Abstract
Acute alcohol intoxication decreases skeletal muscle protein synthesis by impairing mammalian target of rapamycin (mTOR). In 2 studies, we determined whether inhibition of branched-chain amino acid (BCAA) catabolism ameliorates the inhibitory effect of alcohol on muscle protein synthesis by raising the plasma BCAA concentrations and/or by improving the anabolic response to insulin-like growth factor (IGF)-I. In the first study, 4 groups of mice were used: wild-type (WT) and mitochondrial branched-chain aminotransferase (BCATm) knockout (KO) mice orally administered saline or alcohol (5 g/kg, 1 h). Protein synthesis was greater in KO mice compared with WT controls and was associated with greater phosphorylation of eukaryotic initiation factor (eIF)-4E binding protein-1 (4EBP1), eIF4E-eIF4G binding, and 4EBP1-regulatory associated protein of mTOR (raptor) binding, but not mTOR-raptor binding. Alcohol decreased protein synthesis in WT mice, a change associated with less 4EBP1 phosphorylation, eIF4E-eIF4G binding, and raptor-4EBP1 binding, but greater mTOR-raptor complex formation. Comparable alcohol effects on protein synthesis and signal transduction were detected in BCATm KO mice. The second study used the same 4 groups, but all mice were injected with IGF-I (25 μg/mouse, 30 min). Alcohol impaired the ability of IGF-I to increase muscle protein synthesis, 4EBP1 and 70-kilodalton ribosomal protein S6 kinase-1 phosphorylation, eIF4E-eIF4G binding, and 4EBP1-raptor binding in WT mice. However, in alcohol-treated BCATm KO mice, this IGF-I resistance was not manifested. These data suggest that whereas the sustained elevation in plasma BCAA is not sufficient to ameliorate the catabolic effect of acute alcohol intoxication on muscle protein synthesis, it does improve the anabolic effect of IGF-I.
Introduction
Sustained excessive alcohol consumption leads to muscle atrophy characterized by a loss of myofibrillar protein in predominantly fast-twitch skeletal muscle (1). Although alterations in nutrition intake and absorption affect the severity of this wasting syndrome (2), the erosion of lean body mass continues even when nutritional intake is tightly matched (3,4). There is little direct evidence that alcohol abuse accelerates muscle protein degradation, but multiple lines of investigation support a causative role for a decrease in protein synthesis (1). Furthermore, chronic alcohol exposure is not essential, as marked reductions in muscle protein synthesis are observed in a dose- and time-dependent manner after acute (e.g. hours) alcohol intoxication (5,6). This alcohol-induced decrease in protein synthesis is caused by impaired translational efficiency in general and peptide-chain initiation in particular (1). Central to the alcohol-induced decrease in translation initiation is inhibition of mammalian target of rapamycin (mTOR)3 kinase activity, as evidenced by less phosphorylation (e.g. activation) of 2 downstream target proteins: eukaryotic initiation factor (eIF)-4E binding protein-1 (4EBP1) and ribosomal protein S6 kinase (S6K)-1 (5,7–9). The activity of mTOR regulates protein synthesis by integrating inputs from nutrients and growth factors and is now recognized to exist in 2 functional complexes, mTOR complex (mTORC)-1 and mTORC2. The former complex is composed of mTOR, regulatory associated protein of mTOR (raptor), G-protein β-subunit like protein (GβL), and the recently identified proteins proline-rich Akt substrate 40 (PRAS40) and DEPTOR (10).
Muscle protein synthesis is accelerated by growth factors [e.g. insulin and insulin-like growth factor (IGF)-I] and nutrient availability, especially that of branched-chain amino acids (BCAA) (11). Of the BCAA, leucine is the most efficacious in stimulating protein synthesis, and elevated plasma leucine stimulates precursor delivery and rate-controlling signal transduction pathways central to translation initiation (12). Recently, She et al. (13) generated and characterized a murine model in which the gene encoding for the mitochondrial branched-chain aminotransferase isozyme (BCATm) was disrupted. The first step in BCAA metabolism, which is catalyzed by BCATm, involves the transfer of the α-amino group of the BCAA to α-ketoglutarate forming glutamate and the 3 respective branched-chain α-keto acids. Because BCATm has a wide tissue distribution, except in liver and neuronal tissues (14), it helps regulate leucine signaling and interorgan exchange of BCAA metabolites (15). In BCATm knockout (KO) mice, therefore, the peripheral catabolism of BCAA is inhibited and plasma BCAA concentrations rise (13). Importantly, because plasma branched-chain α-keto-acids are not altered in BCATm KO mice, the toxic neurological manifestations observed with prolonged elevation in these BCAA transamination derivatives is prevented (16). Therefore, the present study determined whether sustained elevations in the plasma BCAA concentrations produced by inhibition of BCAA catabolism would ameliorate the reduced muscle protein synthesis observed after acute alcohol intoxication under control conditions. We also assessed whether the elevation of BCAA would enhance the anabolic effect of IGF-I in alcohol-treated mice. Scientific justification for this part of the aim is based on the reported interaction of BCAA and insulin on muscle protein synthesis (17,18).
Materials and Methods
Animal preparation and experimental protocols.
Animal protocols were approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine and adhere to the NIH guidelines. BCATm KO mice have sustained elevations in plasma BCAA but do not manifest elevated levels of either plasma or muscle branched-chain α-keto acids (13). However, the cytosolic isoform of BCAT (BCATc), present in neurons and brain (19), is still active in the BCATm KO mice and the excessive local production of these BCAA metabolites can cause severe neurological derangements (16). To prevent the toxic accumulation of keto acids within neural tissues, both BCATm KO mice and their wild-type (WT) C57BL6 littermates were provided free access to both standard rodent diet (Harlan Teklad 2018; Global 18% protein rodent diet) and a purified BCAA-free diet (Dyets 510081) (13,20). The BCAA-free diet was isonitrogenous and isocaloric relative to that of the Teklad diet. Male WT mice consumed ∼45 and 55%, respectively, of the BCAA-free and standard diet, whereas KO mice consumed 76 and 24%, respectively, of the 2 diets (13). Total food intake and calculated energy intake did not differ between WT and KO mice (13). On the morning of the study, mice were weighed and body fat and lean body mass were measured using 1H-NMR (Bruker Minispec, LF90) (13). For this and all studies, food was removed from the mice at 0700 h and the experiment was performed between 0900 and 1200 h. BCATm and WT mice administered either saline or alcohol were alternated to compensate for differences in the period of time mice were without food. Both BCATm KO and WT mice were between 12 and 14 wk of age at the time of study.
In the first experimental series, the in vivo rate of protein synthesis in the gastrocnemius-plantaris complex (hereafter referred to as skeletal muscle) and liver was determined in WT and BCATm KO mice 1 h after either oral saline gavage (e.g. control condition) or 1 h after oral ethanol (5 g/kg body weight). Hence, 4 groups of mice were used: WT + saline (Sal); WT + alcohol; BCATm KO + Sal, and BCATm KO + alcohol. The alcohol dose and timing were based on preliminary studies demonstrating a reduction in muscle protein synthesis and this model mimics the metabolic effects of binge drinking in humans (21). Mice were feed-deprived after saline or alcohol gavage. Protein synthesis was determined using the flooding-dose technique (22). Mice were injected intraperitoneally with [3H]-l-phenylalanine (150 mmol/L, 30 μCi/mL; 0.1 L/kg body weight) and blood was collected 15 min later to determine phenylalanine concentration and radioactivity. Thereafter, skeletal muscle and liver were rapidly excised, freeze-clamped, and stored at −70°C. Tissue was homogenized and processed exactly as described (22). The rate of protein synthesis, nmol phenylalanine incorporated/(mg protein × h), was calculated by dividing the amount of radioactivity incorporated into protein by the plasma phenylalanine specific radioactivity. The assumption in using this technique to estimate the protein synthetic rate in vivo is the tissue phenylalanine concentration is elevated to a high concentration, thereby limiting any dilution effect of nonradioactive phenylalanine derived from proteolysis on the intracellular-specific radioactivity. Under the condition of elevated plasma phenylalanine concentration (∼1200 μmol/L), the specific radioactivity of the plasma phenylalanine is assumed to be equal to the specific radioactivity of the tRNA-bound phenylalanine. Studies using “isolated”-perfused muscle have shown that at a perfusate phenylalanine concentration > 400 μmol/L, the perfusate, intracellular, and tRNA-bound phenylalanine have the same specific radioactivity within 10 min after the start of perfusion with radioisotopes (23). Furthermore, comparable rates of muscle protein synthesis have been obtained using the flooding-dose method whether calculated based on plasma phenylalanine enrichment or phenylalanyl-tRNA (24). In the present study, the WT and BCATm KO mice did not differ for the plasma phenylalanine-specific activity [9.8 ± 0.4 and 10.7 ± 0.6 Bq/(min × nmol phenylalanine), respectively] or the plasma phenylalanine concentration (1212 ± 143 and 1167 ± 81 μmol/L). The specific radioactivity of the plasma phenylalanine was measured by HPLC analysis of supernatant from trichloracetic acid extracts of plasma (22).
The second study used the same 4 experimental groups as above, except that all mice were injected intraperitoneally with a maximally stimulating dose of IGF-I (25 μg/mouse) 1 h after oral gavage of saline or alcohol (25). Hence, 4 groups of mice were used in this study: WT + Sal + IGF-I; WT + alcohol + IGF-I; BCATm KO + Sal + IGF-I, BCATm KO + alcohol + IGF-I. All groups received IGF-I treatment and no drug control groups were included, because this would be repetitious of the animals included in the first study. Blood and muscle were collected 30 min after IGF-I, as described above.
Western blot analysis.
Fresh tissue was homogenized in ice-cold homogenization buffer consisting of (in mmol/L) 20 HEPES (pH 7.4), 2 EGTA, 50 sodium fluoride, 100 potassium chloride, 0.2 EDTA, 50 β-glycerol phosphate, 1 dithiotreitol, 0.1 phenylmethane-sulphonylfluoride, 1 benzamidine, and 0.5 sodium vanadate and clarified by centrifugation (7,8,26,27). Equal amounts of protein per sample were subjected to standard SDS-PAGE for total and phosphorylated S6K1 (Thr389; Cell Signaling), ribosomal protein S6 (Ser240/Ser245; Cell Signaling), and 4EBP1 (Thr 37/46; Bethyl Laboratories). Total and phosphorylated (Thr246) PRAS40, total and phosphorylated (Ser792) raptor, total and phosphorylated (Thr172) 5′-AMP-activated protein kinase (AMPKα), total and phosphorylated (Ser79) acetyl-CoA carboxylase (ACC), as well as total GβL (mLST8), DEPTOR, and regulated in development and DNA damage responses were also determined by Western blot analysis. The 4EBP1-eIF4E and eIF4G-eIF4E complexes were quantified and autoradiographs developed and quantified (7,8,17,26,27).
Muscle was also homogenized in 3[(3-cholamidopropyl)dimethylammonio]-propanesulfonic acid buffer consisting of (in mmol/L) 40 HEPES, pH 7.5, 120 NaCl, 1 EDTA, 10 pyrophosphate, 10 β-glycerol phosphate, 50 sodium fluoride, 1.5 sodium vanadate, 0.3% 3[(3-cholamidopropyl)dimethylammonio]-propanesulfonic acid, and 1 protease inhibitor cocktail tablet to maintain mTORC1 protein-protein interactions (28). Supernatant was combined with anti-raptor or anti-mTOR antibody and immune complexes isolated with a goat anti-rabbit BioMag IgG beads (PerSeptive Diagnostics).
Determination of plasma metabolite and hormone concentrations.
Plasma insulin and IGF-I concentrations were determined by ELISA (Linco Research and Immunodiagnostic Systems, respectively). Plasma alcohol was also quantified (Analox Instruments). BCAA were determined using reverse-phase HPLC after precolumn derivatization of amino acids with phenylisothiocyanate.
Statistical analysis.
Data for each condition are summarized as means ± SEM where the number of mice per treatment group is indicated in the legend to the figure or table. For 2-group analysis, a 2-tailed Student's t test was performed. For 4-group analysis, statistical evaluation of the data were performed using 2-way ANOVA to determine the effects of BCATm gene deletion, alcohol treatment, and the interaction between the 2. When the interaction was significant (P < 0.05), post hoc analysis was performed using Tukey's multiple-comparison test. When necessary, data were logarithmically transformed to normalize the distribution of residuals and to obtain variance homogeneity. Differences were considered significant when P < 0.05.
Results
Confirmation of BCATm phenotype.
Genotyping indicated deletion of BCATm in KO, but not WT mice (data not shown). Body weight of BCATm KO mice was reduced 16% compared with WT controls (30.2 ± 0.9 vs. 25.1 ± 0.5 g; P < 0.05; n = 20 per group). Lean body mass expressed as a percentage of body weight was greater in BCATm KO mice (81.4 ± 1.1%) compared with WT mice (78.1 ± 1.0%; P < 0.05). Conversely, fat mass as a percent of body weight was less in BCATm KO than WT mice (15. 8 ± 0.8% vs. 19.7 ± 1.2%, respectively; P < 0.05). These body composition differences could not be attributed to altered energy intake as food consumption did not differ between groups (WT = 4.1 ± 0.1 g/d vs. KO = 4.0 ± 0.1 g/d). These data confirm the consistent phenotype of the BCATm KO mice (13). These end points were only determined prior to oral administration of either saline or alcohol because of the acute nature of the alcohol challenge.
Plasma BCCA and hormone concentrations.
BCATm deletion increased the plasma concentrations of leucine (∼10-fold), isoleucine (∼6-fold), and valine (∼4-fold) compared with WT values (Table 1). Alcohol did not alter the plasma concentrations of the BCAA in either WT or BCATm KO mice.
TABLE 1.
| Saline |
Alcohol |
P-value |
|||||
|---|---|---|---|---|---|---|---|
| WT | BCATm KO | WT | BCATm KO | Genotype | Alcohol | Genotype × alcohol | |
| Leucine, μmol/L | 58 ± 10 | 847 ± 164 | 111 ± 31 | 835 ± 144 | <0.0001 | >0.1 | >0.1 |
| Isoleucine, μmol/L | 87 ± 10 | 602 ± 104 | 97 ± 16 | 674 ± 139 | <0.0001 | >0.1 | >0.1 |
| Valine, μmol/L | 144 ± 39 | 772 ± 226 | 162 ± 33 | 792 ± 258 | <0.0001 | >0.1 | >0.1 |
| Insulin, pmol/L | 134 ± 18 | 77 ± 8 | 145 ± 23 | 81 ± 11 | <0.001 | >0.1 | >0.1 |
| IGF-I, pmol/L | 110 ± 11 | 98 ± 12 | 91 ± 12 | 89 ± 10 | >0.1 | >0.1 | >0.1 |
Values are means ± SEM, n = 8–10.
Food was removed from mice at 0700 h and blood was collected between 0900 and 1200 h.
The plasma insulin concentration was reduced 40% in BCATm KO mice compared with WT control mice and there was no alcohol-induced change in insulin (Table 1). Neither deletion of BCATm nor alcohol administration altered the plasma IGF-I concentration. Finally, the plasma alcohol concentration did not differ between WT (74 ± 11 mmol/L) and BCATm KO (64 ± 7 mmol/L) mice.
Muscle protein synthesis.
In vivo-determined protein synthesis in skeletal muscle from BCATm KO mice was 20% greater than in that of WT controls (Table 2). Acute alcohol ingestion decreased protein synthesis 30% in WT mice and 45% in BCATm KO mice compared with their respective control values, so muscle protein synthesis did not differ in WT and BCATm KO mice receiving alcohol. Hepatic protein synthesis was not altered by either the deletion of the BCATm gene and/or acute alcohol intoxication (Table 2).
TABLE 2.
Effect of alcohol on the rate of protein synthesis in WT and BCATm KO mice1
| Saline |
Alcohol |
P-value |
|||||
|---|---|---|---|---|---|---|---|
| WT | BCATm KO | WT | BCATm KO | Genotype | Alcohol | Genotype × alcohol | |
| nmol phenylalanine incorporated · mg protein−1 . h−1 | |||||||
| Muscle | 0.43 ± 0.02b | 0.51 ± 0.02a | 0.29 ± 0.02c | 0.27 ± 0.03c | 0.262 | <0.001 | 0.039 |
| Liver | 12.19 ± 0.78 | 12.65 ± 1.14 | 14.63 ± 1.41 | 13.37 ± 1.36 | NS | NS | NS |
Values are means ± SEM, n = 10. Means in a row with superscripts without a common letter differ, P < 0.05.
4EBP1 phosphorylation and eIF4F complex formation.
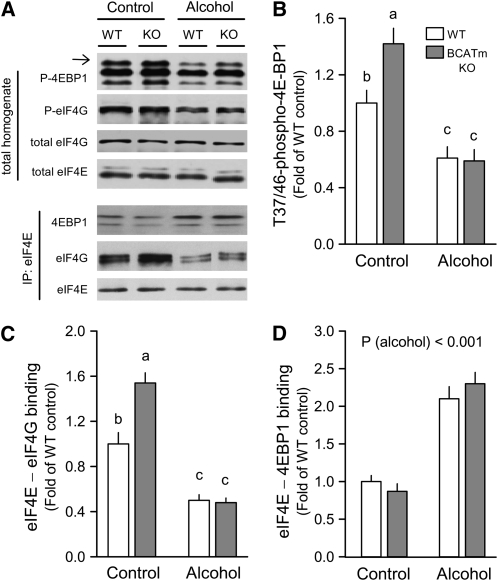
The phosphorylation of 4EBP1, a downstream target protein of mTOR, was quantified as a marker of mTOR kinase activity (29). Thr37/46-phosphorylated 4E-BP1 in muscle of BCATm KO mice was 40% greater than time-matched controls (Fig. 1A,B). However, regardless of the starting point, acute alcohol intoxication decreased 4EBP1 phosphorylation to the same extent. Enhanced 4EBP1 phosphorylation redistributes eIF4E from the inactive eIF4E-4EBP1 complex to the active eIF4E-eIF4G complex (29). In this regard, eIF4E-eIF4G binding under control conditions was greater in BCATm KO mice (Fig. 1A,C). Alcohol increased eIF4E-4EBP1 and decreased eIF4E-eIF4G and the change was comparable in both WT and KO mice (Fig. 1 A,D). In contrast, the phosphorylation of S6K1 (data not shown) and eIF4G (Fig. 1A) in muscle did not differ among the groups.
FIGURE 1 .
Effect of alcohol on 4EBP1 phosphorylation and distribution of eIF4E in skeletal muscle of WT and BCATm KO mice. Representative immunoblots of Thr37/46-phosphorylated (P) 4E-BP1, Ser1108-phosphorylated eIF4G, and total eIF4G and eIF4E in muscle homogenates (A). For Thr37/46 phosphorylated 4EBP1, the most heavily phosphorylated γ-isoform (top band) is identified (arrow on left). There was no statistical difference among groups for phosphorylated eIF4G, total eIF4G, or total eIF4E (data not shown). Also, eIF4E was immunoprecipitated (IP) and representative immunoblots for eIF4G, 4EBP1, and eIF4E present in the IP are shown (A). The β- and α-isoforms for 4EBP1 are so indicated (top and bottom bands, respectively). Densitometric analysis of Western blots for phosphorylated 4EBP1 were normalized to total 4EBP1. (B) Densitometric analysis Western blot data for the amount of eIF4G (C) and 4EBP1 (D) bound to eIF4E, where the amount of eIF4E in the IP did not differ between groups. For all bar graphs, the WT control group was set at 1.0. Values are means ± SEM, n = 9–10. In panels B and C, genotype (P < 0.01), alcohol (P < 0.001), and genotype × alcohol (P < 0.05) were significant. Means without a common letter differ, P < 0.05.
mTORC-1.
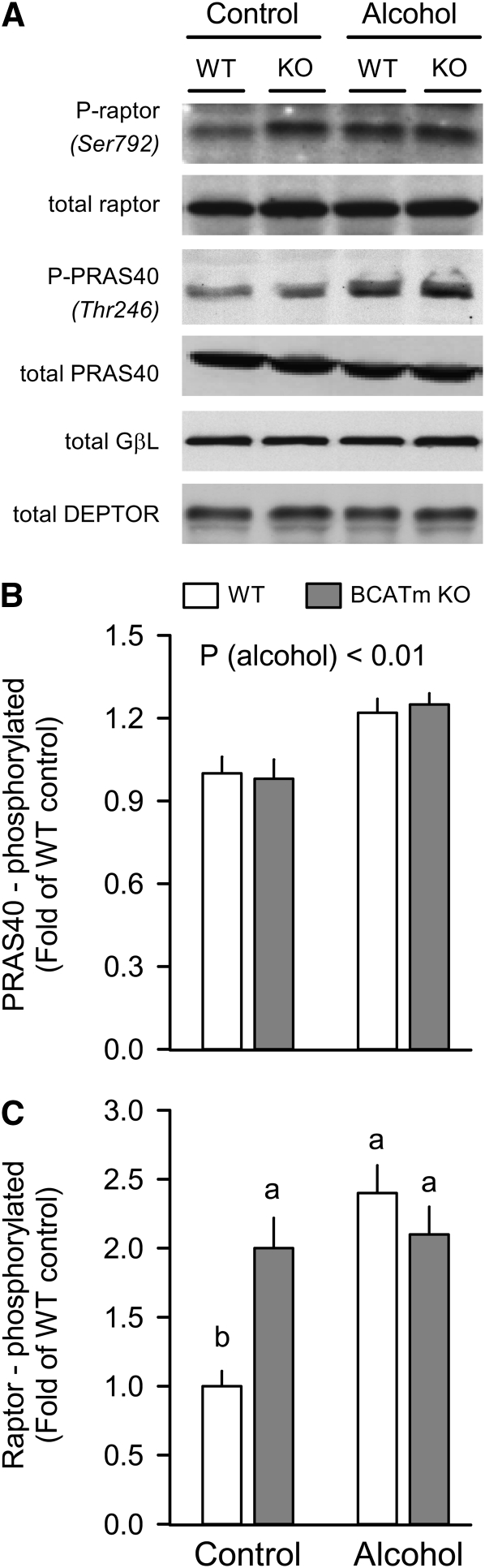
BCATm deletion and/or acute alcohol did not alter the total amount of raptor, PRAS40, GβL, or DEPTOR (Fig. 2A), 4 proteins that, along with mTOR, comprise mTORC1 (10). No difference in PRAS40 phosphorylation was observed between WT and BCATm KO mice under control conditions; however, alcohol increased PRAS40 phosphorylation 20% in both groups (Fig. 2B). Furthermore, raptor phosphorylation was greater in BCATm KO mice under control conditions as well as in both WT and KO mice after alcohol (Fig. 2C).
FIGURE 2 .
Effect of alcohol on PRAS40 and raptor phosphorylation in skeletal muscle of WT and BCATm KO mice. Representative Western blots of Ser792-phosphorylated raptor, total raptor, Thr246-phosphorylated PRAS40, total PRAS40, and total GβL and DEPTOR in muscle homogenates (A). Densitometric analysis of all immunoblots for either phosphorylated PRAS40 (B) or raptor (C), normalized to either total PRAS40 or raptor, respectively, where the value from the WT control group was set at 1.0. For panel B, ANOVA indicated an alcohol (P < 0.001) effect only. For all bar graphs, the WT control group was set at 1.0. Values are means ± SEM, n = 8–10. In panel C, alcohol (P < 0.001) and genotype × alcohol (P < 0.01) were significant. Means without a common letter differ, P < 0.05.
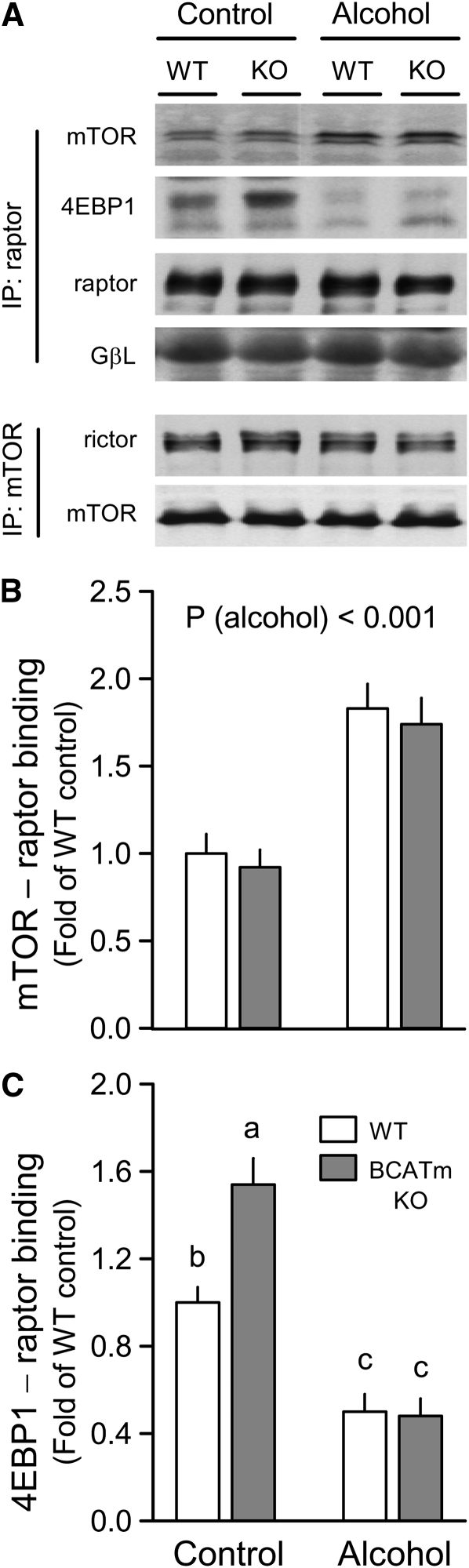
There was no change in the mTOR-raptor interaction under control conditions (Fig. 3A,B), but knockout of BCATm increased 4EBP1-raptor binding (Fig. 3A,C) compared with WT mice. In contrast, alcohol increased mTOR-raptor and decreased the 4EBP1-raptor complex to a comparable extent in muscle from both WT and KO mice. In contrast to the changes in mTORC1, the binding of rictor with mTOR (e.g. mTORC2) did not differ between WT and KO mice under either control conditions or in response to alcohol (Fig. 3A).
FIGURE 3 .
Effect of alcohol on mTOR and 4EBP1 binding with raptor in skeletal muscle of WT and BCATm KO mice. Representative Western blots of various proteins bound to either immunoprecipitated (IP) raptor or mTOR (A). Densitometric analysis of immunoblots for mTOR (B) and 4EBP1 (C) bound to raptor, where the value from the WT control group was set at 1.0. The amount of raptor in the IP did not differ among treatment groups (data not shown). For panel B, ANOVA indicated an alcohol (P < 0.001) effect only. For all bar graphs, the WT control group was set at 1.0. Values are means ± SEM, n = 8–10.In panel C, genotype (P < 0.01), alcohol (P < 0.001), and genotype × alcohol (P < 0.01) were significant. Means without a common letter differ, P < 0.05.
Upstream signal transduction.
Signaling through mTOR is mediated by the activity of AMPK (30). However, the phosphorylation of AMPK and its downstream target ACC did not differ among the 4 groups (data not shown), suggesting this mechanism was not responsible for the abovementioned alcohol effects. Furthermore, the amount of tuberous sclerosis complex (TSC)-1 and TSC2, the phosphorylation of TSC2, and the association of TSC1 with TSC2 did not differ among the 4 groups (data not shown). Finally, although regulated in development and DNA damage responses decreases protein synthesis (31), there was no significant difference between WT and KO mice in the absence or presence of alcohol (data not shown).
IGF-I stimulation.
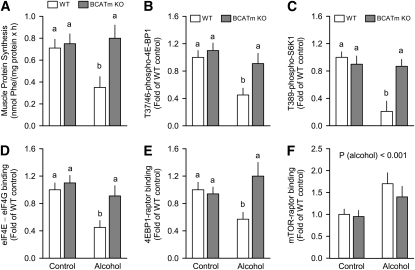
Separate cohorts of WT and BCATm KO mice with or without alcohol were administered a maximally stimulating dose of IGF-I and protein synthesis and mTORC1 activity assessed. The stimulatory effect of IGF-I on muscle protein synthesis was less in alcohol-treated mice than WT controls, but this IGF-I resistance was ameliorated in muscle from alcohol-treated BCATm mice (Fig. 4A). The phosphorylation of 4EBP1 and S6K1 as well as the amount of the eIF4E-eIF4G complex, mTOR-raptor complex, and 4EBP1-raptor complex did not differ between WT and BCATm KO mice injected with IGF-I (Fig. 4, first set of bars in each graph). However, alcohol-treated WT mice injected with IGF-I had less 4EBP1 and S6K1 phosphorylation (Fig. 4B,C), less eIF4E-eIF4G (Fig. 4D) and 4EBP1-raptor (Fig. 4E), and greater mTOR-raptor (Fig. 4F) in muscle than IGF-I-treated WT controls. In contrast, for all endpoints determined, with the exception of the mTOR-raptor binding, alcohol-treated BCATm KO mice injected with IGF-I did not differ from WT control mice injected with IGF-I.
FIGURE 4 .
Effect of IGF-I stimulation on protein synthesis and mTORC1 proteins in skeletal muscle of alcohol-treated WT and BCATm KO mice. All mice in this experiment received an intraperitoneal injection of IGF-I 1 h after oral gavage of either saline or alcohol, as per Methods. Muscle as sampled 30 min after IGF-I stimulation for determination of protein synthesis and assessment of phosphorylated and total proteins. For all bar graphs, the WT control group was set at 1.0. Values are means ± SEM, n = 8. In panels A–E, genotype (P < 0.01), alcohol (P < 0.001), and genotype × alcohol (P < 0.05) were significant. Means without a common letter differ, P < 0.05,
Discussion
Effect of BCATm KO under control conditions.
The plasma concentrations of BCAA in general and leucine in particular were several-fold greater in BCATm KO mice under control (e.g. no alcohol) conditions. This elevation was associated with enhanced protein synthesis and 4EBP1 phosphorylation. Such increases are consistent with those in rats provided chronic dietary leucine supplementation (32). Furthermore, because leucine is transported into but not metabolized in muscle of the BCATm KO mice, these data support the concept that leucine per se and not a metabolite is a direct-acting nutrient signal for stimulation of mTOR and protein synthesis (33). Although elevated plasma leucine may reduce food intake (34), such an anorexic response was not detected in the BCATm KO mice. Indeed, BCATm KO mice ate more food per gram body weight (13). Although leucine is an insulin secretagogue, the plasma insulin concentration was 40% less in BCATm KO mice compared with control WT mice. Such a reduction in insulin might be expected to reduce muscle protein synthesis, but the persistent elevation in BCAA and/or improved insulin sensitivity (13) appears to predominate over the prevailing insulinopenia. Overall, the greater rate of muscle protein synthesis in BCATm KO mice is consistent with their enhanced proportion of lean body mass. These results in the control condition are comparable with previous reports (13).
The current studies also provide a more in-depth analysis of mTORC1 and related signaling elements in BCATm KO mice. Our data indicate that gene deletion increased 4EBP1 phosphorylation and resulted in a redistribution of eIF4E from the inactive eIF4E-4EBP1 complex to the active eIF4E-eIF4G complex, a response consistent with the known release of hyperphosphorylated 4EBP1 from eIF4E (29). Furthermore, raptor phosphorylation was greater in BCATm KO mice under control conditions. This response was unexpected as catabolic, opposed to anabolic, stimuli have been reported to increase raptor phosphorylation (35,36). The reason for this divergent response in the BCATm mice is unclear, but muscle protein synthesis was greater regardless of the underlying mechanism. In contrast, the control level of PRAS40 phosphorylation was unchanged in BCATm KO mice compared with WT mice and again this response was not anticipated, because leucine stimulates PRAS40 phosphorylation in muscle (20). Because PRAS40 is a downstream substrate of Akt, the lack of an increase in PRAS40 phosphorylation may be explained by the mild insulinopenia seen in the BCATm KO mice as opposed to the hyperinsulinemic response normally observed after acute in vivo leucine administration (36). Finally, although we were unable to detect any difference in mTOR-raptor binding between BCATm and WT mice, there was greater binding of 4EBP1 to raptor. This response is consistent with the ability of raptor to function as a scaffold protein and the necessity of 4EBP1 recruitment to the mTOR-raptor complex for optimal stimulation of protein synthesis (28).
Alcohol effect in WT mice.
Acute alcohol intoxication decreases muscle protein synthesis in rats and humans (5–7,37), which is associated with less mTOR kinase activity and evidenced by 4EBP1 hypophosphorylation as well as reduced eIF4E-eIF4G binding. In contradistinction to the response in rats (5,7,8), we did not detect an alcohol-induced decrease in S6K1 or S6 phosphorylation in control WT mice and the reason for this species-specific response in S6K1/S6 is not apparent. Additionally, our work provides novel data on the effect of acute alcohol on mTORC1 complex formation in muscle. For example, alcohol increased raptor phosphorylation, a response similar to that in other catabolic conditions (20,35). Phosphorylation of raptor in an AMPK-dependent manner inhibits mTOR kinase activity (38). Although AMPK activation with 5-aminoimidazole-4-carboxamide-1-β-D- ribonucleoside leads to a comparable increase in raptor phosphorylation (36), we did not detect an alcohol-induced increase in AMPK activity (i.e. no increase in AMPK or ACC phosphorylation). However, because greater raptor phosphorylation was observed in BCATm mice under control conditions (where muscle protein synthesis was increased) and also in WT mice after alcohol ingestion (where muscle protein synthesis was decreased), we interpret these data to suggest this particular phosphorylation event is not central to regulating mTOR kinase activity (39). Finally, we also show that alcohol both increases the amount of mTOR bound to raptor and decreases the amount of 4EBP1 bound to raptor. These data are consistent with the scaffold function of raptor and the necessary recruitment of 4EBP1 prior to its phosphorylation and release. Hence, the alcohol-induced decrease in 4EBP1-raptor matches the observed decrease in 4EBP1 phosphorylation in the total muscle homogenate. Further, these changes are consistent with reports in cell systems exposed to catabolic agents/conditions (40). The alcohol-induced changes in muscle protein synthesis and signal transduction were independent of differences in the circulating concentrations of BCAA, insulin, and IGF-I.
Alcohol effect on BCATm KO mice under control conditions and after IGF-I.
We hypothesized the sustained elevation in leucine and the accompanying stimulation of protein synthesis would ameliorate the alcohol-induced decrease in muscle protein synthesis. However, the inhibitory effect of alcohol on protein synthesis per se and the various mTORC1 signaling components did not differ between WT and BCATm KO mice after acute alcohol intoxication. These results suggest the inhibitory effect of alcohol predominates over the greater availability of leucine and/or BCAA and imply that the alcohol-induced defect in muscle protein synthesis is independent of leucine availability and intracellular metabolism of BCAA.
In the absence of alcohol, IGF-I stimulation of WT and BCATm KO mice yielded comparable levels of 4EBP1 and S6K1 phosphorylation, and eIF4E-eIF4G, mTOR-raptor, and 4EBP1-raptor complex formation did not differ. The comparable response between these groups suggests that because a maximally stimulating dose of IGF-I was administered, an upper limit or “ceiling effect” was likely observed. In the alcohol-treated WT mice, an IGF-I resistance was detected. This blunted IGF-I response in muscle from alcohol-treated mice differs from previous reports in rats, which showed that acute alcohol intoxication impairs IGF-I stimulated phosphorylation of S6K1 but not 4EBP1 (17). The reason for this species-specific response is not clear and was not further investigated. Changes in 1 or more of the 6 IGF binding proteins may also affect IGF-I bioavailability and bioactivity (41); however, whether BCATm deletion and/or alcohol administration altered the circulating concentration of these endogenous IGF-I modulators in mice was not assessed, because the volume of blood collected was limited. Finally, it is noteworthy that the alcohol-induced muscle IGF-I resistance was not seen in BCATm KO mice and is consistent with the improved insulin sensitivity (e.g. improved glucose tolerance) (13). In summary, these data show that the inhibitory effect of acute alcohol intoxication on skeletal muscle protein synthesis in mice persists under control conditions where protein synthetic rates and BCAA supply are elevated. However, the presence of greater BCAA in the circulation did obviate the concomitant IGF-I resistance in skeletal muscle through improved mTORC1 signaling. If a comparable effect of BCAA is operational in more chronic models of alcohol consumption, then we speculate that ameliorating the alcohol-induced IGF-I resistance would blunt the characteristic loss of muscle protein and lean body mass.
Acknowledgments
We thank Anne Pruznak, Gina Deiter, and Jay Nystrom for their expert technical assistance. C.H.L., C.J.L., and T.C.V. developed the research proposal and research design; C.H.L. and T.C.V. performed experiments, analyzed samples, and collected and analyzed data; C.H.L., C.J.L., and T.C.V. wrote the manuscript; all authors read and approved the final draft of the manuscript.
Supported by NIH grants GM38032 (C.H.L.), GM39277 (T.C.V.), and DK062880 (C.J.L.).
Author disclosures: C. H. Lang, C. J. Lynch, and T. C. Vary, no conflicts of interest.
Abbreviations used: ACC, acetyl-CoA carboxylase; AMPK, 5′-AMP-activated protein kinase; BCAA, branched-chain amino acid; BCATc, cytosolic isoform of BCAT; BCATm, mitochondrial branched-chain aminotransferase; eIF, eukaryotic initiation factor; 4EBP1, eukaryotic initiation factor 4E binding protein-1; IGF-I, insulin-like growth factor-I; IP, immunoprecipitated; GβL, G-protein β-subunit like protein; KO, knockout; mTOR, mammalian target of rapamycin; mTORC, mammalian target of rapamycin complex; PRAS40, proline-rich Akt substrate 40; raptor, regulatory associated protein of mammalian target of rapamycin; S6K1, 70-kilodalton ribosomal protein S6 kinase-1; TSC, tuberous sclerosis complex; WT, wild type; WT + Sal, wild-type saline treated.
References
- 1.Lang CH, Frost RA, Summer AD, Vary TC. Molecular mechanisms responsible for alcohol-induced myopathy in skeletal muscle and heart. Int J Biochem Cell Biol. 2005;37:2180–95. [DOI] [PubMed] [Google Scholar]
- 2.Preedy VR, Adachi J, Ueno Y, Ahmed S, Mantle D, Mullatti N, Rajendram R, Peters TJ. Alcoholic skeletal muscle myopathy: definitions, features, contribution of neuropathy, impact and diagnosis. Eur J Neurol. 2001;8:677–87. [DOI] [PubMed] [Google Scholar]
- 3.Lang CH, Wu D, Frost RA, Jefferson LS, Kimball SR, Vary TC. Inhibition of muscle protein synthesis by alcohol is associated with modulation of eIF2B and eIF4E. Am J Physiol. 1999;277:E268–76. [DOI] [PubMed] [Google Scholar]
- 4.Reilly ME, Erylmaz EI, Amir A, Peters TJ, Preedy VR. Skeletal muscle ribonuclease activities in chronically ethanol-treated rats. Alcohol Clin Exp Res. 1998;22:876–83. [PubMed] [Google Scholar]
- 5.Lang CH, Pruznak AM, Deshpande N, Palopoli MM, Frost RA, Vary TC. Alcohol intoxication impairs phosphorylation of S6K1 and S6 in skeletal muscle independently of ethanol metabolism. Alcohol Clin Exp Res. 2004;28:1758–67. [DOI] [PubMed] [Google Scholar]
- 6.Reilly ME, Mantle D, Richardson PJ, Salisbury J, Jones J, Peters TJ, Preedy VR. Studies on the time-course of ethanol's acute effects on skeletal muscle protein synthesis: comparison with acute changes in proteolytic activity. Alcohol Clin Exp Res. 1997;21:792–8. [PubMed] [Google Scholar]
- 7.Lang CH, Frost RA, Deshpande N, Kumar V, Vary TC, Jefferson LS, Kimball SR. Alcohol impairs leucine-mediated phosphorylation of 4E–BP1, S6K1, eIF4G, and mTOR in skeletal muscle. Am J Physiol Endocrinol Metab. 2003;285:E1205–15. [DOI] [PubMed] [Google Scholar]
- 8.Lang CH, Pruznak AM, Nystrom GJ, Vary TC. Alcohol-induced decrease in muscle protein synthesis associated with increased binding of mTOR and raptor: comparable effects in young and mature rats. Nutr Metab (Lond). 2009;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sneddon AA, Koll M, Wallace MC, Jones J, Miell JP, Garlick PJ, Preedy VR. Acute alcohol administration inhibits the refeeding response after starvation in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2003;284:E874–82. [DOI] [PubMed] [Google Scholar]
- 10.Dunlop EA, Tee AR. Mammalian target of rapamycin complex 1: signalling inputs, substrates and feedback mechanisms. Cell Signal. 2009;21:827–35. [DOI] [PubMed] [Google Scholar]
- 11.Vary TC, Lynch CJ. Nutrient signaling components controlling protein synthesis in striated muscle. J Nutr. 2007;137:1835–43. [DOI] [PubMed] [Google Scholar]
- 12.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab. 2006;290:E612–21. [DOI] [PubMed] [Google Scholar]
- 13.She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutson SM, Wallin R, Hall TR. Identification of mitochondrial branched chain aminotransferase and its isoforms in rat tissues. J Biol Chem. 1992;267:15681–6. [PubMed] [Google Scholar]
- 15.Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM. A molecular model of human branched-chain amino acid metabolism. Am J Clin Nutr. 1998;68:72–81. [DOI] [PubMed] [Google Scholar]
- 16.Skvorak KJ. Animal models of maple syrup urine disease. J Inherit Metab Dis. 2009;32:229–46. [DOI] [PubMed] [Google Scholar]
- 17.Kumar V, Frost RA, Lang CH. Alcohol impairs insulin and IGF-I stimulation of S6K1 but not 4E–BP1 in skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E917–28. [DOI] [PubMed] [Google Scholar]
- 18.Suryawan A, O'Connor PM, Bush JA, Nguyen HV, Davis TA. Differential regulation of protein synthesis by amino acids and insulin in peripheral and visceral tissues of neonatal pigs. Amino Acids. 2009;37:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Espinosa MA, Wallin R, Hutson SM, Sweatt AJ. Widespread neuronal expression of branched-chain aminotransferase in the CNS: implications for leucine/glutamate metabolism and for signaling by amino acids. J Neurochem. 2007;100:1458–68. [DOI] [PubMed] [Google Scholar]
- 20.Pruznak AM, Kazi AA, Frost RA, Vary TC, Lang CH. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-beta-D-ribonucleoside prevents leucine-stimulated protein synthesis in rat skeletal muscle. J Nutr. 2008;138:1887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivara FP, Jurkovich GJ, Gurney JG, Seguin D, Fligner CL, Ries R, Raisys VA, Copass M. The magnitude of acute and chronic alcohol abuse in trauma patients. Arch Surg. 1993;128:907–12. [DOI] [PubMed] [Google Scholar]
- 22.Vary TC, Lang CH. Assessing effects of alcohol consumption on protein synthesis in striated muscles. Methods Mol Biol. 2008;447:343–55. [DOI] [PubMed] [Google Scholar]
- 23.McKee EE, Cheung JY, Rannels DE, Morgan HE. Measurement of the rate of protein synthesis and compartmentation of heart phenylalanine. J Biol Chem. 1978;253:1030–40. [PubMed] [Google Scholar]
- 24.Caso G, Ford GC, Nair KS, Garlick PJ, McNurlan MA. Aminoacyl-tRNA enrichment after a flood of labeled phenylalanine: insulin effect on muscle protein synthesis. Am J Physiol Endocrinol Metab. 2002;282:E1029–38. [DOI] [PubMed] [Google Scholar]
- 25.Bark TH, McNurlan MA, Lang CH, Garlick PJ. Increased protein synthesis after acute IGF-I or insulin infusion is localized to muscle in mice. Am J Physiol. 1998;275:E118–23. [DOI] [PubMed] [Google Scholar]
- 26.Lang CH, Frost RA, Svanberg E, Vary TC. IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol Endocrinol Metab. 2004;286:E916–26. [DOI] [PubMed] [Google Scholar]
- 27.Lang CH, Frost RA, Vary TC. Acute alcohol intoxication increases REDD1 in skeletal muscle. Alcohol Clin Exp Res. 2008;32:796–805. [DOI] [PubMed] [Google Scholar]
- 28.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–80. [DOI] [PubMed] [Google Scholar]
- 29.Shah OJ, Anthony JC, Kimball SR, Jefferson LS. 4E–BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab. 2000;279:E715–29. [DOI] [PubMed] [Google Scholar]
- 30.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–85. [DOI] [PubMed] [Google Scholar]
- 31.McGhee NK, Jefferson LS, Kimball SR. Elevated corticosterone associated with food deprivation upregulates expression in rat skeletal muscle of the mTORC1 repressor, REDD1. J Nutr. 2009;139:828–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch CJ, Hutson SM, Patson BJ, Vaval A, Vary TC. Tissue-specific effects of chronic dietary leucine and norleucine supplementation on protein synthesis in rats. Am J Physiol Endocrinol Metab. 2002;283:E824–35. [DOI] [PubMed] [Google Scholar]
- 33.Lynch CJ, Halle B, Fujii H, Vary TC, Wallin R, Damuni Z, Hutson SM. Potential role of leucine metabolism in the leucine-signaling pathway involving mTOR. Am J Physiol Endocrinol Metab. 2003;285:E854–63. [DOI] [PubMed] [Google Scholar]
- 34.Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–54. [DOI] [PubMed] [Google Scholar]
- 35.Frost RA, Nystrom GJ, Lang CH. Endotoxin and interferon-gamma inhibit translation in skeletal muscle cells by stimulating nitric oxide synthase activity. Shock. 2009;32:416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anthony JC, Lang CH, Crozier SJ, Anthony TG, MacLean DA, Kimball SR, Jefferson LS. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab. 2002;282:E1092–101. [DOI] [PubMed] [Google Scholar]
- 37.Pacy PJ, Preedy VR, Peters TJ, Read M, Halliday D. The effect of chronic alcohol ingestion on whole body and muscle protein synthesis–a stable isotope study. Alcohol Alcohol. 1991;26:505–13. [DOI] [PubMed] [Google Scholar]
- 38.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carriere A, Cargnello M, Julien LA, Gao H, Bonneil E, Thibault P, Roux PP. Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr Biol. 2008;18:1269–77. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Kimball SR, Jefferson LS, Shenberger JS. Hydrogen peroxide impairs insulin-stimulated assembly of mTORC1. Free Radic Biol Med. 2009;46:1500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–54. [DOI] [PubMed] [Google Scholar]