Abstract
Maternal vitamin D deficiency has been associated with numerous adverse health outcomes, but its association with fetal growth restriction remains uncertain. We sought to elucidate the association between maternal serum 25-hydroxyvitamin D [25(OH)D] concentrations in early pregnancy and the risk of small-for-gestational age birth (SGA) and explore the association between maternal single nucleotide polymorphisms (SNP) in the vitamin D receptor (VDR) gene and the risk of SGA. We conducted a nested case-control study of nulliparous pregnant women with singleton pregnancies who delivered SGA infants (n = 77 white and n = 34 black) or non-SGA infants (n = 196 white and n = 105 black). Women were followed from <16 wk gestation to delivery. Women's banked sera at <22 wk were newly measured for 25(OH)D and DNA extracted for VDR genotyping. SGA was defined as live-born infants that were <10th percentile of birth weight according to nomograms based on gender and gestational age. After confounder adjustment, there was a U-shaped relation between serum 25(OH)D and risk of SGA among white mothers, with the lowest risk from 60 to 80 nmol/L. Compared with serum 25(OH)D 37.5–75 nmol/L, SGA odds ratios (95% CI) for levels <37.5 and >75 nmol/L were 7.5 (1.8, 31.9) and 2.1 (1.2, 3.8), respectively. There was no relation between 25(OH)D and SGA risk among black mothers. One SNP in the VDR gene among white women and 3 SNP in black women were significantly associated with SGA. Our results suggest that vitamin D has a complex relation with fetal growth that may vary by race.
Introduction
Maternal vitamin D deficiency is a major public health problem. Approximately 2 in 3 pregnant women in the United States have suboptimal vitamin D status, with even higher prevalences reported among black and Mexican-American women (1). Vitamin D deficiency is prevalent even in settings of widespread prenatal vitamin use (2), because prenatal vitamins contain doses of vitamin D that are too low to meaningfully raise serum 25-hydroxyvitamin D [25(OH)D]11 concentrations (3) and dietary intakes of vitamin D among American women are inadequate (4). More than one-half of U.S. women report always or most of the time practicing sun protective behaviors, including using sunscreen or staying in the shade (1), which limit cutaneous production of vitamin D.
Poor vitamin D status during pregnancy has been associated with preeclampsia (5), gestational diabetes (6), and bacterial vaginosis (7), as well as offspring rickets (8), reduced bone density (9), asthma (10), and schizophrenia (11). The link between maternal vitamin D status and fetal growth, as measured most frequently by infant birth weight and birth length, has been explored by a number of investigators with mixed results (12–20). The inconsistency of past findings may be related to the study of continuous birth size rather than a pathologic condition such as growth restriction, or the varied populations studied and methods used. Furthermore, the majority of past studies examined the effect of vitamin D in the 3rd trimester of pregnancy, when fetal growth velocity is greatest (21). Yet, evidence indicates that fetal growth trajectory in late gestation is determined much earlier, most likely in early pregnancy (22). Moreover, the effects of vitamin D status on birth outcome may depend in part on race/ethnicity and maternal or fetal genotype. Some evidence suggests that associations between vitamin D status and adverse health outcomes that are present in whites do not exist among blacks (23–25). Additionally, polymorphisms in vitamin D-metabolizing genes have been related to a number of common, complex conditions for which low 25(OH)D concentration is a risk factor, such as osteoporosis and prostate cancer (26), and a recent study observed that infant vitamin D receptor (VDR) polymorphisms modified the effect of maternal 25(OH)D on offspring size (27). Because fetal growth restriction is associated with perinatal morbidity and mortality (28,29) and later-life chronic disease (30), it is imperative to find modifiable risk factors for this adverse birth outcome.
Our objective was to elucidate the association between maternal serum 25(OH)D concentrations in early pregnancy and the risk of small-for-gestational age birth (SGA), a measure of fetal growth restriction that is defined based on birth weights <10th percentile for a given standard. We also sought to determine the association between maternal single nucleotide polymorphisms (SNP) in the VDR gene and SGA risk.
Methods
We used existing data and banked serum and DNA samples collected as part of a large prospective pregnancy cohort study. Women carrying singleton fetuses were enrolled at <16 wk gestation from outpatient clinics at Magee-Womens Hospital in Pittsburgh, PA (latitude 40° N) and affiliated private practices from 1997 to 2001. The response rate was 72%. After providing informed, written consent, all women completed an interviewer-administered questionnaire at enrollment to collect data on sociodemographic factors, medical history, and health behaviors. Blood samples from nonfasting participants were collected at times of usual blood draws for clinical indications (initial visit, 16–18 wk, 26–29 wk, and predelivery). Maternal serum was processed and DNA was extracted. Both specimens were stored at –80°C. Medical records were abstracted to ascertain antepartum and delivery events and neonatal outcomes. The study was approved by the University of Pittsburgh and Magee-Womens Hospital Institutional Review Boards.
We conducted a nested case-control study among the 1198 women in the cohort who self-identified their race as black or white, were nulliparous, had no preexisting medical conditions, and delivered a live-born infant. In this cohort, there were 124 SGA births (10.4% incidence). Of these, 112 had a maternal blood sample at <22 wk banked and were therefore eligible for the current analysis. We randomly selected 303 non-SGA pregnancies in the cohort to serve as controls. We sought to select 1 sample at <22 wk from each woman, because early pregnancy is likely the time when exposures have the greatest impact on fetal growth trajectory (21). If a woman had more than 1 sample collected at <22 wk gestation, we randomly selected 1 sample using a random number generator. One white control and 2 white cases were excluded because their serum 25(OH)D concentrations were outside the detectable range (discussed below). The final sample size for the primary analyses was 111 SGA cases (n = 77 white and n = 34 black) and 301 controls (n = 196 white and n = 105 black).
Definition of SGA.
SGA births were live-born infants that were <10th percentile of birth weight according to nomograms based on gender and gestational age from a reference population of over 50,000 infants delivered at Magee-Womens Hospital in Pittsburgh, PA. Gestational age was based on a reliable, self-reported estimate of last menstrual period or an ultrasound early in pregnancy. If gestational age estimates were available from both the last menstrual period and the ultrasound, the ultrasound was used for dating.
Quantitation of serum 25(OH)D.
Maternal serum samples were stored from 1997 to 2001 in aliquots at –80°C until assay in 2006. Quantitation of serum 25(OH)D [25(OH)ergocalciferol + 25(OH)cholecalciferol] was performed using a commercial ELISA from Immunodiagnostic Systems Limited and validated against a HPLC method in the laboratory of Dr. James Roberts at the Magee-Womens Research Institute. The ELISA could detect 25(OH)D in the range of 5–300 nmol/L. Three samples were >300 nmol/L and were excluded from this analysis due to probable sample deterioration. No samples in our analysis fell below the detectable range. The inter-assay CV for the ELISA was 10.3%. The ELISA recognized 100% of serum 25(OH)cholecalciferol and 75% of 25(OH)ergocalciferol but did not distinguish between these 2 forms. In our initial HPLC validation, we observed that only 3 of 32 samples (<10%) had any measurable 25(OH)ergocalciferol and within these samples, 25(OH)ergocalciferol accounted for only 10% of the total measurable 25(OH)D.
Details describing our method for validating the results of the ELISA against HPLC were described previously (2). The inter-assay CV for serum 25(OH)cholecalciferol using the HPLC method was 5.8%. The sensitivity of the HPLC method was <10 nmol/L and had a linear range to 1000 nmol/L. The relationship between serum 25(OH) D concentrations obtained from the ELISA and those from HPLC was as follows: slope = 1.14, intercept = 22, r = 0.88. Because studies have shown that the ELISA we used for this analysis overestimates serum 25(OH)D, particularly at concentrations >100 nmol/L (31), we also conducted all analyses limiting the sample to women with values ≤100 nmol/L.
There is no universally acceptable definition of vitamin D deficiency, so we used cut-points suggested recently: <37.5 nmol/L (deficiency), 37.5–75.0 nmol/L (insufficiency), and >75.0 nmol/L (sufficiency) (32,33).
SNP selection.
We used the International Haplotype Map Project Database (34) to identify SNP within a 83.49-kbp region from positions 46,511,588 to 46,595,080 on chromosome 12 (NCBI Build 35 coordinates) that encompassed ∼10 kbp upstream and 10 kbp downstream of the VDR gene. A reference SNP set was initially generated using race-specific genotypes from the HapMap database. We required that a widely studied C/T polymorphism in exon 2 (rs10735810) that introduces a translation start site and protein product that differs in length by 3 amino acids be included in the final SNP set (35). Tagging SNP that capture common variation within the VDR gene region were then selected on the basis of linkage disequilibrium by a pairwise correlation method (36). We forced the Caucasian tagging SNP in the analysis of the HapMap Yoruban genotypes. We required that tagging SNP have a minor allele frequency ≥ 0.05 and predicted the remaining SNP with a minimum r2 = 0.80. A total of 75 tagging SNP were identified for genotyping.
Genotyping.
Genotyping was completed using genomic DNA prepared from whole blood. Genotyping was completed using the fluorogenic 5′-nuclease TaqMan allelic discrimination assay system (Applied Biosystems) under standard conditions on a 7900HT real-time PCR instrument with probes and reagents purchased from Applied Biosystems. Of the 75 SNP identified for genotyping, 70 were successfully genotyped. Eighty-nine of the SGA cases and 265 of the non-SGA controls had DNA available in sufficient quantity and quality for genotyping.
Covariates.
Race/ethnicity was self-reported (white; black). Prepregnancy BMI [weight (kg)/height (m)2] was based on maternal self-report of prepregnancy weight and height at the initial visit. Women self-reported their smoking status in the year before the index pregnancy and in the past 3 mo (nonsmoker, 1–10, ≥10 cigarettes/d). The season of sample collection was defined as winter (December, January, February), spring (March, April, May), summer (June, July, August), and fall (September, October, November). Self-reported data were available for marital status, private insurance, use of periconceptional multivitamins at least once per week, any prepregnancy physical activity, and intensity of physical activity.
We created a socioeconomic index variable by averaging the component scores of 3 self-reported variables: household income, maternal education, and maternal occupation. For each variable, we computed the cumulative percentage distribution, and the midpoint of the percentage interval for each level of the variable constituted the score for that level, as done previously (37). For example, women who reported college as their highest level of education were distributed between the 75th and the 80th percentile, with a midpoint of 77.8. This score was therefore assigned to the education variable for all women in this level. The summary socioeconomic score (SES) was obtained by averaging these 3 scores and dividing by 10. For women with only 2 of 3 reported variables (16%), the mean of the 2 variables was used. This cumulative score was highly correlated with each individual component (r = 0.81–0.87).
Analysis.
Data are presented as mean (95% CI) or odds ratio (OR) (95% CI). Serum 25(OH)D concentrations were skewed, so they were log-transformed before statistical tests were performed. Multivariable logistic regression was used to assess the association between maternal serum 25(OH)D and the risk of SGA birth after confounder adjustment. Serum 25(OH)D was categorized using the deficiency cut-points as well as quartiles defined by the distribution among the non-SGA controls. Because serum 25(OH)D concentrations were curvilinear in the logit of SGA, we tested numerous splines and fractional polynomials to assess the dose-response relation between serum 25(OH)D levels and SGA risk (19). The curvilinear association between serum 25(OH)D and SGA risk in whites was best described using a quadratic spline with knot at 70 nmol/L.
We fit parsimonious regression models by specifying full models with potential confounding variables: race/ethnicity, prepregnancy BMI, smoking, SES, season, maternal age, gestational age at blood sampling, marital status, insurance status, periconceptional multivitamin use, and preconception physical activity. Potential confounding factors were considered to not be influential and were removed from the model if their inclusion did not satisfy our a priori change-in-estimate criterion (>10% change in OR). Effect modification by race/ethnicity was tested using a likelihood ratio test (α = 0.10) and comparing stratum-specific effect estimates for meaningful differences. Prepregnancy BMI, smoking during pregnancy, and SES met our definition of confounding and were included in the race-stratified models.
Finally, as a sensitivity analysis, all analyses were rerun after the exclusion of women with serum 25(OH)D >100 nmol/L because of the concern surrounding the potential error with the assay at high values. Notably, we reassessed the dose-response relation and fit the best new model to the restricted dataset. In the restricted dataset, the best fit of the data among white women was a quadratic term for vitamin D.
For the genotyping analysis, Haploview 4.1 (38) was used to calculate Hardy-Weinberg Equilibrium (HWE) for each SNP (threshold P > 0.001) and to evaluate linkage disequilibrium. HWE was tested in each race group separately. SNP were excluded from subsequent analyses if their minor allele frequency was <1% or they were out of HWE in the sample of controls. Allelic and genotypic distributions were compared among race groups and between cases and controls using the chi-square test. The association between genotype and SGA was analyzed using logistic regression, where SNP were included as linear (additive) terms. Genotype models were analyzed with and without serum 25(OH)D in the model.
Results
Among both white and black women, those who delivered SGA infants tended to be younger, unmarried, of low socioeconomic status, users of cigarettes, and leaner compared with women delivering non-SGA infants (Table 1). Because most women were recruited at their initial visit in the first trimester, the majority of blood samples were from 0 to 13 wk of gestation.
TABLE 1.
Maternal characteristics among SGA age cases and controls1
| Whites |
Blacks |
|||
|---|---|---|---|---|
| Non-SGA controls | SGA cases | Non-SGA controls | SGA cases | |
| Median maternal age, y | 23 [15–42] | 22 [14–36] | 19 [14–35] | 20 [15–35] |
| Maternal age, n (%) | ||||
| <20 y | 47 (24.0) | 18 (23.4) | 56 (53.3) | 16 (47.1) |
| 20–29 y | 105 (53.6) | 50 (64.9) | 42 (40.0) | 17 (50.0) |
| ≥30 y | 44 (22.5) | 9 (11.7) | 7 (6.7) | 1 (2.9) |
| Marital status, n (%) | ||||
| Married | 77 (39.3) | 17 (22.1) | 102 (97.1) | 32 (94.1) |
| Unmarried | 119 (60.7) | 60 (77.9) | 3 (2.9) | 2 (5.9) |
| Private insurance, n (%) | ||||
| No | 110 (62.5) | 52 (72.2) | 84 (84.9) | 27 (84.4) |
| Yes | 66 (37.5) | 20 (27.8) | 15 (15.2) | 5 (15.6) |
| Median composite of maternal SES | 5.3 [0.7–9.4] | 4.2 [0.7–9.3] | 3.9 [0.7–8.5] | 3.3 [0.7–8.5] |
| Quartiles of maternal SES composite variable, n (%) | ||||
| Q1 [median 1.92] | 37 (18.9) | 21 (27.3) | 35 (33.3) | 15 (44.1) |
| Q2 [median 3.82] | 42 (21.4) | 24 (31.2) | 22 (21.0) | 10 (29.4) |
| Q3 [median 5.62] | 42 (21.4) | 14 (18.2) | 40 (38.1) | 7 (20.6) |
| Q4 [median 8.40] | 75 (38.3) | 18 (23.3) | 8 (7.6) | 2 (5.9) |
| Smoking status in the year before the index pregnancy, n (%) | ||||
| Nonsmokers | 91 (46.4) | 27 (35.5) | 63 (60.6) | 22 (68.8) |
| 1–10 cigarettes/d | 38 (19.4) | 16 (21.1) | 32 (30.8) | 9 (28.1) |
| ≥11 cigarettes/d | 67 (34.2) | 33 (43.4) | 9 (8.7) | 1 (3.1) |
| Smoking status in the 3 mo prior to enrollment, n (%) | ||||
| Nonsmokers | 130 (66.3) | 36 (46.8) | 86 (81.9) | 24 (70.6) |
| 1–10 cigarettes/d | 50 (25.5) | 33 (42.8) | 17 (16.2) | 9 (26.5) |
| ≥11 cigarettes/d | 16 (8.2) | 8 (10.4) | 2 (1.9) | 1 (2.9) |
| Prepregnancy BMI, n (%) | ||||
| <18.5 kg/m2 | 17 (8.7) | 10 (13.0) | 3 (2.9) | 4 (11.7) |
| 18.5–24.9 kg/m2 | 109 (55.6) | 48 (62.3) | 50 (47.5) | 16 (47.1) |
| 25.0–29.9 kg/m2 | 43 (21.9) | 13 (16.9) | 26 (24.8) | 5 (14.7) |
| ≥30.0 kg/m2 | 27 (13.8) | 6 (7.8) | 26 (24.8) | 9 (26.5) |
| Periconceptional multivitamin use at least once/wk, n (%) | ||||
| Yes | 88 (44.9) | 42 (54.6) | 72 (68.6) | 23 (67.6) |
| No | 108 (55.1) | 35 (45.4) | 33 (31.4) | 11 (32.4) |
| Preconception leisure-time physical activity, n (%) | ||||
| None | 106 (54.1) | 51 (66.2) | 71 (68.3) | 21 (61.8) |
| Yes, low intensity | 13 (6.6) | 5 (6.5) | 9 (8.7) | 3 (8.8) |
| Yes, medium intensity | 42 (21.4) | 12 (15.6) | 16 (15.4) | 6 (17.7) |
| Yes, high intensity | 35 (17.9) | 9 (11.7) | 8 (7.6) | 4 (11.7) |
| Season of blood sampling, n (%) | ||||
| Winter | 38 (19.4) | 17 (22.1) | 25 (23.8) | 7 (20.6) |
| Spring | 54 (27.6) | 23 (29.9) | 38 (36.2) | 14 (41.2) |
| Summer | 50 (25.5) | 20 (26.0) | 18 (17.1) | 8 (23.5) |
| Fall | 54 (27.6) | 17 (22.0) | 24 (22.9) | 5 (14.7) |
| Median birthweight centile | 57.1 [11.7–100] | 5.4 [0.1–9.9] | 53.0 [13.1–98.8] | 5.3 [0.1–9.9] |
| Median gestational age of blood sampling, wk | 10.4 [4.7–21.0] | 10.1 [5.6–20.4] | 10.4 [5.3–20.4] | 9.1 [5.4–19.6] |
| Gestational age of blood sampling, n (%) | ||||
| 0–13 wk | 135 (68.9) | 52 (67.5) | 66 (62.9) | 23 (67.7) |
| 14 to <22 wk | 61 (31.1) | 25 (32.5) | 39 (37.1) | 11 (32.3) |
| Large-for-gestational age,2n (%) | 31 (15.8) | – | 12 (11.4) | – |
| Delivery <37 wk, n (%) | 15 (7.7) | 6 (7.8) | 10 (9.5) | 4 (11.8) |
| Preeclampsia,3n (%) | 9 (4.6) | 4 (5.2) | 1 (1.0) | 2 (5.9) |
Values are n (%) or medians [range]. Missing data were as follows: insurance status was missing for 10 white controls, 5 white cases, 6 black controls, and 2 black cases; year-prior smoking status was missing for 1 white SGA case; preconception physical activity was missing for 1 black control.
Large-for-gestational age was defined as live-born infants that were >90th percentile of birthweight according to nomograms based on gender and gestational age from a reference population of over 50,000 infants delivered at Magee-Womens Hospital in Pittsburgh, PA.
Preeclampsia was defined as new-onset hypertension and proteinuria after 20 wk of gestation.
The association between serum 25(OH)D and risk of SGA varied by race/ethnicity (P = 0.01). Among white mothers, mean maternal serum 25(OH)D at <22 wk did not differ between SGA cases [geometric mean (95% CI): 73.2 (69.7, 76.8) nmol/L] and controls [71.5 (64.0, 79.9) nmol/L]. However, white mothers with SGA infants were more likely than white controls to have serum 25(OH)D <37.5 nmol/L (deficiency) and >75 nmol/L (sufficiency) (P < 0.0001) (Table 2). Case mothers were also more likely to be in the lowest and highest quartile of serum 25(OH)D concentrations (P < 0.05) (Table 2). These results did not change after limiting the analysis to women with serum 25(OH)D ≤100 nmol/L (n = 217; data not shown).
TABLE 2.
Association between maternal vitamin D status and the risk of SGA by race/ethnicity
| Controls | SGA | Unadjusted OR (95% CI) | Adjusted OR1 (95% CI) | |
|---|---|---|---|---|
| n (%) | ||||
| White women | ||||
| Serum 25(OH)D at <22 wk2 | ||||
| <37.5 nmol/L | 3 (1.5) | 8 (10.4) | 10.6 (2.6, 42.5) | 7.5 (1.8, 31.9) |
| 37.5 – 75 nmol/L | 107 (54.6) | 27 (35.1) | 1.0 (ref) | 1.0 (ref) |
| >75 nmol/L | 86 (43.9) | 42 (54.5) | 1.9 (1.1, 3.4) | 2.1 (1.2, 3.8) |
| Quartile 1 [21.0–58.0 nmol/L] | 49 (25.0) | 25 (32.5) | 3.1 (1.3, 7.6) | 2.7 (1.1, 6.8) |
| Quartile 2 [58.1–71.4 nmol/L] | 49 (25.0) | 8 (10.4) | 1.0 (ref) | 1.0 (ref) |
| Quartile 3 [71.5–90.6 nmol/L] | 49 (25.0) | 15 (19.5) | 1.9 (0.7, 4.8) | 1.9 (0.7, 5.1) |
| Quartile 4 [90.7–245.0 nmol/L] | 49 (25.0) | 29 (37.6) | 3.6 (1.5, 8.7) | 3.9 (1.6, 9.7) |
| Black women | ||||
| Serum 25(OH)D at <22 wk | ||||
| <37.5 nmol/L | 48 (45.7) | 17 (50.0) | 1.4 (0.6, 3.1) | 1.5 (0.6, 3.5) |
| 37.5–75 nmol/L | 50 (47.6) | 13 (38.2) | 1.0 (ref) | 1.0 (ref) |
| >75 nmol/L | 7 (6.7) | 4 (11.8) | 2.2 (0.6, 8.7) | 2.2 (0.5, 9.0) |
| Quartile 1 [13.8–30.0 nmol/L] | 27 (25.7) | 11 (32.4) | 1.8 (0.6, 5.5) | 1.7 (0.5, 5.5) |
| Quartile 2 [30.1–38.8 nmol/L] | 26 (24.8) | 6 (17.7) | 1.0 (ref) | 1.0 (ref) |
| Quartile 3 [40.4–49.3 nmol/L] | 26 (24.8) | 5 (14.7) | 0.8 (0.2, 3.1) | 0.8 (0.2, 3.2) |
| Quartile 4 [49.4–137.2 nmol/L] | 26 (24.8) | 12 (35.3) | 2.0 (0.7, 6.1) | 1.8 (0.5, 5.8) |
Adjusted for prepregnancy BMI, smoking during pregnancy, and SES. Additional adjustment for season, maternal age, gestational age at blood sampling, marital status, insurance status, smoking in the year before pregnancy, periconceptional multivitamin use, or preconception physical activity had no meaningful impact on the results.
Distribution differs by case status, P < 0.0001 (Pearson chi-squared test).
Among white mothers, vitamin D deficiency and sufficiency were both associated with increased risk of SGA, independent of pregravid BMI, smoking, and SES (Table 2). Excluding women with serum 25(OH)D >100 nmol/L did not meaningfully change the results for the vitamin D-sufficient group [serum 25(OH)D 75.1–100 nmol/L; adjusted OR 2.4 (1.2, 4.7)]. In quartile analysis, women in the bottom quartile and upper quartile were ∼2.7 and 3.9 times as likely, respectively, as women in the second quartile to have an SGA infant after confounder adjustment. These results also remained significant even after limiting the analysis to serum 25(OH)D ≤100 nmol/L [upper quartile adjusted OR 9.3 (95% CI: 2.8, 30.6)].
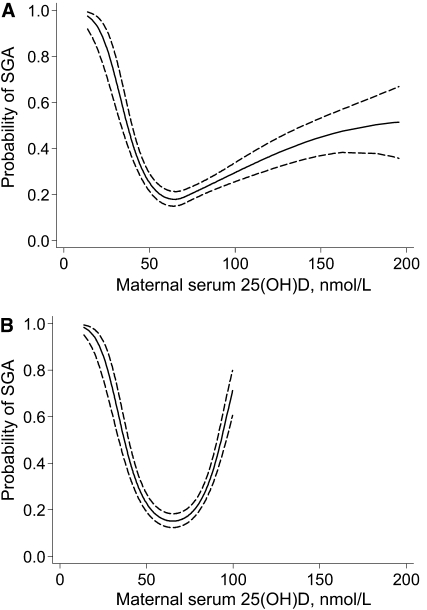
The U-shaped nature of the relation between serum 25(OH)D at <22 wk and risk of SGA was confirmed with spline regression (Fig. 1A; P < 0.01 for spline terms). Indeed, this model suggested a steep downward trend in the probability of SGA from 20 to 70 nmol/L and then a significant gradual increase in risk at concentrations >70 nmol/L. The U-shape did not change after the exclusion of observations >100 nmol/L (n = 217; Fig. 1B) or additional adjustment for other measured covariates.
FIGURE 1 .
Unadjusted association between the probability of SGA births and serum 25(OH)D concentrations among white women (A; n = 273) and white women with a 25(OH)D ≤ 100 nmol/L (B; n = 217) at <22 wk. The point estimates were derived from logistic regression models with serum 25(OH)D concentrations specified as a quadratic spline with knot at 70 nmol/L (P = 0.006; A) or quadratic term (P < 0.0001; B). The solid line represents the point estimate and the dotted lines represent the 95% confidence bands.
Vitamin D deficiency and insufficiency were pervasive among black women, and their geometric mean concentration was almost one-half that of white women (39.8 vs. 72.7 nmol/L). Among black women, the mean maternal serum 25(OH)D concentration at <22 wk did not differ [SGA cases: 39.8 (36.7, 43.2) vs. non-SGA controls: 39.8 (33.6, 47.0) nmol/L] and the distribution of vitamin D status did not vary by case status (Table 2). Likewise, there was no association between maternal serum 25(OH)D and the risk of SGA after confounder adjustment (Table 2), regardless of how the 25(OH)D was specified in the model (e.g. linear, categorical, spline). These results did not change when we excluded serum 25(OH)D >100 nmol/L.
The results for white and black women did not meaningfully differ when pregnancies with preeclampsia, preterm birth, or infants with a birth weight >90th percentile were excluded.
VDR gene sequence variation was significantly associated with risk of SGA and differed by race (Table 3; Supplemental Table 1). Among white women, a single SNP (rs11168292) demonstrated a significant association with SGA, whereas among black women, 3 SNP in this gene (rs11168287, rs3782905, and rs2239179) were significant. Inclusion of serum 25(OH)D concentration in these models revealed that, for both black and white women, VDR genotype and vitamin D concentration have independent contributions to risk of SGA.
TABLE 3.
Association between VDR SNP and the risk of SGA birth by race/ethnicity1
| OR (95% CI) | P-value | Model r2 | Model adjusted r2 | |
|---|---|---|---|---|
| White women | ||||
| Model 1 | 2.6 | 3.8 | ||
| rs11168292 | 1.8 (1.1, 3.0) | 0.02 | ||
| Model 2 | 8.0 | 12.0 | ||
| rs11168292 | 1.7 (1.1, 2.8) | 0.04 | ||
| Serum 25(OH)D at <22 wk | ||||
| <37.5 nmol/L | 14.0 (2.7, 73.0) | 0.005 | ||
| 37.5–75 nmol/L | 1.0 (ref) | |||
| >75 nmol/L | 2.0 (1.1, 3.7) | 0.17 | ||
| Black women | ||||
| Model 1 | 12.3 | 18.6 | ||
| rs11168287 | 0.4 (0.2, 0.9) | 0.03 | ||
| rs3782905 | 5.7 (1.8, 17.7) | 0.004 | ||
| rs2239179 | 0.3 (0.1, 0.6) | 0.003 | ||
| Model 2 | 14.0 | 21.0 | ||
| rs11168287 | 0.4 (0.2, 0.8) | 0.02 | ||
| rs3782905 | 5.3 (1.8, 17.4) | 0.003 | ||
| rs2239179 | 0.3 (0.1, 0.7) | 0.004 | ||
| Serum 25(OH)D at <22 wk | ||||
| <37.5 nmol/L | 1.4 (0.5, 3.9) | 0.61 | ||
| 37.5–75 nmol/L | 1.0 (ref) | |||
| >75 nmol/L | 3.4 (0.6, 17.4) | 0.18 |
Additive models were analyzed for each SNP. OR represent the increase in odds associated with the addition of 1 allele.
Discussion
We observed that maternal vitamin D status at <22 wk gestation had a U-shaped relation with SGA risk in white women. Among whites, the risk of SGA declined precipitously as serum 25(OH)D increased to ∼70 nmol/L, after which the rise in risk was less steep, but still significant, even after excluding women with serum 25(OH)D >100 nmol/L. Thus, the lowest risk of SGA among whites was at serum 25(OH)D concentrations from ∼60 to 80 nmol/L. No association was found among black women.
To our knowledge, our study is the first to examine the relation between maternal serum 25(OH)D in early pregnancy and risk of fetal growth restriction. Brooke et al. (12) conducted a randomized, double-blinded, placebo-controlled trial among 126 Asian mothers living in Britain and found that compared with placebo, supplementation with 1000 iu (25 μg) vitamin D/d starting in the 3rd trimester reduced the risk of SGA (15 vs. 28%; P < 0.05) and increased 25(OH)D concentrations (mean 168.0 vs. 16.2 nmol/L; P < 0.001). In another trial, supplementation with 600,000 iu (15 mg) twice in gestational mo 7 and 8 reduced the incidence of low birth weight (<2500 g) compared with placebo (4 vs. 19%) among 200 Indian pregnant women (18). Serum 25(OH)D was not assessed.
Other previous studies of vitamin D and fetal growth examined measures of size at birth on a continuum with mixed results. One vitamin D supplementation trial and 4 observational studies of 25(OH)D levels, most of which studied late-pregnancy exposure, found no relation with birth weight or other measures of size at birth (13–16,20,39). In contrast, another 3rd-trimester vitamin D supplementation trial and an observational study of maternal vitamin D intake found positive associations with birth weight (17,19). However, the problem with studying birth weight on a continuum is that it is not an intrinsically pathologic outcome. The relation between vitamin D and fetal growth or size may not have as close a relationship in accounting for variation in normal as it would in accounting for variation in a pathophysiologic condition like growth restriction. The conflicting findings may also be due to the study of vitamin D in late pregnancy, rather than early pregnancy, when factors affecting fetal growth have the greatest impact (40).
Vitamin D has a biologically plausible role in fetal growth. In terms of placental development and function, the vitamin D-activating enzyme CYP27B1 as well as VDR are present in human placenta (41). 1,25-Dihydroxyvitamin D, the active form of vitamin D, acting through the VDR and the cAMP/protein kinase A signaling pathway, regulates human chorionic gonadotropin expression and secretion in human synctiotrophoblast (42) and increases placental sex steroid production (43). Vitamin D is also important in glucose/insulin metabolism and homeostasis (44) and so may play a role in glucose availability for transplacental transport and fetal usage. As a regulator of calcium homeostasis and transport, calcitrol also can influence fetal growth directly through influences on skeletal muscle and bone development (45).
The elevated risk of SGA among vitamin D-sufficient white women that we observed previously remained after excluding serum 25(OH)D levels >100 nmol/L, which might have a greater likelihood of being misclassified because of the assay method we used (31). The increased risk with vitamin D sufficiency has not been observed previously and its potential mechanisms remain uncertain. Notably, others have observed U-shaped associations between 25(OH)D and risk of atopic disorders (46,47) and allergic response (48), lower-extremity function (49), prostate cancer (50), and colorectal cancer (51), which suggests that vitamin D may have dual effects. The U-shaped relationship we observed is further supported by time series analyses of birth weight. Such analyses point to nonlinear risk curves, such as those for vitamin D, in underpinning well-known seasonal variability in infant birth weight (52). However, we cannot rule out residual confounding as a potential explanation for our finding, e.g. by intake of fatty fish, which may raise serum 25(OH)D concentrations (53) while also increasing exposure to growth-restricting organic pollutants (54). We lacked data on other dietary confounders to address this possibility. Research to understand the mechanisms underlying this U-shaped relation is a high priority. Because the definition of vitamin D sufficiency (typically >75 nmol/L) was selected based on parathyroid hormone levels, calcium transport, and bone outcomes (33,55), it is possible that these definitions do not apply to SGA and other pregnancy outcomes. Importantly, researchers should explore whether genotypic differences in the study population may explain this U-shaped relation.
To our knowledge, ours is the first study to assess serum 25(OH)D in relation to SGA risk among black women. Future studies with large samples of black women are needed to confirm or refute our finding of a lack of an association. The reason for the lack of association remains unclear. It may have been due to a relatively small sample of black cases and/or relatively narrow distribution of serum 25(OH)D among black women. However, others have also observed a differential association between vitamin D and bone health and diabetes by race/ethnicity (23–25). Scientists have postulated that blacks are less sensitive to the negative impacts of vitamin D deficiency. For instance, although secondary hyperparathyroidism results from vitamin D deficiency in blacks, there is a net increase in bone mass and decreased risk of osteoporosis that is observed in blacks compared with whites (25). This race difference indicates that the ability of parathyroid hormones to induce bone turnover is lower among blacks than whites (25). Researchers speculate that blacks may have experienced skeletal and renal adaptations to vitamin D deficiency that explain their lack of association between vitamin D and bone health (23,25). Whether this applies to pregnancy outcomes is unknown. It is possible that exposures other than vitamin D deficiency may contribute to the higher risk of growth restriction in black infants compared with white infants (56).
Recently, Morley et al. (27) observed that sequence variation in the VDR gene modified the effect of maternal vitamin D deficiency on infant size at birth. These investigators noted that low 25(OH)D concentrations were associated with lower birthweight infants only among infants that were either homozygous for the FokI major allele or heterozygotes. Although we did not have the power to study the modifying effect of VDR SNP in our analysis, our data suggest an association of sequence variation in the VDR gene with the risk of SGA. However, we did not find a relation between SGA and 2 commonly studied variants in the VDR gene, BsmI (rs1544410) and FokI (rs2228570), which previously have been associated with complex phenotypes, including cancer, diabetes, and fracture (26), or SNP in linkage disequilibrium with these variants. These findings should be viewed as preliminary; the number of cases within each race stratum limited our power. Similarly, our sample size limited our exploration of SNP to those with a predicted minor allele frequency of ≥5%. Thus, we cannot exclude the possible association of less common variants in the VDR gene and risk of SGA. Additionally, we explored only the contribution of maternal genotype to risk of SGA. It is certainly possible that the fetal VDR gene plays a role in regulation of fetal growth and thus also deserves study. Despite these limitations, these findings highlight the importance of future studies with larger sample size, mother-child pairs, and genotyping that includes less common variants in the VDR gene. None of the associated SNP are known to be potentially functional and thus may be in linkage disequilibrium with the causal variant(s). These data also support the performance of more fundamental, mechanistic work exploring the influence of VDR sequence variants on the function of the receptor itself with respect to binding affinity and downstream signal transduction.
Our study was limited by its reliance on the Immunodiagnostic Systems Limited ELISA for assessing serum 25(OH)D, which overestimates serum 25(OH)D >100 nmol/L. We did not have the resources to reanalyze the samples with an assay that is more accurate at high levels. Nevertheless, the overestimation of serum 25(OH)D would apply equally to cases and controls and would not systematically bias our results. Further, we observed similar U-shaped relations when results were limited to values ≤100 nmol/L. Our outcome, SGA, is an imperfect measure of fetal growth restriction, which is defined as the failure of an infant to exercise its genetic growth potential (57). By definition, SGA cases will include some infants that are constitutionally small rather than pathologically small and, conversely, exclude some infants that have failed to achieve their growth potential (57). We used a local population-based birthweight standard to define SGA rather than a national standard, such as those of Alexander et al. (58), but the results were not meaningfully different after applying this national standard (data not shown). Nevertheless, compared with birthweight-based growth standards, customized fetal growth trajectories are able to provide a more precise estimate of pathologic growth (59–61) and should be explored in future studies of maternal vitamin D status.
We also did not have the resources to measure biomarkers of the vitamin D endocrine system, parathyroid hormone concentration, or other functional indicators of maternal vitamin D status. Unmeasured confounders or error in the measurement of existing confounding factors may have biased our results. Future studies of vitamin D and growth restriction would benefit from using serial ultrasound measurements, the gold standard assessment, quantifying other vitamin D-related biomarkers, and considering a wide range of dietary and lifestyle confounding factors.
Our results suggest that vitamin D deficiency is associated with SGA in white women and a more modest increased risk may also exist for higher serum 25(OH)D levels. Additional studies are needed to refute or replicate these findings given the high prevalence of vitamin D deficiency in U.S. pregnant women.
Supplementary Material
Acknowledgments
We thank John McGrath for helpful discussions about these findings. L.M.B., J.M.C., J.M.R., M.L.M., and H.N.S. designed the research; J.M.Z. and J.M.R. conducted research and provided essential materials; L.M.B. and M.E.C. analyzed data; L.M.B., J.M.C., and H.N.S. wrote the paper; M.S.P. conducted the literature review; and L.M.B. had primary responsibility for final content. All authors read and approved the final manuscript.
Supported by NIH grants PPG 2P01 HD30367, 5M01 RR00056, and P30 DK046204. Dr. Bodnar was supported by NIH grant K01 MH074092 and R01 HD056999. Dr. Simhan was supported by NIH grants R01 HD041663 and R01 HD052732.
Author disclosures: L. M. Bodnar, J. M. Catov, J. M. Zmuda, M. E. Cooper, M. S. Parrott, J. M. Roberts, M. L. Marazita, and H. N. Simhan, no conflicts of interest.
Supplemental Table 1 is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: 25(OH)D, 25-hydroxyvitamin D; HWE, Hardy-Weinberg Equilibrium; OR, odds ratio; SES, socioeconomic score; SGA, small-for-gestational age; SNP, single nucleotide polymorphism; VDR, vitamin D receptor.
References
- 1.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88:1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137:447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10. [DOI] [PubMed] [Google Scholar]
- 4.Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr. 2005;135:2478–85. [DOI] [PubMed] [Google Scholar]
- 5.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92:3517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C, Qiu C, Hu FB, David RM, van Dam RM, Bralley A, Williams MA. Maternal plasma 25-hydroxyvitamin d concentrations and the risk for gestational diabetes mellitus. PLoS One. 2008;3:e3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr. 2009;139:1157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–52. [DOI] [PubMed] [Google Scholar]
- 9.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. [DOI] [PubMed] [Google Scholar]
- 10.Camargo CA Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Kleinman K, Gillman MW. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGrath J. Does 'imprinting' with low prenatal vitamin D contribute to the risk of various adult disorders? Med Hypotheses. 2001;56:367–71. [DOI] [PubMed] [Google Scholar]
- 12.Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, Robinson VP, Winder SM. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. BMJ. 1980;280:751–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunvand L, Quigstad E, Urdal P, Haug E. Vitamin D deficiency and fetal growth. Early Hum Dev. 1996;45:27–33. [DOI] [PubMed] [Google Scholar]
- 14.Farrant HJ, Krishnaveni GV, Hill JC, Boucher BJ, Fisher DJ, Noonan K, Osmond C, Veena SR, Fall CH. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur J Clin Nutr. 2009;63:646–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mallet E, Gugi B, Brunelle P, Henocq A, Basuyau JP, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstet Gynecol. 1986;68:300–4. [DOI] [PubMed] [Google Scholar]
- 16.Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab. 2006;91:906–12. [DOI] [PubMed] [Google Scholar]
- 17.Mannion CA, Gray-Donald K, Koski KG. Association of low intake of milk and vitamin D during pregnancy with decreased birth weight. CMAJ. 2006;174:1273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marya RK, Rathee S, Dua V, Sangwan K. Effect of vitamin D supplementation during pregnancy on foetal growth. Indian J Med Res. 1988;88:488–92. [PubMed] [Google Scholar]
- 19.Marya RK, Rathee S, Lata V, Mudgil S. Effects of vitamin D supplementation in pregnancy. Gynecol Obstet Invest. 1981;12:155–61. [DOI] [PubMed] [Google Scholar]
- 20.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardosi JMFM. Customized growth curves. Clin Obstet Gynecol. 1997;40:715–22. [DOI] [PubMed] [Google Scholar]
- 22.Bloomfield FH, Oliver MH, Harding JE. The late effects of fetal growth patterns. Arch Dis Child Fetal Neonatal Ed. 2006;91:F299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–8. [DOI] [PubMed] [Google Scholar]
- 24.Bell NH, Greene A, Epstein S, Oexmann MJ, Shaw S, Shary J. Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest. 1985;76:470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cosman F, Nieves J, Dempster D, Lindsay R. Vitamin D economy in blacks. J Bone Miner Res. 2007;22 Suppl 2:V34–8. [DOI] [PubMed] [Google Scholar]
- 26.Zmuda JM, Cauley JA, Ferrell RE. Molecular epidemiology of vitamin D receptor gene variants. Epidemiol Rev. 2000;22:203–17. [DOI] [PubMed] [Google Scholar]
- 27.Morley R, Carlin JB, Pasco JA, Wark JD, Ponsonby AL. Maternal 25-hydroxyvitamin D concentration and offspring birth size: effect modification by infant VDR genotype. Eur J Clin Nutr. 2009;63:802–4. [DOI] [PubMed] [Google Scholar]
- 28.M Kady S, Gardosi J. Perinatal mortality and fetal growth restriction. Best Pract Res Clin Obstet Gynaecol. 2004;18:397–410. [DOI] [PubMed] [Google Scholar]
- 29.Jarvis S, Glinianaia SV, Torrioli MG, Platt MJ, Miceli M, Jouk PS, Johnson A, Hutton J, Hemming K, et al. Cerebral palsy and intrauterine growth in single births: European collaborative study. Lancet. 2003;362:1106–11. [DOI] [PubMed] [Google Scholar]
- 30.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71:S1344–52. [DOI] [PubMed] [Google Scholar]
- 31.Carter GD, Carter R, Jones J, Berry J. How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem. 2004;50:2195–7. [DOI] [PubMed] [Google Scholar]
- 32.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- 33.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–22. [DOI] [PubMed] [Google Scholar]
- 34.International HapMap Project. International HapMap Project, release 20. 2009. [cited 2009 Jun 20]. Available from: http://www.hapmap.org.
- 35.Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–56. [DOI] [PubMed] [Google Scholar]
- 36.Roeder K, Bacanu SA, Sonpar V, Zhang X, Devlin B. Analysis of single-locus tests to detect gene/disease associations. Genet Epidemiol. 2005;28:207–19. [DOI] [PubMed] [Google Scholar]
- 37.Myrianthopoulos NC, French KS. An application of the U.S. Bureau of the Census socioeconomic index to a large, diversified patient population. Soc Sci Med. 1968;2:283–99. [DOI] [PubMed] [Google Scholar]
- 38.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. [DOI] [PubMed] [Google Scholar]
- 39.Prentice A, Jarjou LM, Goldberg GR, Bennett J, Cole TJ, Schoenmakers I. Maternal plasma 25-hydroxyvitamin D concentration and birthweight, growth and bone mineral accretion of Gambian infants. Acta Paediatr. 2009;98:1360–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bloomfield FH, Harding JE. Experimental aspects of nutrition and fetal growth. Fetal Matern Med Rev. 1998;102:91–107. [Google Scholar]
- 41.Henry HL, Norman AW. Vitamin D: metabolism and biological actions. Annu Rev Nutr. 1984;4:493–520. [DOI] [PubMed] [Google Scholar]
- 42.Barrera D, Avila E, Hernandez G, Mendez I, Gonzalez L, Halhali A, Larrea F, Morales A, Diaz L. Calcitriol affects hCG gene transcription in cultured human syncytiotrophoblasts. Reprod Biol Endocrinol. 2008;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barrera D, Avila E, Hernandez G, Halhali A, Biruete B, Larrea F, Diaz L. Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol. J Steroid Biochem Mol Biol. 2007;103:529–32. [DOI] [PubMed] [Google Scholar]
- 44.Billaudel B, Labriji-Mestaghanmi H, Sutter BC, Malaisse WJ. Vitamin D and pancreatic islet function. II. Dynamics of insulin release and cationic fluxes. J Endocrinol Invest. 1988;11:585–93. [DOI] [PubMed] [Google Scholar]
- 45.DeLuca HF. The vitamin D story: a collaborative effort of basic science and clinical medicine. FASEB J. 1988;2:224–36. [PubMed] [Google Scholar]
- 46.Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, Jarvelinb MR. Infant vitamin D supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci. 2004;1037:84–95. [DOI] [PubMed] [Google Scholar]
- 47.Wjst M, Hypponen E. Vitamin D serum levels and allergic rhinitis. Allergy. 2007;62:1085–6. [DOI] [PubMed] [Google Scholar]
- 48.Hypponen E, Berry DJ, Wjst M, Power C. Serum 25-hydroxyvitamin D and IgE: a significant but nonlinear relationship. Allergy. 2009;64:613–20. [DOI] [PubMed] [Google Scholar]
- 49.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, Dawson-Hughes B. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80:752–8. [DOI] [PubMed] [Google Scholar]
- 50.Tuohimaa P, Tenkanen L, Ahonen M, Lumme S, Jellum E, Hallmans G, Stattin P, Harvei S, Hakulinen T, et al. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries. Int J Cancer. 2004;108:104–8. [DOI] [PubMed] [Google Scholar]
- 51.Platz EA, Hankinson SE, Hollis BW, Colditz GA, Hunter DJ, Speizer FE, Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and adenomatous polyps of the distal colorectum. Cancer Epidemiol Biomarkers Prev. 2000;9:1059–65. [PubMed] [Google Scholar]
- 52.McGrath J, Barnett A, Eyles D, Burne T, Pedersen CB, Mortensen PB. The impact of nonlinear exposure-risk relationships on seasonal time-series data: modelling Danish neonatal birth anthropometric data. BMC Med Res Methodol. 2007;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brustad M, Sandanger T, Aksnes L, Lund E. Vitamin D status in a rural population of northern Norway with high fish liver consumption. Public Health Nutr. 2004;7:783–9. [DOI] [PubMed] [Google Scholar]
- 54.Halldorsson TI, Meltzer HM, Thorsdottir I, Knudsen V, Olsen SF. Is high consumption of fatty fish during pregnancy a risk factor for fetal growth retardation? A study of 44,824 Danish pregnant women. Am J Epidemiol. 2007;166:687–96. [DOI] [PubMed] [Google Scholar]
- 55.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2005 period linked birth/infant death data set. Hyattsville (MD): National Center for Health Statistics; 2008. [PubMed]
- 57.Goldenberg RL, Cliver SP. Small for gestational age and intrauterine growth restriction: definitions and standards. Clin Obstet Gynecol. 1997;40:704–14. [DOI] [PubMed] [Google Scholar]
- 58.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. [DOI] [PubMed] [Google Scholar]
- 59.Zhang X, Platt RW, Cnattingius S, Joseph KS, Kramer MS. The use of customised versus population-based birthweight standards in predicting perinatal mortality. BJOG. 2007;114:474–7. [DOI] [PubMed] [Google Scholar]
- 60.Gardosi J, Francis A. Adverse pregnancy outcome and association with small for gestational age birthweight by customized and population-based percentiles. Am J Obstet Gynecol. 2009;201:28 e1–8. [DOI] [PubMed]
- 61.Gardosi J, Clausson B, Francis A. The value of customised centiles in assessing perinatal mortality risk associated with parity and maternal size. BJOG. 2009;116:1356–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.