC/PBS.
Introduction
Interstitial cystitis/painful bladder syndrome (IC/PBS) is a chronic bladder pain disease with unknown etiology. The incidence of IC/PBS varies greatly, ranging from 2 to 200 in 100,000, and the number of symptomatic patients is projected to be as high as 5000 per 100,000. While bladder pain is the hallmark feature of IC/PBS, proposed treatments, both medical and surgical, have had limited clinical utility in this debilitating disease (Payne et al., 2007).
One mechanism by which bladder pain is induced is postulated to involve chronic tissue inflammation that can lead to functional changes in C-fiber afferents (Yoshimura and Birder, 2007). Hyperactivity and emergence of mechanosensitivity of C-fiber afferents may lead to pain sensation in response to normal nonnoxious distension of the bladder. Histochemical analysis of bladders from patients with IC/PBS revealed marked edema, vasodilation, proliferation of nerve fibers, and infiltration of mast cells (Johansson et al., 1997; Theoharides et al., 2001) that have also been observed in chemically induced cystitis in animals, in which increased urinary frequency is initiated by sensitizing mechanosensitive afferents and/or recruitment of afferents normally unresponsive to mechanical stimulation (Häbler et al., 1990; Sengupta and Gebhart, 1994; Dmitrieva and McMahon, 1996; Dmitrieva et al., 1997; Yoshimura and Birder, 2007).
It has been well documented that enkephalinergic mechanisms in the brain and spinal cord have inhibitory effects on the micturition reflex, and that exogenous enkephalins or opiate drugs applied to the sacral spinal cord can depress micturition (Dray and Metsch, 1984; Hisamitsu and de Groat, 1984; Booth et al., 1985; Dray et al., 1985; de Groat et al., 1986a, 1993). Endogenous enkephalins are expressed in bladder afferent and efferent pathways to inhibit micturition (Glazer and Basbaum, 1980; de Groat et al., 1986b; de Groat, 1987). Preproenkephalin (PPE) A is one of three genes that encode endogenous opioid peptides. Its main product, enkephalin, is synthesized in a variety of central and peripheral neurons. PPE gene knockout mice demonstrate an altered response to painful stimuli in the formalin test, which supports the role of endogenous opioids, such as enkephalins, in nociceptive processing (Konig et al., 1996). In patients with IC/PBS, frequent or unremitting pain may also require pain management with long-acting opioids such as morphine (MS Contin, Oramorph) or oxycodone (OxyContin) (Erickson, 1999; Ratner, 2001). However, the use of systemic opioid therapy has been limited because of its untoward side effects, tolerance, and dependency (Foley, 1993; Way, 1993). Thus, there remains an unmet need to deliver therapeutic peptides to the bladder afferents in a manner that releases biologically relevant levels of the opioids, which block chronic pain, without the unwanted side effects or tolerance attributed to the current drug therapies.
Herpes simplex virus type 1 (HSV-1) represents a viral vector system with several biological features that make it attractive for gene delivery to the peripheral or visceral nervous system (Fink and Glorioso, 1997; Wilson et al., 1999; Fink et al., 2000). Replication-defective mutants have been created that are deleted for specific viral immediate-early (IE) genes that abrogate the ability of the virus to replicate but enable these vectors to rapidly establish a “latent-like” state within neurons and other cell types with which the virus comes in contact. The potential utility of HSV gene therapy for treating peripheral nervous system (PNS) disease in vivo has been shown by expression of a number of genes to date (Antunes Bras et al., 1998; Wilson et al., 1999; Braz et al., 2001; Goins et al., 2001; Goss et al., 2001, 2002a,b; Yamada et al., 2001; Chattopadhyay et al., 2002a,b; Hao et al., 2003, 2005; Liu et al., 2004; Sasaki et al., 2004; Yeomans et al., 2004, 2006; Meunier et al., 2005; Wolfe et al., 2007; Yang et al., 2008).
The present study examines whether targeted and localized expression of enkephalin in afferent nerves, which innervate the bladder, using HSV vector-based gene transfer, can reduce bladder pain and urinary frequency induced by chemical bladder irritation. We used a replication-defective HSV-1 vector, SHPE (Goss et al., 2001), engineered to carry the human PPE (hPPE) transgene to the bladder and its sensory nerves. Our current results demonstrate the presence of the vector in both the bladder and dorsal root ganglia (DRG; sixth lumbar [L6] and first sacral [S1]), using quantitative polymerase chain reaction (qPCR), and successful vector-mediated transgene expression in the bladder as well as its afferent pathways originating from L6 and S1 DRG as measured by histochemical staining and real-time (RT)-PCR. Physiologically, inhibitory enkephalinergic effects on bladder hyperactivity in urethane-anesthetized rats treated with intravesical capsaicin, as well as capsaicin-induced bladder nociceptive behavior in freely moving, unanesthetized rats, were shown after bladder inoculation with SHPE, but not the lacZ-expressing SHZ control vector.
Materials and Methods
Vectors
The SHPE vector was created by the introduction of a plasmid that contained the human cytomegalovirus (CMV) promoter/enhancer, simian virus 40 (SV40) intron, human PPE cDNA, and SV40 poly(A) sequence with a loxP site into the thymidine kinase locus of a replication-defective vector deleted for the ICP4 immediate-early gene (Goins et al., 2001; Goss et al., 2001). The Escherichia coli lacZ gene was introduced into an ICP4– replication-defective virus in a similar fashion to create the recombinant virus SHZ, which was used as a control virus (Mester et al., 1995). Viral stocks were prepared with the E5 cell line (Deluca et al., 1985) in 10-layer Nunc Cell Factories (Thermo Fisher Scientific, Waltham, MA) at a multiplicity of infection (MOI) of 0.01, and purified by tangential flow filtration and ion-exchange chromatography as previously described (Ozuer et al., 2002a,b; Wechuck et al., 2002; Jiang et al., 2004). Titers for both SHPE and SHZ were determined in triplicate on E5 cells according to standard protocols (Goins et al., 2002).
Viral infection
All experiments were performed on female Sprague-Dawley rats (250–300 mg; Hilltop Animal Care, Pittsburgh, PA) in accordance with the requirements and recommendations in the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, 1985) approved by the University of Pittsburgh (Pittsburgh, PA) Institutional Animal Care and Use Committee (IACUC). Under isoflurane anesthesia, a low midline incision was performed to expose the bladder and 20 μl of viral suspension (total, 5.0 × 108 plaque-forming units [PFU]) of SHPE or SHZ was injected at four different sites (5 μl at each point) on the bladder wall, using a 30-gauge Hamilton syringe. In the study in which nociceptive behavior and bladder capacity were simultaneously recorded, 20 μl of viral suspension (low-titer group, 7.0 × 107 PFU; high-titer group, 8.7 × 108 PFU) was injected into the bladder wall in the same manner.
Histochemistry
One week after bladder injection with the SHZ control vector, animals were killed and bladders and DRG were harvested. Cryostat sections (10 μm) of bladder and DRG were mounted on gelatin-coated slides and the slides were fixed for 1 min in 1.5% glutaraldehyde (Sigma-Aldrich, St. Louis, MO), rinsed twice in phosphate-buffered saline, and incubated overnight at 37°C in β-galactosidase substrate [0.4 mg/ml 5-bromo-chloro-3-indolyl-β-d-thiogalactopyranoside (X-Gal; Roche, Indianapolis, IN), 1 mM MgCl2, 5 mM K4Fe(CN)6, and 5 mM K3Fe(CN)6 in phosphate-buffered saline]. After several washes in phosphate-buffered saline, sections were counterstained with eosin.
Viral genome and transgene quantitation in target tissues
Seven, 14, and 30 days postinjection, bladder tissue and L6 and S1 DRG from rats injected with SHPE (n = 3) or SHZ were removed and snap-frozen at –80°C. DNA was isolated with a QIAamp DNA mini kit (Qiagen, Valencia, CA). We quantified the viral genome number per 800 ng of total tissue DNA with a GeneAmp 5700 real-time PCR sequence detection system (Applied Biosystems, Foster City, CA). We used Primer Express software (Applied Biosystems) to design the forward (5′-ATTTGGGAAACCTGCAAGGA-3′) and reverse (5′-GGGTGCTGGTGCCATCTT-3′) primers, as well as the TaqMan fluorogenic probe (5′-TCCTGCAGCTGTCCAAACCAGAGCT-3′, with 6-FAM at the 5′ end and TAMRA at the 3′ end), specific to the human PPE sequence. We amplified tissue DNA samples for 50 cycles (95°C for 15 sec/60°C for 60 sec) and this was quantified by comparison with a standard curve representing a known amount of virus. All samples were run in triplicate. At various times postinjection of SHPE, RT-PCR amplification with a radioactive probe specific to the human PPE cDNA sequence produced DNA products that were separated on a 1.2% agarose gel and stained with ethidium bromide. Negative controls included SHZ-injected animals.
Cystometrograms
One week after intramural bladder injection of either SHPE or SHZ, animals were given subcutaneous urethane anesthesia (1.14 g/kg). With a lower midline abdominal incision, we exposed the bladder and inserted PE-50 tubing through the dome and into the bladder. Saline was then infused transvesically at 0.04 ml/min; rats voided spontaneously per urethra. A software package (WinDaq; DATAQ Instruments, Akron, OH) was used for data collection and data manipulation.
After a baseline was established with saline infusion, we infused capsaicin (15 μM in 10% ethanol 10% Tween 80, and 80% saline) into the bladder at 0.04 ml/min to acutely promote bladder hyperactivity in SHPE (n = 10) and SHZ (n = 9) rats. After establishing bladder hyperactivity, we administered (SHPE, n = 7; SHZ, n = 6) opioid receptor antagonists to delineate an opioid effect of this response to intravesical capsaicin. First, we gave naloxone methiodide (Sigma-Aldrich), which does not pass through the blood–brain barrier (BBB), at 5 mg/kg body weight, intravenously. One hour after naloxone methiodide administration, we gave rats naloxone hydrochloride (Sigma-Aldrich), which passes through the BBB, at 5 mg/kg body weight, intravenously.
Simultaneous evaluation of nociceptive behavior and bladder capacity
In previous studies (Craft et al., 1993; Saitoh et al., 2008), intravesical instillation of resiniferatoxin, a capsaicin analog, induced two types of nociceptive behavior: abdominal licking (licking) and immobility with pointing of their nose toward the lower abdomen without licking (freezing). In this study, therefore, these two nociceptive behaviors were counted to evaluate bladder pain induced by intravesical application of capsaicin. The methods for simultaneous recordings of nociceptive behavior and voided volume in freely moving, uncatheterized rats followed those described in another study (Saitoh et al., 2008). In brief, 2 weeks after bladder injection with SHPE (n = 6) or SHZ (n = 6), rats were placed in metabolic cages for at least 1 hr for acclimation. Rats were then placed in a Bollman-type restraining device (KN-326; Natsume Seisakusho, Tokyo, Japan). A polyethylene tube (PE-50; Clay Adams Division of Becton Dickinson, Parsippany, NJ ) was inserted into the bladder through the urethra, and residual urine was withdrawn. Capsaicin (1 mM) was then instilled into the bladder via the catheter in a volume of 0.6 ml and kept there for 1 min. Thereafter, the transurethral catheter was removed and rats were placed back in the metabolic cage. Licking and freezing were scored by a blinded observer for 15 min that was divided into 5-sec intervals. When licking or freezing occurred during each 5-sec interval, it was scored as one positive event. Simultaneously, micturition patterns were recorded for 75 min and the average voided volume was defined as the total urine volume divided by the number of micturitions.
Statistics
The nonparametric Mann–Whitney U test was used to test for differences between SHPE and SHZ intercontraction intervals in cystometry, sensory ganglial PPE transgene levels, nociceptive behavior, and voided volume in a metabolic cage study. Parametric analyses were done within groups (SHPE and SHZ) after specific treatments (capsaicin, naloxone) in cystometry.
Results
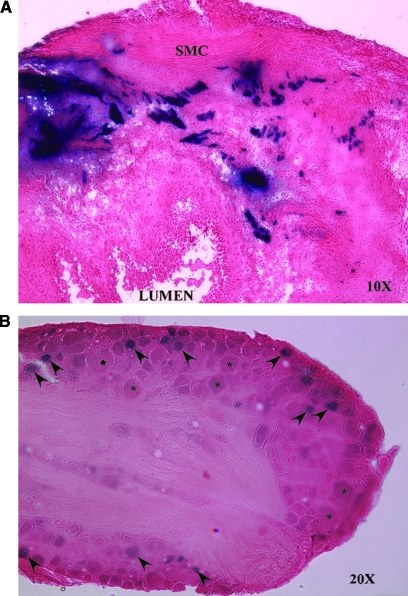
Initial experiments were designed to address the ability of replication-defective HSV vectors to deliver and express transgene in the bladder and bladder afferent nerves after injection of the vector into the bladder wall. These first experiments exploited the SHZ vector, as this control vector readily expresses the E. coli lacZ transgene (Mester et al., 1995) instead of the PPE gene. After injection of the vector into four sites within the bladder wall, we observed histochemical activity for β-galactosidase in both the bladder and L6 DRG 7 days after intramural bladder injection of SHZ. No staining was visualized in the L4 sensory ganglia, or in saline-injected controls (data not shown). Bladder wall sections displayed transgene expression predominantly within the smooth muscle cell layer at 1 week (Fig. 1A). L6 and S1 DRG sections demonstrated staining of small- and medium-sized cell bodies (Fig. 1B). No expression was detected in larger cell bodies (denoted by asterisks in Fig. 1B).
FIG. 1.
β-Galactosidase staining in rat bladder and L6 DRG after SHZ vector injection into the bladder wall. (A) A section of X-Gal-stained/eosin-counterstained SHZ-injected rat bladder (original magnification, ×10), with β-galactosidase activity seen in the wall of the bladder (blue). The bladder lumen and smooth muscle cell (SMC) layers are shown. (B) β-Galactosidase staining in a section of L6 sensory DRG (original magnification, ×20). Note positive staining in small- and medium-sized cell bodies (arrowheads), with sparing of large cell bodies (asterisks).
We had shown that a vector (SHZ) expressing a reporter gene injected into the bladder resulted in transgene expression in both bladder and DRG neurons after bladder wall vector injection. Next, we examined expression of the therapeutic product in target tissues. We studied SHPE vector-mediated expression of the transgene in various tissues by RT-PCR, using primers specific for the human PPE cDNA (Fig. 2) that fail to hybridize to the endogenous rat PPE sequence. The 70-bp hPPE product of the amplification was found (Fig. 2, arrow) to be present in RNA isolated from the bladder and L6 and S1 DRG of rats 1 week after SHPE injection, whereas SHZ-injected animals showed no amplification product from bladder samples (Fig. 2) or any DRG (data not shown), supporting expression of the therapeutic gene in the target tissues by SHPE but not the SHZ control vector.
FIG. 2.
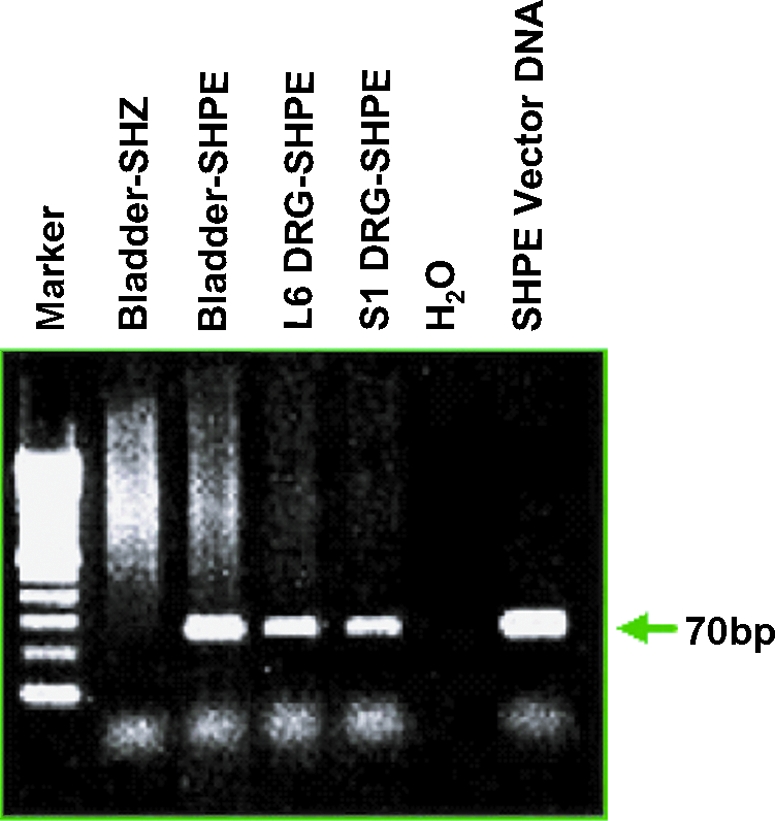
RT-PCR analysis of HSV SHPE vector-mediated human preproenkephalin (hPPE) gene expression in bladder and DRG. Gel electrophoresis of PCR amplification products was performed with primers specific for the human preproenkephalin sequence. Amplification product (70 bp) was detected (arrowhead) in the bladder, L6, and S1 DRG of SHPE-injected rats 1 week postinjection. SHZ-injected animals showed no amplification product from bladder samples. Color images available online at www.liebertonline.com/hum.
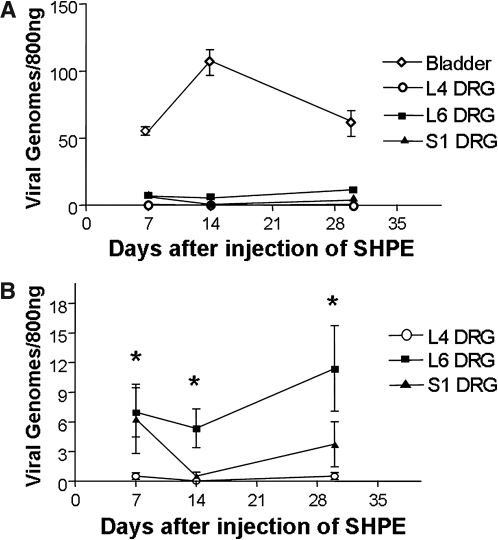
We also wanted to correlate hPPE expression with the presence of HSV vector. Therefore we examined the number of viral genomes in bladder and DRG tissue 7, 14, and 30 days after bladder injection, using quantitative real-time PCR (Fig. 3). At all time points, mean bladder viral vector genome numbers were greater than the number of vector genomes present in DRG samples (Fig. 3A). A significant difference was demonstrated, between viral genomes/800 ng of DNA from L4 DRG and L6 DRG, at 7 days (0.5 ± 0.2 and 6.97 ± 1.43, p < 0.05), 14 days (0.03 ± 0.02 and 4.75 ± 1.55, p < 0.05), and 30 days (0.68 ± 0.25 and 11.4 ± 4.33, p < 0.05) (Fig. 3B). This result is consistent with L6 innervation of the bladder in rats. Vector genomes were also higher in S1 DRG than in L4 DRG, which does not contain bladder afferent neurons; however, the differences were not statistically significant at any of the three time points examined.
FIG. 3.
Quantitative PCR analysis for the presence of HSV vector genomes in SHPE-injected rat bladder and L4, L6, and S1 DRG tissues. (A) The number of viral vector genomes present in the bladder was 1–2 logs higher than in DRG. (B) The number of vector genomes found in L6 DRG neurons was significantly higher (*p < 0.05) than that observed for L4 DRG cells. An increase trend was noted in L6 versus S1 levels, although results were not statistically significant. Data are shown as means ± SEM.
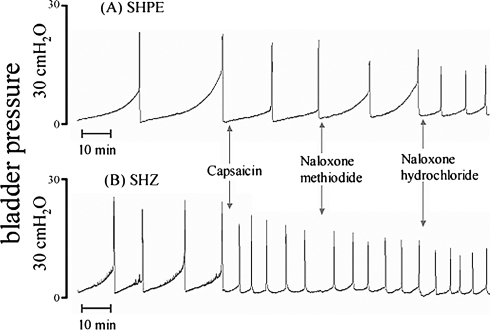
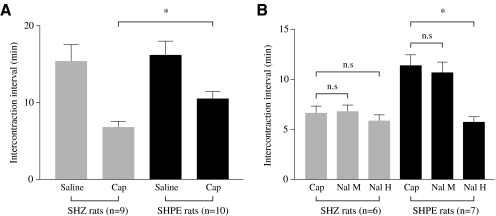
Now that we had demonstrated the presence of viral vector genomes and therapeutic gene expression in the bladder and bladder afferent pathways, we designed assays to evaluate the therapeutic effect of PPE expression on bladder responses to nociceptive stimuli, using two methodologies (cystometry and metabolic cage measurements). In the first series of experiments, we performed cystometries on urethane-anesthetized rats 1 week after bladder inoculation with either SHPE or SHZ, as shown by the cystometrograms (CMG) for SHPE- and SHZ-injected rats depicted in Fig. 4. During saline infusion, no significant differences in intercontraction intervals (ICIs) were observed between the two groups (Fig. 5). Thereafter, when 15 μM capsaicin was continuously infused into the bladder after a baseline was established with saline, both SHPE- and SHZ-injected rats showed bladder hyperactivity as evidenced by significant reductions in ICI (36.9 and 61.3% decrease, respectively). However, the reduction of ICI in the SHPE-injected rats was significantly smaller than that obtained with the SHZ control vector-injected rats (ICI, 10.4 ± 2.8 vs. 6.8 ± 2.3 min after capsaicin, respectively; p < 0.01) (Fig. 5A).
FIG. 4.
Representative example of in vivo continuous cystometrograms (CMGs) of urethane-anesthetized rats, with CMG performed 1 week after mural injection of SHPE (A) or SHZ (B). The SHPE-treated rat showed a smaller reduction in intercontraction interval (ICI) in response to 15 μM intravesical capsaicin infusion versus the SHZ-injected rat. Naloxone hydrochloride (5 mg/kg, intravenous) antagonized this response in the SHPE-treated rat, with little effect on the SHZ control vector-injected rat. However, naloxone methiodide (5 mg/kg, intravenous) showed little affect on ICI in both SHPE and SHZ-treated rats, demonstrating that the SHPE-mediated effects occurred centrally.
FIG. 5.
Intercontraction intervals (ICIs) in urethane-anesthetized rats injected 1 week previously with SHPE or SHZ. (A) Both SHPE- and SHZ-treated rats showed a reduction in ICI after capsaicin treatment; however, this reduction was significantly smaller in SHPE rats. (B) The SHPE-mediated effect on the ICI was reversed only by naloxone hydrochloride (Nal H), not by naloxone methiodide (Nal M). Asterisks denote statistically significant differences; n.s., nonsignificant. The number of animals in each treatment group is denoted in parentheses. Columns and bars represent means ± SEM.
While continuing capsaicin infusion, rats were treated with two types of naloxone to establish whether the antinociceptive effect on capsaicin-induced bladder hyperactivity in SHPE-injected rats was opioid dependent (Figs. 4 and 5). Administration of naloxone hydrochloride (Nal H) significantly antagonized the antinociceptive effect of SHPE vector-mediated expression of hPPE, which is cleaved to Met- and Leu-enkephalin (10.4 ± 2.8 min before naloxone hydrochloride to 5.4 ± 1.7 min after naloxone hydrochloride; p < 0.01); however, naloxone methiodide (Nal M) did not antagonize the antinociceptive effect (10.4 ± 2.8 min before naloxone methiodide to 9.8 ± 3.5 min after naloxone methiodide) (Fig. 5B). No change was seen in the SHZ-injected rats after administration of either naloxone (6.8 ± 2.3 min before naloxones to 6.8 ± 1.6 min after naloxone methiodide, and to 5.8 ± 1.6 min after naloxone hydrochloride), consistent with the inability of the control vector to produce factors that alter bladder hyperactivity induced by nociceptive stimuli (Fig. 5B).
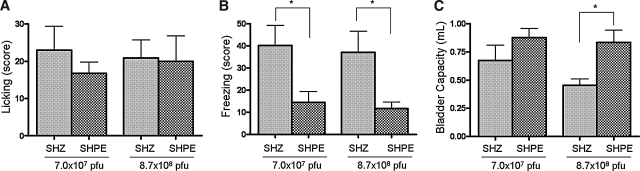
In addition to performing cystometric studies on anesthetized rats treated with the enkephalin vector and in which bladder hyperactivity was induced with capsaicin, we also employed metabolic cage studies to further assess the effect of SHPE vector treatment on nociceptive behavior and bladder function in freely moving, unanesthetized rats. In addition, to evaluate behavior and bladder activity in the uncatheterized condition, we used a brief application (1 min) of capsaicin at a concentration of 1 mM, which was higher than the concentration (15 μM) used for continuous infusion (1–2 hr) in cystometry. We also evaluated the effects of SHPE treatment 2 weeks after the bladder injection in order to examine whether the effects of hPPE gene transfer last more than 1 week, as consistent with HSV vector-mediated transgene expression that lasted up to 4 weeks (Goins et al., 2001; Sasaki et al., 2004). We have found that the number of freezing events during a 15-min period after intravesical capsaicin was significantly reduced in SHPE-injected rats compared with SHZ-injected rats, by 64% in the low-titer group (40.2 ± 9.0 vs. 14.4 ± 4.9, respectively; p < 0.05) and 68% in the high-titer group (37.0 ± 9.6 vs. 11.7 ± 3.0, respectively; p < 0.05) (Fig. 6B). However, there was no significant difference in capsaicin-induced licking behavior between SHZ- and SHPE-injected rats (low-titer group, 23.0 ± 6.4 vs. 16.8 ± 3.0; high-titer group, 20.1 ± 4.8 vs. 20.0 ± 6.8, respectively) (Fig. 6A). In addition, the average voided volume during the 75-min interval after intravesical instillation of capsaicin was significantly larger in SHPE-injected rats compared with SHZ-treated rats in the high-titer group (0.84 vs. 0.46 ml, respectively; p < 0.05) (Fig. 6C). In the low-titer group, bladder capacity tended to increase in SHPE-injected rats compared with SHZ-injected rats (0.88 vs. 0.67 ml, respectively), but was not significantly different (Fig. 6C).
FIG. 6.
HSV vector-mediated hPPE effects on nociceptive behavior and bladder capacity after intravesical instillation of capsaicin. The scores for licking (A) and freezing (B) behaviors were counted as one positive event when it occurred during a 5-sec interval, with the total observation time being 15 min. Rats with vector-mediated hPPE expression displayed a significant reduction (*p < 0.05) in capsaicin-induced freezing behavior compared with SHZ-injected rats at both doses tested, whereas licking behavior was not significantly altered. (C) Bladder capacity was observed for 75 min after intravesical instillation of capsaicin. In the high-titer group, bladder capacity was significantly increased in SHPE-injected rats compared with SHZ-injected rats (*p < 0.05). Columns and bars represent means ± SEM.
Discussion
Endogenous enkephalins have been identified by immunohistochemical techniques in sensory afferent terminals in the brain and spinal cord (Glazer and Basbaum, 1980; de Groat et al., 1986b; de Groat, 1987), and are thought to colocalize with excitatory neurotransmitters, such as substance P, in the nociceptive pathway at the spinal cord level (de Groat et al., 1986b; de Groat, 1987). Data from Yoshimura and North (1983) and Mudge and colleagues (1979) support both the direct inhibitory effect of opiates on sensory nerve conduction and neurotransmitter release in vitro. Yaksh and associates (1980) also demonstrated reduced substance P release in spinal cord neurons after administration of intrathecal morphine in anesthetized rats. Exogenous administration of enkephalin or opiate drugs produced a reduction in the micturition reflexes in rats and cats (Dray and Metsch, 1984; Hisamitsu and de Groat, 1984; Dray et al., 1985; de Groat et al., 1986a), as well as antinociceptive effects in a behavioral rat model (Craft et al., 1995). More recently, studies have demonstrated that the DNA for PPE, the precursor to the enkephalin class of opioid peptides, can be successfully transferred into DRG cells via their peripheral terminal fields, using HSV vectors (Goss et al., 2001, 2002b; Hao et al., 2003), with antinociceptive results that can be blocked by intrathecal naloxone administration. In all instances vector-mediated enkephalin expression induced a block in the natural nociceptive response that was transient whether examined in the chronic formalin test, the bone cancer model, or even the spinal nerve ligation (SNL) model. Moreover, successful readministration of the vector led to an even more vigorous response compared with the initial vector injection. However, it still remained transient in nature, dissipating between 14 and 21 days postinjection. Together, these data suggest, given the successful transduction of the target tissue and possible physiologic relevance, that gene therapy with hPPE may be useful in the treatment of bladder-hypersensitive disorders such as IC/PBS.
In this study we have demonstrated successful delivery of the hPPE cDNA by replication-defective HSV vectors in the bladder afferent pathways of rats. Because it is known that enkephalins and other opioid drugs applied in the sacral spinal cord depress bladder activities (Dray and Metsch, 1984; Hisamitsu and de Groat, 1984; Booth et al., 1985; Dray et al., 1985; de Groat et al., 1986a, 1993), it seems reasonable to assume that after PPE gene therapy, enkephalin expressed at central terminals of bladder afferent nerves in the spinal cord could directly exert inhibitory effects on spinal neurons involved in the micturition reflex and bladder nociceptive sensation. The concept that HSV vector-based gene therapy with PPE has an antinociceptive effect, regardless of etiology, is novel and may have important clinical implications as other current drug therapies have not proven effective in the entire patient population with IC/PBS.
Because of the difficulty in demonstrating enkephalinergic immunohistochemical staining, as reported by others (de Groat et al., 1986b; Pohl et al., 1994), we chose to evaluate marker gene (lacZ) transfer in the rat bladder and DRG tissue. The selective staining in small- and medium-sized cell bodies of the L6 DRG may reflect specific uptake of the virus in bladder afferents known to be of this size (Yoshimura and Birder, 2007), which has been previously noted for wild-type HSV infection of mouse footpad, where peripheral inoculation of virus resulted in the presence of virus in small- and medium-sized C-fibers and Aδ-fibers but not the larger myelinated Aβ-fibers (Yang et al., 2000; Margolis et al., 2007).
Results of both RT-PCR for the human enkephalin gene and quantitative PCR for vector genomes complement the previously described histochemical findings. PPE transgene levels in the bladder and L6 DRG were consistent with uptake by afferent nerves of either viral particles or PPE after synthesized by the vector in the bladder where there were 1–2 logs greater viral genomes in the bladder compared with DRG. Thus, the presence of vector-mediated enkephalins in L6 and S1 DRG may be due to transcription of the human PPE gene cassette from viral genomes present within DRG neurons or the result of expression in the bladder and retrograde transport of the peptides to the bladder afferent nerves, or both. Our current assays cannot rule out either mechanism. Interestingly, injection of SHPE into the bladder demonstrated a trend of increased PPE transgene levels in L6 more than S1 DRG tissue (Fig. 2), consistent with prior reports that bladder innervation is found to be more numerous in L6 DRG than in S1 DRG in rats (Keast and de Groat, 1992; Su et al., 1997). Sustained transgene levels at 1 month were observed in both bladder and L6 DRG; however, the strongest levels were observed from 7 to 14 days postinjection. The CMV promoter used to drive hPPE in the background of SHPE is known to maintain expression during this period of time, as shown by Wilson and colleagues (1999). However, we have also detected expression from this promoter after 14 days; the levels have been reduced as expression still seems to be transient from this promoter (Goins et al., 2001; Goss et al., 2001, 2002a; Sasaki et al., 2004). The issue of duration of expression needs to be explored further, and the mechanism of promoter shutoff, in the background of the replication-defective HSV vector, needs to be better defined.
In this study, cystometric analyses showed a physiologic antinociceptive effect due to PPE gene transfer. Another important point is that although bladder hyperactivity was altered, normal voiding did not appear to be affected by viral infection or expression of the hPPE therapeutic gene, consistent with the findings of Wilson and colleagues (1999) in the peripheral nervous system; this suggests that baseline sensation remains intact with hPPE gene transfer. Furthermore, the augmented response to intravesical capsaicin in SHPE-injected rats was antagonized by naloxone hydrochloride, but not by naloxone methiodide, which is a quaternary salt opioid receptor antagonist that does not pass the blood–brain barrier (BBB), suggesting that HSV-mediated enkephalin gene transfer exerts its effects in the central nervous system rather than in the periphery. It is possible, but unlikely, that the reduced ICI we observed could be due to the effects of naloxone hydrochloride at the supraspinal level, because the differential response in control (SHZ) and SHPE-treated rats given intravesical capsaicin as an acute bladder irritant suggests that the effect was opioid mediated in SHPE rats, probably because of HSV vector-mediated enkephalin expression in afferent nerves. Thus, it seems reasonable to assume that HSV vector-mediated enkephalin gene therapy suppressed bladder irritation induced by capsaicin via activation of naloxone-sensitive opioid receptors in the spinal cord.
Last, we further assessed the effect of SHPE vector treatment on nociceptive behavior and bladder function in freely moving, unanesthetized rats, situations that more closely mimic IC/PBS in human patients, and found that nociceptive freezing behavior induced by intravesical application of capsaicin was significantly reduced in SHPE-injected rats compared with SHZ-injected rats. At the same time, voided volume that was evaluated simultaneously with nociceptive behavior was significantly increased in SHPE-injected rats compared with SHZ-injected rats. However, there was no difference in capsaicin-induced licking behavior between SHZ- and SHPE-injected rats. This is probably due to the fact that licking behavior is induced by stimulation of urethral afferents in the pudendal nerve rather than bladder afferents, because previous studies by us and others have demonstrated that pudendal nerve transection significantly reduces licking behavior induced by intravesical application of capsaicin (Lecci et al., 1994) or resiniferatoxin (Saitoh et al., 2008). This also provides evidence that the effects of PPE gene transfer after SHPE bladder inoculation are limited to bladder afferent pathways and neither the vector nor the transgene is having pleiotropic effects on other nontarget sites.
In conclusion, this study demonstrated proof of concept for the use of gene therapy to treat visceral pain. The results of the present study indicate that (1) HSV vectors injected into the bladder wall were transported through bladder afferent pathways to L6 and S1 DRG, where bladder afferent nerves originate, and these viral genomes expressed human PPE; (2) bladder hyperactivity induced by nociceptive stimuli (i.e., capsaicin) can be reduced via naloxone-dependent opioid mechanisms, presumably at the spinal cord level, after SHPE bladder inoculation; and (3) nociceptive freezing behavior induced by intravesical capsaicin was also suppressed in association with increased bladder capacity after SHPE but not SHZ control vector treatment. Our data illustrate that enkephalin gene therapy for bladder pain response is not only feasible but achieves a physiological decrease in bladder irritative response. This technique of gene transfer using replication-defective HSV vectors may offer new hope for the treatment of refractory IC/PBS. However, because this study used only an acute bladder irritation model and HSV injection before bladder pain induction, further studies using chronic bladder irritation models and postirritation injection paradigms of HSV vectors are necessary.
Acknowledgments
This project was supported by NIH P01 DK44935, DK57267, and DK68557; the Interstitial Cystitis Association; and the Fishbein Family IC Research Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- Antunes Bras J.M. Epstein A.L. Bourgoin S. Hamon M. Cesselin F. Pohl M. Herpes simplex virus 1-mediated transfer of preproenkephalin A in rat dorsal root ganglia. J. Neurochem. 1998;70:1299–1303. doi: 10.1046/j.1471-4159.1998.70031299.x. [DOI] [PubMed] [Google Scholar]
- Booth A.M. Hisamitsu T. Kawatani M. de Groat W.C. Regulation of urinary bladder capacity by endogenous opioid peptides. J. Urol. 1985;133:339–342. doi: 10.1016/s0022-5347(17)48935-x. [DOI] [PubMed] [Google Scholar]
- Braz J. Beaufour C. Coutaux A. Epstein A.L. Cesselin F. Hamon M. Pohl M. Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons. J. Neurosci. 2001;21:7881–7888. doi: 10.1523/JNEUROSCI.21-20-07881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M. Goins W. Glorioso J.C. Mata M. Fink D.J. HSV-mediated gene transfer of NGF in cisplatin neuropathy. Mol. Ther. 2002a;5:S244. [Google Scholar]
- Chattopadhyay M. Wolfe D. Huang S. Goss J. Glorioso J.C. Mata M. Fink D.J. In vivo gene therapy of pyridoxine-induced neuropathy by HSV-mediated gene transfer of neurotrophin-3. Ann. Neurol. 2002b;51:19–27. doi: 10.1002/ana.10061. [DOI] [PubMed] [Google Scholar]
- Craft R.M. Carlisi V.J. Mattia A. Herman R.M. Porreca F. Behavioral characterization of the excitatory and desensitizing effects of intravesical capsaicin and resiniferatoxin in the rat. Pain. 1993;55:205–215. doi: 10.1016/0304-3959(93)90149-J. [DOI] [PubMed] [Google Scholar]
- Craft R.M. Henley S.R. Haaseth R.C. Hruby V.J. Porreca F. Opioid antinociception in a rat model of visceral pain: Systemic versus local drug administration. J. Pharmacol. Exp. Ther. 1995;275:1535–1542. [PubMed] [Google Scholar]
- de Groat W.C. Neuropeptides in pelvic afferent pathways. Experientia. 1987;43:801–813. doi: 10.1007/BF01945358. [DOI] [PubMed] [Google Scholar]
- de Groat W.C. Kawatani M. Hisamitsu T. Booth A.M. Roppolo J.R. Thor K. Tuttle P. Nagel J. Neural control of micturition: The role of neuropeptides. J. Auton. Nerv. Syst. Suppl. 1986a:369–375. [Google Scholar]
- de Groat W.C. Lowe I.P. Kawatani M. Morgan C.W. Kuo D. Roppolo J.R. Nagel J. Identification of enkephalin immunoreactivity in sensory ganglia cells. J Auton. Nerv. Syst. Suppl. 1986b:361–368. [Google Scholar]
- de Groat W.C. Yoshimura N. Booth A.M. Neurophysiology of Micturition. In: Maggi C.A., editor. The Autonomic Nervous System, Vol. 3: Nervous Control of the Urogenital System. Harwood Academic Publishers; London: 1993. pp. 123–456. [Google Scholar]
- Deluca N.A. Schaffer P.A. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Mol. Cell. Biol. 1985;5:1997–2008. doi: 10.1128/mcb.5.8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva N. McMahon S.B. Sensitization of visceral afferents by nerve growth factor in the adult rat. Pain. 1996;66:87–97. doi: 10.1016/0304-3959(96)02993-4. [DOI] [PubMed] [Google Scholar]
- Dmitrieva N. Shelton D. Rice A.S. McMahon S.B. The role of nerve growth factor in a model of visceral inflammation. Neuroscience. 1997;78:449–459. doi: 10.1016/s0306-4522(96)00575-1. [DOI] [PubMed] [Google Scholar]
- Dray A. Metsch R. Inhibition of urinary bladder contractions by a spinal action of morphine and other opioids. J. Pharmacol. Exp. Ther. 1984;231:254–260. [PubMed] [Google Scholar]
- Dray A. Nunan L. Wire W. Central δ-opioid receptor interactions and inhibition of reflex urinary bladder contractions in the rat. Br. J. Pharmacol. 1985;85:717–726. doi: 10.1111/j.1476-5381.1985.tb10569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson D.R. Interstitial cystitis: Update on etiologies and therapeutic options. J. Womens Health Gend. Based Med. 1999;8:745–758. doi: 10.1089/152460999319075. [DOI] [PubMed] [Google Scholar]
- Fink D.J. Glorioso J.C. Engineering herpes simplex virus vectors for gene transfer to neurons. Nat. Med. 1997;3:357–359. doi: 10.1038/nm0397-357. [DOI] [PubMed] [Google Scholar]
- Fink D.J. Deluca N.A. Yamada M. Wolfe D.P. Glorioso J.C. Design and application of HSV vectors for neuroprotection. Gene Ther. 2000;7:115–119. doi: 10.1038/sj.gt.3301118. [DOI] [PubMed] [Google Scholar]
- Foley K.M. Opioid analgesics in clinical pain management. In: Akil H., editor; Simon E.J., editor. Opioid II. Springer-Verlag; Berlin: 1993. pp. 697–744. [Google Scholar]
- Glazer E.J. Basbaum A.I. Leucine enkephalin: Localization in and axoplasmic transport by sacral parasympathetic preganglionic neurons. Science. 1980;208:1479–1481. doi: 10.1126/science.6155697. [DOI] [PubMed] [Google Scholar]
- Goins W.F. Yoshimura N. Phelan M.W. Yokoyama T. Fraser M.O. Ozawa H. Bennett N., JR. de Groat W.C. Glorioso J.C. Chancellor M.B. Herpes simplex virus mediated nerve growth factor expression in bladder and afferent neurons: potential treatment for diabetic bladder dysfunction. J. Urol. 2001;165:1748–1754. [PubMed] [Google Scholar]
- Goins W.F. Krisky D.M. Wolfe D.P. Fink D.J. Glorioso J.C. Development of replication-defective herpes simplex virus vectors. Methods Mol. Med. 2002;69:481–507. doi: 10.1385/1-59259-141-8:481. [DOI] [PubMed] [Google Scholar]
- Goss J.R. Mata M. Goins W.F. Wu H.H. Glorioso J.C. Fink D.J. Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene Ther. 2001;8:551–556. doi: 10.1038/sj.gt.3301430. [DOI] [PubMed] [Google Scholar]
- Goss J.R. Goins W.F. Lacomis D. Mata M. Glorioso J.C. Fink D.J. Herpes simplex-mediated gene transfer of nerve growth factor protects against peripheral neruropathy in streptozotocin-induced diabetes in the mouse. Diabetes. 2002a;51:2227–2232. doi: 10.2337/diabetes.51.7.2227. [DOI] [PubMed] [Google Scholar]
- Goss J.R. Harley C.F. Mata M. O'Malley M.E. Goins W.F. Hu X.-P. Glorioso J.C. Fink D.J. Herpes vector-mediated expression of proenkephalin reduces pain-related behavior in a model of bone cancer pain. Ann. Neurol. 2002b;52:662–665. doi: 10.1002/ana.10343. [DOI] [PubMed] [Google Scholar]
- Häbler H.J. Jänig W. Koltzenburg M. Activation of unmyelinated afferent fibers by mechanical stimuli and inflammation of the urinary bladder in the cat. J. Physiol. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S. Mata M. Goins W. Glorioso J.C. Fink D.J. Transgene-mediated enkephalin release enhances the effect of morphine and evades tolerance to produce a sustained antiallodynic effect in neuropathic pain. Pain. 2003;102:135–142. doi: 10.1016/s0304-3959(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Hao S. Mata M. Wolfe D. Huang S. Glorioso J.C. Fink D.J. Gene transfer of glutamic acid decarboxylase reduces neuropathic pain. Ann. Neurol. 2005;57:914–918. doi: 10.1002/ana.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamitsu T. de Groat W.C. The inhibitory effect of opioid peptides and morphine applied intrathecally and intracerebroventricularly on the micturition reflex in the cat. Brain Res. 1984;298:51–65. doi: 10.1016/0006-8993(84)91146-6. [DOI] [PubMed] [Google Scholar]
- Institute for Laboratory Animal Research, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. National Academies Press; Washington, D.C.: 1985. [Google Scholar]
- Jiang C. Wechuck J.B. Goins W.F. Krisky D.M. Wolfe D.P. Ataai M.M. Glorioso J.C. Immobilized cobalt affinity chromatography provides a novel, efficient method for HSV gene vector purification. J. Virol. 2004;78:8994–9006. doi: 10.1128/JVI.78.17.8994-9006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S.L. Ogawa K. Fall M. The pathology of interstitial cystitis. In: Sant G.R., editor. Interstitial Cystitis. Lippincott-Raven; Philadelphia, PA: 1997. pp. 143–151. [Google Scholar]
- Keast J.R. de Groat W.C. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J. Comp. Neurol. 1992;319:615–623. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- Konig M. Zimmer A.M. Steiner H. Holmes P.V. Crawley J.N. Brownstein M.J. Zimmer A. Pain responses, anxiety, and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- Lecci A. Giuliani S. Lazzeri M. Benaim G. Turini D. Maggi C.A. The behavioral response induced by intravesical instillation of capsaicin rats is mediated by pudendal urethral sensory fibers. Life Sci. 1994;55:429. doi: 10.1016/0024-3205(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Liu J. Wolf D. Hao S. Huang S. Glorioso J.C. Mata M. Fink D.J. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Mol. Ther. 2004;10:57–66. doi: 10.1016/j.ymthe.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Margolis T.P. Imai Y. Yang L. Vallas V. Krause P.R. Herpes simplex virus type 2 (HSV-2) establishes latent infection in a different population of ganglionic neurons than HSV-1: Role of latency-associated transcripts. J. Virol. 2007;81:1872–1878. doi: 10.1128/JVI.02110-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mester J.C. Pitha P.M. Glorioso J.C. Antiviral activity of herpes simplex virus vectors expressing murine α1-interferon. Gene Ther. 1995;2:187–196. [PubMed] [Google Scholar]
- Meunier A. Latremoliere A. Mauborgne A. Bourgoin S. Kayser V. Cesselin F. Hamon M. Pohl M. Attenuation of pain-related behavior in a rat model of trigeminal neuropathic pain by viral-driven enkephalin overproduction in trigeminal ganglion neurons. Mol. Ther. 2005;11:608–616. doi: 10.1016/j.ymthe.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Mudge A.W. Leeman S.E. Fischbach G.D. Enkephalin inhibits release of substance P from sensory neurons in culture and decreases action potential duration. Proc. Natl. Acad. Sci. U.S.A. 1979;76:526–530. doi: 10.1073/pnas.76.1.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozuer A. Wechuck J.B. Goins W.F. Wolfe D. Glorioso J.C. Ataai M.M. Effects of genetic background and culture conditions on production of herpesvirus-based gene therapy vectors. Biotechnol. Bioeng. 2002a;77:685–692. doi: 10.1002/bit.10162. [DOI] [PubMed] [Google Scholar]
- Ozuer A. Wechuck J.B. Russell B. Wolfe D. Goins W.F. Glorioso J.C. Ataai M.M. Evaluation of infection parameters in the production of herpes simplex virus type 1 (HSV-1) vectors for gene therapy. Biotechnol. Prog. 2002b;18:476–482. doi: 10.1021/bp010176k. [DOI] [PubMed] [Google Scholar]
- Payne C.K. Joyce G.F. Wise M. Clemens J.Q. Urologic Diseases in America Project: Interstitial cystitis and painful bladder syndrome. J. Urol. 2007;177:2042–2049. doi: 10.1016/j.juro.2007.01.124. [DOI] [PubMed] [Google Scholar]
- Pohl M. Collin E. Bourgoin S. Conrath M. Benoliel J.J. Nevo I. Hamon M. Giraud P. Cesselin F. Expression of preproenkephalin A gene and presence of Met-enkephalin in dorsal root ganglia of the adult rat. J. Neurochem. 1994;63:1226–1234. doi: 10.1046/j.1471-4159.1994.63041226.x. [DOI] [PubMed] [Google Scholar]
- Ratner V. Interstitial cystitis: A chronic inflammatory bladder condition. World J. Urol. 2001;19:157–159. doi: 10.1007/pl00007096. [DOI] [PubMed] [Google Scholar]
- Saitoh C. Chancellor B.C. de Groat W.C. Yoshimura N. Effects of intravesical instillation of resiniferatoxin on bladder function and nociceptive behavior in freely moving, conscious rats. J. Urol. 2008;179:359–364. doi: 10.1016/j.juro.2007.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K. Chancellor M.B. Goins W.F. Phelan M.W. Glorioso J.C. de Groat W.C. Yoshimura N. Gene therapy using replication-defective herpes simplex virus vectors expressing nerve growth factor in a rat model of diabetic cystopathy. Diabetes. 2004;53:2723–2730. doi: 10.2337/diabetes.53.10.2723. [DOI] [PubMed] [Google Scholar]
- Sengupta J.N. Gebhart G.F. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J. Neurophysiol. 1994;72:2420–2430. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- Su X. Sengupta J.N. Gebhart G.F. Effects of opioids on mechanosensitive pelvic nerve afferent fibers innervating the urinary bladder of the rat. J. Neurophysiol. 1997;77:1566–1580. doi: 10.1152/jn.1997.77.3.1566. [DOI] [PubMed] [Google Scholar]
- Theoharides T.C. Kempuraj D. Sant G.R. Mast cell involvement in interstitial cystitis: A review of human and experimental evidence. Urology. 2001;57(Suppl. 6A):47–55. doi: 10.1016/s0090-4295(01)01129-3. [DOI] [PubMed] [Google Scholar]
- Way E.L. Opioid tolerance and physical dependence and their relationship. In: Akil H., editor; Simon E.J., editor. Opioid II. Springer-Verlag; Berlin: 1993. pp. 573–596. [Google Scholar]
- Wechuck J.B. Ozuer A. Goins W.F. Wolfe D. Oligino T. Glorioso J.C. Ataai M.M. Effect of temperature, composition, and cell passage on production of herpes-based viral vectors. Biotechnol. Bioeng. 2002;79:112–119. doi: 10.1002/bit.10310. [DOI] [PubMed] [Google Scholar]
- Wilson S.P. Yeomans D.C. Bender M.A. Lu Y. Goins W.F. Glorioso J.C. Antihyperalgesic effects of infection with preproenkephalin-encoding herpes virus. Proc. Natl. Acad. Sci. U.S.A. 1999;96:3211–3216. doi: 10.1073/pnas.96.6.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe D. Hao S. Hu J. Srinivasan R. Goss J. Mata M. Fink D.J. Glorioso J.C. Engineering an endomorphin-2 gene for use in neuropathic pain therapy. Pain. 2007;133:29–38. doi: 10.1016/j.pain.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Yaksh T.L. Jessell T.M. Gamse R. Mudge A.W. Leeman S.E. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature. 1980;286:155–157. doi: 10.1038/286155a0. [DOI] [PubMed] [Google Scholar]
- Yamada M. Natsume A. Mata M. Oligino T. Goss J. Glorioso J. Fink D.J. Herpes simplex virus vector-mediated expression of Bcl-2 protects spinal motor neurons from degeneration following root avulsion. Exp. Neurol. 2001;168:225–230. doi: 10.1006/exnr.2000.7597. [DOI] [PubMed] [Google Scholar]
- Yang H. McNearney T.A. Chu R. Lu Y. Ren Y. Yeomans D.C. Wilson S.P. Westlund K.N. Enkephalin-encoding herpes simplex virus-1 decreases inflammation and hotplate sensitivity in a chronic pancreatitis model. Mol. Pain. 2008;4:8. doi: 10.1186/1744-8069-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. Voytek C.C. Margolis T.P. Immunohistochemical analysis of primary sensory neurons latently infected with herpes simplex virus type 1. J. Virol. 2000;74:209–217. doi: 10.1128/jvi.74.1.209-217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans D.C. Jones T. Laurito C.E. Lu Y. Wilson S.P. Reversal of ongoing thermal hyperalgesia in mice by a recombinant herpesvirus that encodes human preproenkephalin. Mol. Ther. 2004;9:24–29. doi: 10.1016/j.ymthe.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Yeomans D.C. Lu Y. Laurito C.E. Peters M.C. Vota-Vellis G. Wilson S.P. Pappas G.D. Recombinant herpes vector-mediated analgesia in a primate model of hyperalgesia. Mol. Ther. 2006;13:589–597. doi: 10.1016/j.ymthe.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Yoshimura N. Birder L.A. Interstitial cystitis and related painful bladder syndromes: Pathophysiology. In: Pasricha P.J., editor; Willis W.D., editor; Gebhart G.F., editor. Chronic Abdominal and Visceral Pain: Theory and Practice. Informa Healthcare USA; New York: 2007. pp. 495–520. [Google Scholar]
- Yoshimura M. North R.A. Substantia gelatinosa neurons hyperpolarized in vitro by enkephalin. Nature. 1983;305:529–530. doi: 10.1038/305529a0. [DOI] [PubMed] [Google Scholar]