Abstract
It has been suggested that high doses of β-carotene limit its conversion to vitamin A, yet this effect has not been well established in humans. A feeding study was conducted in a randomized crossover design in which volunteers consumed 2 doses of deuterium-labeled β-carotene on 2 occasions, with β-carotene and vitamin A response assessed by plasma area under the concentration time curve (AUC). Seven volunteers (4 men, 3 women) consumed each of 2 doses of β-carotene-d8 and provided serial blood samples for 37 d after each dose. β-Carotene doses were 20 and 40 mg. Plasma β-carotene-d8 was assessed by HPLC-MS. Plasma retinol (ROH)-d4, which was derived from the β-carotene-d8, was evaluated by GC-MS after saponification to convert retinyl esters to ROH prior to the formation of the trimethylsilylether. The plasma AUC for β-carotene-d8 increased 2-fold from the 20-mg dose to the 40-mg dose. The plasma AUC for ROH-d4 increased 36% from the 20-mg dose to the 40-mg dose. These results establish that, in humans, β-carotene conversion to vitamin A decreases as the dietary dose increases.
Introduction
Vitamin A deficiency continues to impose a major health impact throughout much of the developing world. The negative consequences of vitamin A deficiency include impaired resistance to infection, xerophthalmia, blindness, and increased risk of mortality. The WHO estimates that 190 million preschool-aged children and 19 million pregnant women suffer from vitamin A deficiency (1).
β-Carotene is the primary plant-based source of dietary vitamin A; thus, a clear understanding of factors influencing its potential for utilization as vitamin A has important public health implications. A variety of factors have been found to influence β-carotene absorption, including food matrix (2,3), amount of coingested fat (4), and type of coingested lipid (5–7). Two studies have suggested that dose may influence the conversion of β-carotene to vitamin A. First, Brubacher and Weiser (8) showed that β-carotene conversion to vitamin A is dose dependent in rats. Later, Tang et al. (9) published a report of β-carotene conversion to vitamin A in a single female participant after she consumed 2 doses of vitamin A separated by 2.5 y. At the higher dose of 126 mg, the retinol (ROH)7 equivalency was 55 to 1 (mg of β-carotene to mg of retinol) compared to 3.8 to 1 at the lower dose of 6 mg. However, the influence of dose has not been investigated in a well-controlled feeding study. This study is an important advancement past previous work in that it involves multiple participants, β-carotene doses closer to that found in the diet, and a protocol in which the doses were administered only weeks apart and in a randomized treatment order. The efficiency of conversion of β-carotene to vitamin A has important implications with respect to the role of β-carotene in ameliorating vitamin A deficiency.
Our objective in this study was to determine how absorption of β-carotene and its utilization as vitamin A is influenced by dose. To meet this objective, we conducted a human feeding study in which volunteers consumed isotopically labeled β-carotene at 2 dose levels. Blood was collected for several weeks following the doses and plasma labeled β-carotene and vitamin A responses were used as indices of absorption and bioconversion.
Experimental Procedures
Study design.
Seven healthy adults (4 men, 3 women) participated in a β-carotene dose-response study. Participants had a body weight (mean ± SD) of 72 ± 8 kg, plasma β-carotene concentration of 0.43 ± 0.2 μmol/L, and mean ROH concentration of 1.6 ± 0.5 μmol/L. This study was conducted according to the guidelines established in the Declaration of Helsinki. Participants gave informed, written consent, and all procedures were approved by the Johns Hopkins University Bloomberg School of Public Health Committee on Human Research.
The study was conducted in a randomized crossover design in which volunteers consumed a single dose of deuterium-labeled β-carotene, followed by a 37-d blood collection period and a 17-d break. Doses were 20 and 40 mg and dose sequence was randomly assigned. The blood collection schedule was as follows: dose day hours 0 (baseline), 1, 2, 3, 4, 5, 6, 8, 10, 12, d 1 morning (24 h post dose) and afternoon (32 h post dose), and morning fasting samples on d 3, 4, 6, 9, 11, 13, 17, 20, 23, 26, 30, and 37.
During the study, participants consumed a controlled diet prepared by the Beltsville Human Nutrition Research Center. The diet contained 15% of energy from protein, 32% from fat, and the balance from carbohydrate. The diet provided 2 mg/d β-carotene, which is equivalent to the mean daily β-carotene intake in the US (10). The vitamin A content of the diet was dependent on calorie level, with 730 ROH equivalents/8.36 MJ. This was slightly higher than the mean daily intake (547 ROH equivalents/d) in the US and provided the Recommended Dietary Allowance (10). During the dosing days, lunch and dinner were provided at 1700 and 2200 h, respectively, and the foods provided were free of carotenoids and vitamin A. Vitamins and other supplements were prohibited throughout the study.
Reagents.
All trans-β-carotene-10, 10', 19, 19, 19, 19', 19', 19'-d8 (BC-d8), all-trans-retinyl-10, 19, 19, 19-d4 acetate (retinyl-d4 acetate), and all-trans-retinyl-10, 14, 19, 19, 19, 20, 20, 20-d8 acetate (retinyl-d8 acetate) were purchased from Cambridge Isotope Labs. All trans-β-carotene-U 13C40 (13C-BC) was purchased from Martek. All solvents used were HPLC grade.
The method for plasma extraction and analysis was that of Pawlosky et al. (11). All sample preparations and analyses were performed under subdued light. 13C-BC (internal standard) was added to thawed plasma, which was then extracted 3 times with hexane after ethanol precipitation of proteins. Combined hexane layers were dried under nitrogen and redissolved in HPLC mobile phase.
Samples were analyzed for labeled and unlabeled β-carotene using a Beckman model-110 HPLC system fitted with a 250- × 0.46-mm 5-μm C-18 Microsorb column (Rainin Instrument). The HPLC mobile phase consisted of CH3CN:CH2Cl2:CH2OH (65:25:10) with 0.1% diisopropylethylamine. The HPLC effluent was delivered directly into a Hewlett-Packard 5989B particle beam quadrupole mass spectrometer (Agilent Technologies) operated in the negative ionization mode with methane as the reagent gas. Single ion monitoring was used to collect parent ion [M]− data for mass:charge ratio (m/z) 536 for unlabeled β-carotene, m/z 544 for all trans-β-carotene-10, 10', 19, 19, 19, 19', 19', 19'-d8, and m/z 576 for 13C-BC. Quantification was performed against external standard curves for unlabeled β-carotene and β-carotene-d8.
A second extraction was performed as above to isolate ROH and retinyl esters from plasma, with all-trans-ROH-10, 14, 19, 19, 19, 20, 20, 20-d8 (ROH-d8) (hydrolyzed from retinyl-d8 acetate) as the internal standard. Extracts were saponified with KOH to convert retinyl esters to ROH. Samples were applied to conditioned NH2 SPE cartridges (500 mg/2.8 mL, Alltech Biotechnology) and eluted with hexane:isopropanol (90:10). Trimethylsilyl (TMS) ether derivatives were formed with N,O-bis-(TMS)-trifluoroacetamide and pyridine (1:1). ROH derivatives were analyzed by GC-MS using a Hewlett Packard 6890 gas chromatograph with a DB-1 Durabond methyl siloxane column (15-m × 250-μm i.d., 0.1-μm film thickness, J&W Scientific) and a Hewlett Packard 5973 quadrupole mass spectrometric detector. Analytes were ionized by negative chemical ionization with methane. Single ion monitoring was used to detect fragment ions [M-90 (TMSOH)]− at m/z 268 for ROH, m/z 272 for ROH-d4, and m/z 276 for ROH-d8. Quantification was performed against external standard curves for TMS derivatives of unlabeled ROH and ROH-d4.
Calculations and statistics.
Areas under the plasma concentration time curves were calculated by the trapezoid method in Microsoft Excel 2007. Areas for the second experimental phase were mathematically corrected for the presence of labeled analyte from the first experimental phase. Area under the curve values were compared between dose level using a repeated-measures ANOVA with SigmaStat v. 3.1, with P < 0.05 considered significant. Pearson product correlations were determined using SigmaStat v. 3.1.
Results
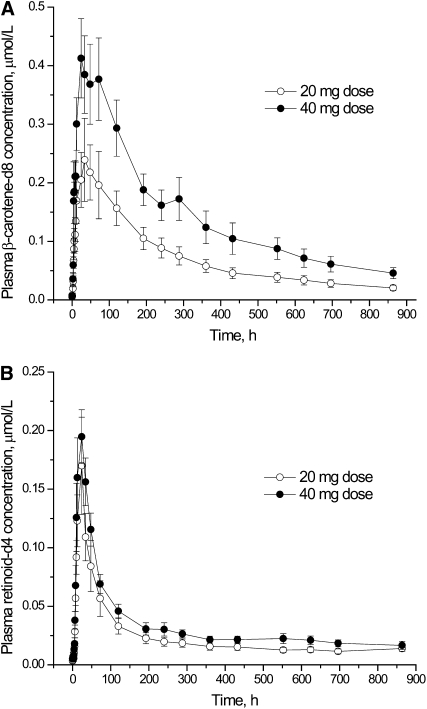
The β-carotene response was assessed by plasma appearance of β-carotene-d8 and ROH-d4, which represented both retinyl esters and ROH derived from β-carotene-d8, because sample preparation included saponification. After participants consumed β-carotene-d8, labeled β-carotene (Fig. 1A) and ROH plus retinyl ester (Fig. 1B) were measurable in plasma throughout the sampling period. This was true for both the 20- and 40-mg doses. After each dose, there was a rapid rise in both plasma-labeled β-carotene and ROH plus retinyl ester, followed by a rapid decline. By the end of the sampling period, plasma analytes had nearly returned to 0 (baseline).
FIGURE 1 .
Plasma labeled β-carotene concentration (A) and labeled ROH plus retinyl ester (B) as a function of time after participants ingested 20 or 40 mg of β-carotene-d8. Values are means ± SE, n = 7.
Area under the curve (AUC) calculations for β-carotene-d8 doubled from the 20-mg dose to the 40-mg dose (Table 1). AUC calculations for plasma labeled ROH plus retinyl ester did not increase to the same magnitude as the increase in dose (Table 1). Plasma AUC for ROH plus retinyl ester increased by only 36% from the 20-mg dose to the 40-mg dose. The sum of the AUC values for β-carotene plus retinyl esters and ROH increased 47% from the low dose to the high dose.
TABLE 1.
AUC in participants after ingestion of 20 or 40 mg of β-carotene-d81
| 20-mg dose | 40-mg dose | |
|---|---|---|
| Plasma analyte | μmol/L·h | |
| β-Carotene | 61.4 ± 13.4 | 124.6 ± 21.7* |
| Retinol plus retinyl ester | 18.3 ± 4.4 | 24.9 ± 2.3* |
Values are means ± SEM, n = 7. *Different from the 20-mg dose, P < 0.05.
Variabilities in plasma responses were greater at the lower dose than the higher dose, especially for retinoid AUC, which was particularly susceptible to dose and saturation. The plasma β-carotene AUC ranged from 29 to 134 μmol/L·h (CV = 58%) for the 20-mg dose and from 55 to 208 μmol/L·h (CV = 46%) for the 40-mg dose. Plasma retinoid AUC ranged from 6 to 40 μmol/L·h (CV = 64%) for the 20-mg dose and from 18 to 33 μmol/L·h (CV = 24%) for the 40-mg dose.
The correlation coefficient describing the relationship between the baseline plasma ROH concentration and the ROH AUC for the 40-mg dose was 0.743 (P = 0.055). This relationship was weaker for the 20-mg dose (r = 0.53; P = 0.2).
Discussion
Our purpose in this study was to investigate the effect of the size of the oral dose of β-carotene on its conversion to ROH. β-Carotene absorption was assessed by AUC for labeled β-carotene after a bolus dose of β-carotene-d8. Conversion of β-carotene to vitamin A was assessed by AUC for labeled ROH after saponification of plasma samples. The AUC for β-carotene-d8 doubled with doubling of the dose. In contrast, the AUC for retinoid-d4 increased only 36% with doubling of the β-carotene dose, suggested that some process associated with conversion of β-carotene to vitamin A was unable to handle the increased demand.
Plasma kinetic curves observed in this study were in accord with previously published data (12–14) and with previously proposed mechanisms of β-carotene absorption, metabolism, and distribution. Inspection of individual subject curves showed that, for most participants, β-carotene-d8 produced 2 plasma peaks, as observed previously (15–19), with the first representing β-carotene being transported by intestinally derived, triglyceride-rich lipoproteins and the second peak representing β-carotene primarily in LDL (19,20).
Plasma concentrations of labeled β-carotene and labeled vitamin A were also in accord with data from previous studies. Mean peak concentrations for labeled β-carotene were 0.25 μmol/L and 0.45 μmol/L for the 20- and 40-mg doses, respectively. Mean peak concentrations for the labeled vitamin A were 0.17 μmol/L and 0.21 μmol/L for the 20- and 40-mg doses, respectively. These values are in accord with peak values reported previously (9,13,14,19).
A previously published dose-response study with lycopene showed saturation of lycopene absorption at increasing doses (21). A tomato beverage was administered to adult males at increasing serving sizes, delivering lycopene doses ranging from 10 to 120 mg. Compartmental modeling showed that absorption of lycopene for 10, 30, and 60 mg was ∼34, 14, and 7%, respectively. Thus, lycopene over the same dose range as that studied in the present experiment displayed reduced absorption efficiencies with increasing dose.
Two previous studies suggested that β-carotene conversion to vitamin A may be limited at high doses. First, Brubacher and Weiser (8) determined the vitamin A equivalence of β-carotene in rats fed varying levels of β-carotene. In this study, the vitamin A equivalence of β-carotene decreased with increasing dose. In a case study, an adult female consumed a 126-mg dose and a 6-mg dose of β-carotene 2.5 y apart, and the vitamin A equivalence of those doses was determined by the stable isotope reference method (9). The vitamin A equivalence of the β-carotene at the high dose was significantly less than that at the low dose. To confirm this phenomenon of limited conversion of β-carotene to vitamin A, a randomized crossover study of healthy adults was necessary and our data support that indeed vitamin A formation from β-carotene is limited with increasing β-carotene dose. This phenomenon explains the lack of observed vitamin A toxicity with high β-carotene intakes.
The dose dependence of carotenoid uptake and secretion by CaCo-2 cells was investigated by During et al. (22). The process of β-carotene uptake and secretion into chylomicrons was concentration dependent and saturable. Saturation occurred at concentrations above what would be equivalent to the range of typical intakes. Those authors predicted that saturation of absorption would be achieved with dietary intakes equivalent to ∼100 mg/d, which is more than the highest dose administered in this study.
Conversion of β-carotene to retinal in enterocytes is facilitated by the enzyme 15, 15' monooxygenase, with a reported Km for β-carotene of ∼1–6 μmol/L and a Vmax of ∼100–370 pmol β-carotene cleaved·mg protein−1·h−1 (23,24). During et al. (22) estimated that a 5-mg oral dose would result in ∼200 pmol/cm2 of surface absorption. Based on the size and morphology of an enterocyte as determined by scanning electron microscopy (25), the maximum concentration of β-carotene in the enterocyte after a 5-mg dose would be on the order of 25 μmol/L if the entire dose were absorbed instantaneously, and the maximum β-carotene concentration would be 100 μmol/L for instantaneous absorption of a 20-mg dose. However, the distribution of absorption over time and the metabolism of β-carotene by 15, 15' monooxygenase would result in an enterocyte β-carotene concentration less than the maximum theoretical concentration. If the concentration of β-carotene in the enterocyte is reduced to ∼10% the maximum value by time distributed absorption and continuous metabolism of absorbed β-carotene, then the 20-mg dose would provide an enterocyte concentration of 10 μmol/L, which would be in a range where saturation might take place, based on the range of the Km values reported for 15, 15' monooxygenase metabolism of β-carotene. Given that we were comparing and integrating data from isolated enzyme assays, cell culture experiments, and human feeding studies, it is remarkable that these data produced such a consistent picture of the effects of β-carotene dose on its intestinal absorption and conversion to vitamin A.
One weakness of this study is that the doses administered, although in the range of values found in a serving of certain foods, were somewhat high. The 20-mg dose provided the amount of β-carotene present in 230 g raw carrot, 360 g raw spinach, or 211 g cooked sweet potato. Thus, dietary intakes are likely to be similar to or lower than the doses studied here, and it is possible that conversion of β-carotene to vitamin A is less limited at smaller doses. An additional weakness is that absolute values for β-carotene absorption and conversion to vitamin A could not be determined from this type of analysis. When a single oral dose is administered, determination of absolute absorption requires collection and analysis of feces or analysis through compartmental modeling. In the future, these data may be subjected to compartmental modeling to retrieve that information. This, however, does not weaken the overall conclusion of the study.
In conclusion, this study demonstrates that efficiency of β-carotene conversion to vitamin A in humans is reduced at increasing doses. These results explain why vitamin A toxicity is not observed in individuals consuming large amounts of β-carotene. This is important information for future revisions of vitamin A equivalency values for β-carotene.
Acknowledgments
J.A.N. designed research; J.A.N. and D.J.H. conducted research; J.A.N., A.C.K., D.J.H., R.P., V.P.F., and E.H.H. participated in method development; D.J.H., V.P.F., and R.P. analyzed samples; J.A.N., D.J.H., V.P.F., and R.P. analyzed data; J.A.N. and E.H.H. interpreted data; J.A.N. wrote the paper; and J.A.N. had primary responsibility for final content. All authors read and approved the final manuscript.
Supported by the USDA and NIH grants R01DK044498 and R01HL049879.
Author disclosures: J. A. Novotny, D. J. Harrison, R. Pawlosky, V. P. Flanagan, E. H. Harrison, and A. C. Kurilich, no conflicts of interest.
Abbreviations used: AUC, area under the plasma concentration time curve; 13C-BC, all trans-β-carotene-U 13C40; m/z, mass:charge ratio; ROH, retinol; TMS, trimethylsilyl.
References
- 1.WHO. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. WHO Global Database on Vitamin A Deficiency. Geneva: WHO; 2009.
- 2.Brown ED, Micozzi MS, Craft NE, Bieri JG, Beecher G, Edwards BK, Rose A, Taylor PR, Smith JC Jr. Plasma carotenoids in normal men after a single ingestion of vegetables or purified beta-carotene. Am J Clin Nutr. 1989;49:1258–65. [DOI] [PubMed] [Google Scholar]
- 3.Micozzi MS, Brown ED, Edwards BK, Bieri JG, Taylor PR, Khachik F, Beecher GR, Smith JC Jr. Plasma carotenoid response to chronic intake of selected foods and beta-carotene supplements in men. Am J Clin Nutr. 1992;55:1120–5. [DOI] [PubMed] [Google Scholar]
- 4.Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, White WS. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr. 2004;80:396–403. [DOI] [PubMed] [Google Scholar]
- 5.Borel P, Tyssandier V, Mekki N, Grolier P, Rochette Y, Alexandre-Gouabau MC, Lairon D, Azais-Braesco V. Chylomicron beta-carotene and retinyl palmitate responses are dramatically diminished when men ingest beta-carotene with medium-chain rather than long-chain triglycerides. J Nutr. 1998;128:1361–7. [DOI] [PubMed] [Google Scholar]
- 6.Huo T, Ferruzzi MG, Schwartz SJ, Failla ML. Impact of fatty acyl composition and quantity of triglycerides on bioaccessibility of dietary carotenoids. J Agric Food Chem. 2007;55:8950–7. [DOI] [PubMed] [Google Scholar]
- 7.Hu X, Jandacek RJ, White WS. Intestinal absorption of beta-carotene ingested with a meal rich in sunflower oil or beef tallow: postprandial appearance in triacylglycerol-rich lipoproteins in women. Am J Clin Nutr. 2000;71:1170–80. [DOI] [PubMed] [Google Scholar]
- 8.Brubacher GB, Weiser H. The vitamin A activity of beta-carotene. Int J Vitam Nutr Res. 1985;55:5–15. [PubMed] [Google Scholar]
- 9.Tang G, Qin J, Dolnikowski GG, Russell RM. Vitamin A equivalence of beta-carotene in a woman as determined by a stable isotope reference method. Eur J Nutr. 2000;39:7–11. [DOI] [PubMed] [Google Scholar]
- 10.Panel on Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Uses of Dietary Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: 2001.
- 11.Pawlosky RJ, Flanagan VP, Novotny JA. A sensitive procedure for the study of beta-carotene-d8 metabolism in humans using high performance liquid chromatography-mass spectrometry. J Lipid Res. 2000;41:1027–31. [PubMed] [Google Scholar]
- 12.Ho CC, de Moura FF, Kim SH, Burri BJ, Clifford AJ. A minute dose of 14C-beta-carotene is absorbed and converted to retinoids in humans. J Nutr. 2009;139:1480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickenbottom SJ, Follett JR, Lin Y, Dueker SR, Burri BJ, Neidlinger TR, Clifford AJ. Variability in conversion of beta-carotene to vitamin A in men as measured by using a double-tracer study design. Am J Clin Nutr. 2002;75:900–7. [DOI] [PubMed] [Google Scholar]
- 14.Lin Y, Dueker SR, Burri BJ, Neidlinger TR, Clifford AJ. Variability of the conversion of beta-carotene to vitamin A in women measured by using a double-tracer study design. Am J Clin Nutr. 2000;71:1545–54. [DOI] [PubMed] [Google Scholar]
- 15.Dueker SR, Lin Y, Buchholz BA, Schneider PD, Lame MW, Segall HJ, Vogel JS, Clifford AJ. Long-term kinetic study of beta-carotene, using accelerator mass spectrometry in an adult volunteer. J Lipid Res. 2000;41:1790–800. [PubMed] [Google Scholar]
- 16.Lemke SL, Dueker SR, Follett JR, Lin Y, Carkeet C, Buchholz BA, Vogel JS, Clifford AJ. Absorption and retinol equivalence of beta-carotene in humans is influenced by dietary vitamin A intake. J Lipid Res. 2003;44:1591–600. [DOI] [PubMed] [Google Scholar]
- 17.Kostic D, White WS, Olson JA. Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses. Am J Clin Nutr. 1995;62:604–10. [DOI] [PubMed] [Google Scholar]
- 18.Ho CC, de Moura FF, Kim SH, Clifford AJ. Excentral cleavage of beta-carotene in vivo in a healthy man. Am J Clin Nutr. 2007;85:770–7. [DOI] [PubMed] [Google Scholar]
- 19.Novotny JA, Dueker SR, Zech LA, Clifford AJ. Compartmental analysis of the dynamics of beta-carotene metabolism in an adult volunteer. J Lipid Res. 1995;36:1825–38. [PubMed] [Google Scholar]
- 20.Parker RS. Absorption, metabolism, and transport of carotenoids. FASEB J. 1996;10:542–51. [PubMed] [Google Scholar]
- 21.Diwadkar-Navsariwala V, Novotny JA, Gustin DM, Sosman JA, Rodvold KA, Crowell JA, Stacewicz-Sapuntzakis M, Bowen PE. A physiological pharmacokinetic model describing the disposition of lycopene in healthy men. J Lipid Res. 2003;44:1927–39. [DOI] [PubMed] [Google Scholar]
- 22.During A, Hussain MM, Morel DW, Harrison EH. Carotenoid uptake and secretion by CaCo-2 cells: beta-carotene isomer selectivity and carotenoid interactions. J Lipid Res. 2002;43:1086–95. [DOI] [PubMed] [Google Scholar]
- 23.Paik J, During A, Harrison EH, Mendelsohn CL, Lai K, Blaner WS. Expression and characterization of a murine enzyme able to cleave beta-carotene. The formation of retinoids. J Biol Chem. 2001;276:32160–8. [DOI] [PubMed] [Google Scholar]
- 24.Grolier P, Duszka C, Borel P, Alexandre-Gouabau MC, Azais-Braesco V. In vitro and in vivo inhibition of beta-carotene dioxygenase activity by canthaxanthin in rat intestine. Arch Biochem Biophys. 1997;348:233–8. [DOI] [PubMed] [Google Scholar]
- 25.Marsh MN, Peters TJ, Brown AC. Observations of isolated enterocytes and of their subcellular components using transmission and scanning electron microscopy. Gut. 1971;12:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]