Abstract
Increased adiposity is associated with increased storage of several fat-soluble nutrients. However, the extent to which vitamin K is stored in fat and the association between vitamin K status and adiposity are unknown. Our objectives in this study were to determine whether vitamin K is stored in human adipose tissue and the association between vitamin K status and body fat in older men and women. In study A, the vitamin K concentration of subcutaneous and visceral adipose tissue was quantified in samples taken from 16 gastric bypass patients [13 women, 3 men, age 40 ± 10 y (mean ± SD)] using HPLC. In study B, cross-sectional associations between percent body fat (%BF) and circulating measures of vitamin K status were examined in 260 women and 183 men [age = 68 ± 5 y]. The phylloquinone (K1) concentrations in subcutaneous and visceral adipose tissue were 148.2 ± 71.8 and 175 ± 112 nmol/kg, respectively, which is higher than the reported concentrations of other organs known to store vitamin K. There was an inverse association between %BF and plasma K1 in women (P-trend < 0.001). Higher %BF was associated with greater circulating concentrations of uncarboxylated prothrombin, indicative of lower hepatic utilization of vitamin K in both men (P-trend = 0.02) and women (P-trend = 0.002) but not with the percentage of undercarboxylated osteocalcin. Adipose tissue contained high concentrations of vitamin K, and increased adiposity was associated with poorer vitamin K status in older adults. Additional studies are needed to further explore the relationships among body fat, storage of vitamin K in adipose tissue, and implications for vitamin K status and function.
Introduction
Vitamins A, D, and E, and carotenoids all appear to be concentrated and stored in adipose tissue in humans (1–4). Because overweight and obese individuals are at increased risk for insufficiency of these nutrients (5–8), it has been proposed that adiposity may influence circulating concentrations, metabolism, and ultimately end-organ action of fat-soluble vitamins. For example, evidence suggests that the hypovitaminosis D associated with obesity may be due to the sequestration of vitamin D in adipose tissue, thereby limiting its bioavailability (9,10).
In contrast to the other fat-soluble nutrients, little is known about the association between vitamin K and adiposity. Given that vitamin K is fat soluble, it is physiologically plausible that adipose tissue may serve as a storage site for vitamin K as well. Recently, different forms of vitamin K were detected in large amounts in the adipose tissue of mice (11). Although prior research has demonstrated that human tissue concentrations of vitamin K differ considerably (12), the extent to which vitamin K is stored in human adipose tissue is not known.
Our purpose in the present analyses was 2-fold: to measure the extent to which vitamin K is stored in fat, and to assess the association between circulating measures of vitamin K status and body fat in older men and women. This was achieved using 2 different studies. In study A, the vitamin K concentrations in subcutaneous and visceral adipose tissue were measured in samples collected from obese individuals undergoing gastric bypass surgery. In study B, we examined the cross-sectional associations between percent body fat (%BF)7 and several biomarkers of vitamin K status [plasma phylloquinone (K1), percent uncarboxylated osteocalcin (%ucOC), and protein-induced in vitamin K absence-factor II (prothrombin) (PIVKA-II)] in older, community-dwelling women and men. We hypothesized that vitamin K would be stored in adipose tissue and that increased body fat would be associated with lower vitamin K status.
Methods
Study A
Study participants.
Obese men and women scheduled to undergo gastric bypass surgery were recruited as part of a study originally designed to assess the association between the vitamin D concentration in adipose tissue and the serum concentration of 25-hydroxyvitamin D in obese individuals. All participants were recruited from the Obesity Consultation Center at Tufts Medical Center and signed a written informed consent. This study was approved by the Institutional Review Board at Tufts University Medical Center. Exclusion criteria for the study are described elsewhere (4).
Participants were enrolled 1–2 wk prior to gastric bypass surgery during a preoperative clinic visit. At this visit, a medical history interview was conducted and a nonfasting blood sample was drawn. BMI was calculated using weight (kg)/height (m2). At the time of surgery, abdominal subcutaneous and visceral adipose tissue were collected, cut into pieces weighing ∼500 mg, placed in plastic vials, and stored frozen at −70°C until analysis. Sixteen patients who had sufficient visceral [mean number of samples per visceral fat depot = 5.5 (range: 1–10)] and subcutaneous [mean number of samples per subcutaneous fat depot = 2.3 (range: 1–6)] adipose tissue and plasma samples were used in these analyses.
Measurement of vitamin K in adipose tissue.
The extraction and chromatography solvents used were all of HPLC grade (Fisher Scientific). Lipase and solvents were purchased from Sigma Chemical, with the exception of ammonium hydroxide and glacial acetic acid, which were purchased from JT Baker. Zinc powder (−100 mesh) was purchased from Alfa Aesar. The internal standard, K1(25), was purchased from GL Synthesis.
The method used to extract vitamin K from adipose tissue was modified from a method previously described to extract vitamin K from human milk (13). Prior to homogenization, visceral and subcutaneous adipose tissue samples were thawed, patted dry, and sectioned into 0.08-g pieces for analysis. The pieces were weighed, then transferred to a small glass test tube and 1.0 mL of Dubecco's PBS was added. Adipose tissue was homogenized for 2 min using a Power Gen 125 Homogenizer (Fisher Scientific). A 250-μL aliquot of tissue homogenate (∼20 mg fat) was transferred to a 50-mL polypropylene centrifuge tube to which 50 μL of K1(25) internal standard (88.7 nmol/L) was added. We added 10 μL of 200 g/L albumin solution and 200 μL of an aqueous solution of sodium taurocholate (0.05 mol/L), calcium chloride (0.1 mol/L), and sodium chloride (0.15 mol/L) to each sample, followed by 1.2 mL of 0.2 mol/L Tris buffer (pH 7.7) that contained 4.08 mg of porcine pancreas, type II lipase. The samples were incubated at 37°C in a shaking water bath for 45 min at 100 strokes/min. Upon removal from the bath, 4 mL of reagent alcohol, 2 mL dH2O, and 200 μL of an ammonium hydroxide solution (50 g/L) were added. Samples were vortexed for 5 s, 7.5 mL of hexane was added, and samples were vortexed for an additional 2 min. Samples were centrifuged and the upper hexane layer was transferred to a clean 13- × 100-mm borosilicate tube. The hexane layer was then evaporated completely and residues were reconstituted in 0.5 mL of hexane. The hexane solutions were further processed by solid phase extraction (14) on silica columns followed by solid phase extraction on C18 columns. The residues from the C18 columns were each reconstituted with 30 μL of methylene chloride and 170 μL of aqueous methanol. K1, menaquinone-4 (MK-4), and dihydrophylloquinone (DK1) were detected using HPLC, as described previously (14). The 3 forms of vitamin K differ in the saturation of the side-chain. To assess the variability in vitamin K content within adjacent regions of visceral adipose tissue, 5 contiguous visceral fat samples randomly chosen from 4 patients were extracted and analyzed. Insufficient samples were available to conduct a similar analysis of subcutaneous fat samples.
Circulating measures.
Plasma concentrations of K1, MK-4, and DK1 were measured by reversed-phase HPLC using postcolumn reduction, followed by fluorometric detection (15). The lower limits of detection of this HPLC assay were 0.02 nmol/L, 0.07 nmol/L, and 0.02 nmol/L for plasma K1, MK-4, and DK1, respectively. Plasma triglycerides were analyzed on a COBAS Mira (Roche Instruments).
Statistical analyses.
Plasma and adipose tissue concentrations of K1, MK-4, and DK1 were transformed using the natural log to reduce skewness. Student's t test for paired samples was used to determine whether the vitamin K concentrations in plasma and in subcutaneous and visceral adipose tissue were different. Spearman correlation coefficients were used to assess the relations between subcutaneous and visceral adipose tissue vitamin K concentrations and between plasma K1 and DK1 with their concentrations in subcutaneous and visceral adipose tissue. MK-4 was not detected in the circulation, so we were unable to examine these correlations of that form of vitamin K. Because blood samples for study A were drawn from nonfasting participants, and given that circulating K1 varies with triglyceride-rich lipoproteins, we corrected plasma K1 and DK1 for circulating triglycerides in our analyses. Analyses for study A were carried out using SAS (v 9.1) and results were considered significant at P < 0.05.
Study B
Study participants.
The participation criteria of this study have been described elsewhere (16). Briefly, 452 community-dwelling older men and postmenopausal women (aged 60–80 y) were recruited to participate in a randomized, controlled trial to assess the influence of vitamin K supplementation on age-related bone loss and progression of coronary artery calcification. This study was approved by the Institutional Review Board at Tufts University and all participants signed written informed consent. The sample for the present cross-sectional analyses included 443 (260 female and 183 male) participants, for whom all measures of vitamin K status were available.
Vitamin K status.
All blood samples were fasting, drawn between 0700 and 1000 h. Dedicated aliquots of plasma and serum were stored at −80°C and protected from light until the time of analysis. PIVKA-II, a functional measure of hepatic utilization of vitamin K, was analyzed in plasma using enzyme-linked immunoassay (Diagnostica Stago). Serum uncarboxylated osteocalcin (ucOC) and total osteocalcin were analyzed using a RIA method (17). The antibody recognizes both carboxylated osteocalcin and ucOC. Carboxylated osteocalcin was separated from ucOC by adsorption on hydroxyapatite as described (17). The %ucOC, a functional measure of skeletal vitamin K status, was calculated as (ucOC concentration/total osteocalcin concentration) × 100. In addition, dietary intakes of K1 were assessed using the Harvard FFQ (18). Plasma triglycerides were analyzed using the COBAS Mira (Roche Instruments).
Body composition.
%BF was measured from a whole body dual x-ray absorptiometry scan using a GE-Lunar model Prodigy scanner (enCORE 2002; version 6.10.029). BMI was calculated as kg/m2 from measures of height and weight.
Statistical analyses.
Means reported in Table 3 are in the original scale. For analyses beyond descriptive statistics, plasma K1 concentrations were transformed using the natural log to reduce skewness. Pearson correlations were used to determine whether plasma K1 correlated with PIVKA-II and %ucOC. Differences in participant characteristics and in the circulating measures of vitamin K status, across sex-specific tertiles of %BF, were examined using ANOVA. We subsequently used ANCOVA to analyze the differences in measures of vitamin K status across tertiles of %BF, adjusted for age, triglycerides, dietary K1 intake, and smoking. If there was a main effect for measures of vitamin K status, individual tertiles were then compared using Tukey's honestly significant difference test (HSD). Because measures of vitamin K status varied according to %BF tertile, we also examined associations between plasma K1 and the functional measures of PIVKA-II and %ucOC according to tertile of %BF in men and women separately, using multiple linear regression to test for an interaction between tertile of %BF times plasma K1, with respect to the functional measures. Significant interactions between %BF tertile times plasma K1 with respect to PIVKA-II and %ucOC were observed in women (P ≤ 0.01). Therefore, we stratified subsequent regression models to examine the association between plasma K1 and PIVKA-II and %ucOC in tertiles of %BF separately in men and women. Analyses for study B were also carried out using SAS v. 9.1 and results were considered significant at P < 0.05.
TABLE 3.
Measures of vitamin K status according to tertile of %BF in men and women in study B
| Women |
Men |
|||||||
|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | P-trend1 | Tertile 1 | Tertile 2 | Tertile 3 | P-trend1 | |
| n | 83 | 89 | 88 | 60 | 62 | 61 | ||
| Range %BF | 15.2–37.7 | 37.8–44.4 | 44.5–57.0 | 10.7–25.7 | 25.8–31.7 | 31.8–45.7 | ||
| Plasma K1, nmol/L | ||||||||
| Unadjusted2 | 1.4 ± 0.2a | 1.1 ± 0.1a,b | 0.8 ± 0.1b | 0.002 | 1.1 ± 0.1 | 1.6 ± 0.4 | 1.1 ± 0.1 | 0.74 |
| Adjusted3 | 1.5 ± 0.1a | 1.1 ± 0.1a,b | 0.8 ± 0.1b | <0.001 | 1.4 ± 0.2 | 1.5 ± 0.2 | 0.8 ± 0.2 | 0.63 |
| PIVKA-II, μg/L | ||||||||
| Unadjusted2 | 2.2 ± 0.1a | 2.4 ± 0.1a,b | 2.6 ± 0.1b | 0.002 | 2.2 ± 0.1a | 2.4 ± 0.1a,b | 2.7 ± 0.1b | 0.002 |
| Adjusted3 | 2.1 ± 0.1a | 2.3 ± 0.1a,b | 2.6 ± 0.1b | 0.002 | 2.3 ± 0.1a | 2.4 ± 0.1a,b | 2.7 ± 0.1b | 0.02 |
| %ucOC | ||||||||
| Unadjusted2 | 41.1 ± 1.9 | 43.5 ± 1.9 | 43.6 ± 1.6 | 0.55 | 36.0 ± 2.1 | 40.6 ± 2.1 | 37.4 ± 1.8 | 0.26 |
| Adjusted3 | 41.4 ± 1.9 | 43.5 ± 1.8 | 43.2 ± 1.8 | 0.68 | 35.9 ± 2.1 | 40.6 ± 2.0 | 37.6 ± 2.0 | 0.27 |
Unadjusted analyses are based on 1-way ANOVA. Adjusted analyses are based on analysis of covariance, adjusted for age, vitamin K intake, smoking, and triglycerides. When the main effect was significant, individual tertiles were compared using Tukey's HSD.
Data are means ± SE. Within gender, means in a row with superscripts without a common letter differ, P < 0.05.
Data are least-square means ± SE, adjusted for age, vitamin K intake, smoking, and triglycerides. Within gender, means in a row with superscripts without a common letter differ, P < 0.05.
Results
Study A.
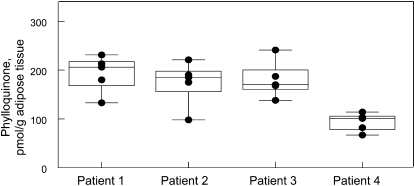
The participants (13 females, 3 males, 100% Caucasian) of Study A were all obese adults (Table 1). Plasma concentrations of MK-4 and DK1 in subcutaneous and visceral adipose tissue were lower than that of K1 (P < 0.001). However, concentrations of K1, MK-4, and DK1 did not differ between subcutaneous and visceral abdominal compartments. Visceral and subcutaneous adipose tissue concentrations of K1 (r = 0.83, P < 0.001), MK-4 (r = 0.60, P = 0.01), and DK1 (r = 0.69, P = 0.003) were positively correlated. Plasma concentrations of K1 (corrected for triglycerides) were not correlated with subcutaneous adipose tissue K1, visceral adipose tissue K1, or BMI (Spearman r ranged from −0.21 to 0.41; P ≥ 0.13). Similarly, plasma DK1 was not correlated with subcutaneous adipose tissue DK1, visceral adipose tissue DK1, or BMI (Spearman r ranged from 0.13 to 0.23 corrected for triglycerides; P ≥ 0.40). Among 4 patients, the intra-individual:inter-individual variability ratio for visceral adipose tissue K1 was 1.03 (Fig. 1).
TABLE 1.
Characteristics of 13 women and 3 men in study A1
| Mean ± SD | Range | |
|---|---|---|
| Age, y | 40.4 ± 10.4 | 21–58 |
| Weight, kg | 145 ± 22.0 | 113–194 |
| Height, cm | 169 ± 8.8 | 155–185 |
| BMI, kg/m2 | 50.6 ± 5.7 | 41.7–60.6 |
| Plasma triglycerides,23mmol/L | 1.7 ± 0.8 | 0.9 – 3.1 |
| Plasma vitamin K | ||
| K1, nmol/L | 1.4 ± 0.9 | 0.3–3.4 |
| K1/triglycerides,2nmol/mmol | 0.8 ± 0.5 | 0.3–20.0 |
| DK1, nmol/L | 0.4 ± 0.6 | nd4–1.9 |
| DK1/triglycerides,2nmol/mmol | 0.2 ± 0.3 | nd–0.8 |
| MK-4, nmol/L | nd5 | nd5 |
| Subcutaneous adipose tissue vitamin K | ||
| K1, nmol/kg | 148 ± 71.8 | 51.2–285 |
| DK1, nmol/kg | 40.0 ± 30.2 | 11.9–131 |
| MK-4, nmol/kg | 2.4 ± 4.3 | nd5–10.3 |
| Visceral adipose tissue vitamin K | ||
| K1, nmol/kg | 175 ± 112 | 46.8–431 |
| DK1, nmol/kg | 48.5 ± 36.2 | 3.4–160 |
| MK-4, nmol/kg | 3.1 ± 6.0 | nd5–20.3 |
Values are mean ± SD (range), n = 16 unless indicated otherwise.
n = 15.
Lower detectable limit is <0.05 mmol/L.
nd, Not detectable <0.02 nmol/L.
nd, not detectable <0.07 nmol/L.
FIGURE 1 .
Variability of K1 concentrations in adipose tissue in 4 patients from Study A. Five contiguous visceral adipose tissue samples were analyzed per patient. Each data point is represented by a circle. When fewer than 5 points are shown, there was overlap of at least 2 points. The median and interquartile ranges are represented by the box and the whiskers represent the minimum and maximum measures for each patient.
Study B.
The 260 women and 183 men in Study B were older (age range 65–80 y), community-dwelling adults. The women and men in the middle tertile of %BF would be considered overweight based on having a mean BMI >25 kg/m2 and those in the highest tertile would be considered obese, based on having a mean BMI >30 kg/m2 (Table 2). After adjustment for age, vitamin K intake, triglycerides, and smoking status, women in the highest tertile of %BF had lower plasma K1 (P-trend < 0.001) and women in the highest tertile had higher PIVKA-II compared with women in the lower tertiles (P-trend = 0.002). Men in the highest tertile of %BF also had higher PIVKA-II compared with men in the lower tertiles (P-trend = 0.02). However, there was no association between tertile of %BF and plasma K1 in men (P = 0.64) and the %ucOC did not differ according to tertile of %BF in men or women (P ≥ 0.27, adjusted for age, vitamin K intake, smoking, and triglycerides) (Table 3). Plasma K1 was inversely correlated with PIVKA-II and %ucOC in men and women (Pearson r ranged from −0.29 to −0.18; P ≤ 0.02).
TABLE 2.
Participant characteristics of study B, according to tertile of %BF1
| Women |
Men |
|||||||
|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | P-value2 | Tertile 1 | Tertile 2 | Tertile 3 | P-value2 | |
| n | 83 | 89 | 88 | 60 | 62 | 61 | ||
| Range %BF | 15.2–37.7 | 37.8–44.4 | 44.5–57.0 | 10.7–25.7 | 25.8–31.7 | 31.8–45.7 | ||
| Age, y | 68 ± 5 | 68 ± 5 | 68 ± 5 | 0.90 | 70 ± 6 | 69 ± 6 | 68 ± 5 | 0.05 |
| Body fat, % | 32.0 ± 4.8 | 41.1 ± 1.9 | 47.8 ± 2.7 | <0.001 | 21.0 ± 3.4 | 28.8 ± 1.9 | 36.0 ± 3.5 | <0.001 |
| BMI, kg/m2 | 23.1 ± 2.8 | 27.4 ± 3.3 | 33.0 ± 4.8 | <0.001 | 24.4 ± 2.1 | 27.7 ± 2.8 | 31.8 ± 4.1 | <0.001 |
| Weight, kg | 59 ± 8 | 71 ± 9 | 86 ± 13 | <0.001 | 74 ± 8 | 84 ± 11 | 96 ± 14 | <0.001 |
| K1 intake, μg/d | 208 ± 129 | 196 ± 106 | 182 ± 133 | 0.40 | 157 ± 79 | 142 ± 75 | 148 ± 91 | 0.60 |
| Serum total osteocalcin, μg/L | 9.6 ± 3.1 | 9.0 ± 3.7 | 8.3 ± 2.7 | 0.04 | 7.9 ± 2.6 | 8.0 ± 2.6 | 7.3 ± 2.4 | 0.23 |
| Plasma triglycerides,4mmol/L | 1.14 ± 0.64 | 1.35 ± 0.71 | 1.34 ± 0.59 | 0.05 | 0.99 ± 0.38 | 1.39 ± 1.06 | 1.53 ± 0.79 | <0.001 |
| Smokers, n (%) | 8 (10) | 3 (4) | 2 (2) | 0.063 | 3 (5) | 3 (5) | 5 (8) | 0.673 |
Data are mean ± SD, unless otherwise indicated.
Based on 1-way ANOVA overall F-statistic, unless otherwise indicated.
Based on chi-square analysis.
Lower detectable limit is 0.05 mmol/L.
In women, the associations between plasma K1 and PIVKA-II and %ucOC, were partially dependent on %BF. The magnitude of the inverse association between plasma K1 and the functional measures of PIVKA-II and %ucOC varied across tertiles of %BF. In men, plasma K1 was inversely associated with PIVKA-II only among those in the highest tertile of %BF [unstandardized β-coefficient (unstd β) = −0.66; P = 0.02) (Table 4). In contrast, there was no association between %ucOC and plasma K1 in the highest tertile.
TABLE 4.
Associations between (ln) plasma K1 and functional measures of vitamin K status according to tertile of %BF in older men and women in study B
| Women |
Men |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 |
Tertile 2 |
Tertile 3 |
Tertile 1 |
Tertile 2 |
Tertile 3 |
|||||||
| 83 (15.2–37.7)1 |
89 (37.8–44.4) |
88 (44.5–57.0) |
60 (10.7–25.7) |
62 (25.8–31.7) |
61 (31.8–45.7) |
|||||||
| Unstd β2 | P-value | Unstd β | P-value | Unstd β | P-value | Unstd β | P-value | Unstd β | P-value | Unstd β | P-value | |
| (ln) Plasma K1, nmol/L | ||||||||||||
| PIVKA-II, μg/L | −0.41 | 0.03 | −0.36 | 0.08 | −0.52 | 0.02 | −0.18 | 0.44 | −0.21 | 0.31 | −0.66 | 0.02 |
| %ucOC | −10.4 | 0.01 | −18.6 | < 0.001 | −1.9 | 0.66 | −9.7 | 0.04 | −10.3 | 0.02 | 2.2 | 0.65 |
n (range %BF).
Unstd β based on multiple linear regression. For all models, the outcome was the functional measure of vitamin K status (PIVKA-II or %ucOC), and the primary predictor was (ln) plasma K1, adjusted for age, smoking status, and triglycerides.
Discussion
In study A, we established that various forms of vitamin K are present in human adipose tissue. In the cross-sectional analyses of study B, corrected for dietary intakes of K1, older women with higher body fat had lower vitamin K status, as measured by lower plasma K1 and higher PIVKA-II, compared with women with lower body fat. Men in the highest tertile of %BF had higher PIVKA-II, suggesting increased adiposity is associated with lower vitamin K status. Although being overweight or obese are risk factors for insufficiencies of other fat-soluble nutrients (5), to the best of our knowledge, this is the first report to show that increased body fat may also be associated with lower vitamin K status.
K1, the principal form of vitamin K in the Western diet, is found primarily in green leafy vegetables (19), and it is plausible that overweight and obese persons consume less of these foods. However, in our sample (study B) vitamin K intake did not differ across tertiles of %BF and we adjusted for vitamin K intake, which suggests that lower vitamin K status among those with higher body fat is not entirely explained by differences in dietary intake.
K1 appears to accumulate in human adipose tissue at much higher concentrations compared with the reported concentrations in other human organs, including the liver (12). It has been postulated that adipose tissue sequesters other fat-soluble nutrients, thereby reducing their bioavailability (9,10), and it is possible that the sequestration of K1 in adipose tissue may contribute to the lower vitamin K status among those with higher %BF. For the other fat-soluble vitamins, positive correlations between circulating concentrations and adipose tissue concentrations have been reported (4,20). However, our results suggest this is not the case for K1. Given the inter-individual variability in adipose tissue K1, our sample may not have been large enough to detect significant associations between circulating and adipose tissue concentrations. Alternatively, large intra-individual variation in circulating K1 is attributed to the strong influence of dietary K1 intake within the previous 24 h on K1 concentrations (21). The K1 concentrations of adipose tissue are less likely to respond rapidly to dietary fluctuations, thereby attenuating the serum-adipose tissue correlations.
MK-4 and DK1 were also present in adipose tissue, although at much lower concentrations than K1. MK-4 is the primary form of vitamin K found in several tissues, including brain and kidney (12). Circulating concentrations are generally nondetectable in humans when intakes are low, as would be expected among adults consuming a Western diet (19). Tissue-specific conversion of K1 to MK-4 has been reported in rats, although the precise mechanisms are unclear (22). The high concentration of K1 relative to MK-4 may suggest that tissue-specific conversion to MK-4 does not occur in adipose tissue. Instead, any MK-4 measured in adipose tissue may be attributed to MK-4 obtained from the diet (23). DK1, a form of vitamin K that results from the commercial hydrogenation of certain vegetable oils, is abundantly found in foods that contain hydrogenated oils. The circulating concentration of DK1, which is also low compared with K1, correlates positively with trans-fatty acid intake (24). Our results suggest that circulating DK1 concentrations are not correlated with adipose tissue concentrations, as was also the case for K1. Unfortunately, we had an insufficient sample to measure longer chain menaquinones, such as MK-7 and MK-9, so it is possible that different forms of vitamin K have different associations with circulating forms.
Although PIVKA-II and %ucOC are both functional estimates of vitamin K status, each measure reflects different tissue stores of vitamin K. Circulating concentrations of both measures increase when vitamin K intakes are low. However, PIVKA-II concentrations are more readily restored during dietary repletion, compared with %ucOC, suggesting preferential utilization of vitamin K by the liver to maintain coagulation (25). Both measures were inversely correlated with plasma K1 in the men and women in our sample, as has been reported by others (26). However, in women, %BF influenced the association between plasma K1 and both PIVKA-II and %ucOC. The inverse association between plasma K1 and PIVKA-II was stronger among women with a higher %BF, whereas the association between plasma K1 and %ucOC was not significant among women in the highest tertile of %BF. It is plausible that the storage of K1 in adipose tissue may in part explain this observation. We were unable to measure PIVKA-II and %ucOC in study A, which would have provided more insight into the relationship between the functional measures of vitamin K status and the storage of K1 in adipose tissue, so further studies are needed to explore the extent to which the storage of vitamin K in adipose tissue influences functional vitamin K status.
The associations between adiposity and measures of vitamin K status were more evident among women than men. Although there was a significant trend for increasing PIVKA-II across tertiles of %BF in both men and women, the trend for lower plasma K1 across tertiles of %BF was not significant in men. However, the data suggest that plasma K1 was also lower among men in the highest tertile of %BF. As expected, the %BF was much higher in women, compared with men, which may have contributed to the differential response (27). Storage of vitamin K in fat may reduce bioavailability of vitamin K to other organs. However, because %BF influenced the association between plasma K1 and the functional measures of vitamin K status only in women, gender differences may warrant further exploration. Although vitamin K absorption and function are not known to differ between men and women (21,28), recent evidence suggests men and women respond differently to vitamin K supplementation in metabolic processes influenced by weight or body fat, such as insulin resistance (27).
To the best of our knowledge, this study is the first to report that increased body fat is associated with lower vitamin K status in older adults and that vitamin K is stored in human adipose tissue. However, there are certain limitations to consider. Although the amount of vitamin K in human adipose tissue was quantified in study A, due to limited resources, we were unable to measure the PIVKA-II or %ucOC in circulation, so we cannot speculate on the associations between the vitamin K concentration of adipose tissue and functional measures of vitamin K status. Blood samples in study A were drawn from nonfasting participants and circulating concentrations of vitamin K vary according to recent intakes. In circulation, vitamin K is transported on triglyceride-rich lipoproteins, so circulating measures are highly correlated with triglyceride concentrations (28). Therefore, we corrected our analyses for triglycerides, which is considered an appropriate indication of vitamin K status (15). The plasma vitamin K concentrations in study A would have been lower if measured following an overnight fast. The patients in study A were required to participate in dietary counseling as a prerequisite to gastric bypass surgery and may have increased their intakes of green leafy vegetables as a result. Unfortunately, because dietary intake data are not available for study A, we cannot account for vitamin K intake in that study. Analyses in study B are cross-sectional, so causation cannot be determined. Future metabolic studies designed to examine the circulating measures of vitamin K status as well as the vitamin K concentration of adipose tissue in obese and nonobese men and women may clarify our outcomes. As the participants in both studies were primarily Caucasian, these findings may not be generalized to other ethnic groups.
In conclusion, we found high adipose tissue concentrations of K1 and, combined with functional measures of vitamin K status, individuals with higher adiposity appear to be at risk for vitamin K insufficiency. Together, these observations may provide important insight into recent observations suggesting novel roles for vitamin K in obesity-related health outcomes (27,29). The potential inter-relationships among vitamin K, adiposity, and weight-related chronic disease merits investigation, utilizing studies that are designed to elucidate underlying mechanisms.
Acknowledgments
M.K.S., S.L.B., and E.S. designed the research and drafted the manuscript; C.W. and J.W.P. collected the data; M.K.S. analyzed the data; and C.M.G., J.W.P., C.W., and B.D.H. contributed to interpretation of the data and reviewed the manuscript. All authors have read and approved the final version of this manuscript.
Supported by the USDA Agricultural Research Service under Cooperative Agreement no. 58-1950-7-707 and by the NIH (AG14759, HL69272, AR47869, P30AG21332). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Author disclosures: M. K. Shea, S. L. Booth, C. M. Gundberg, J. W. Peterson, C. Waddell, B. Dawson-Hughes, and E. Saltzman, no conflicts of interest.
Study B was registered at clinicaltrials.gov as NCT00183001.
Abbreviations used: %BF, percent body fat; DK1, dihydrophylloquinone; HSD, honestly significant difference; MK-4, menaquinone-4; PIVKA-II, protein induced in vitamin K absence factor-II; K1, phylloquinone; ucOC, uncarboxylated osteocalcin; %ucOC, percent uncarboxylated osteocalcin; Unstd β, unstandardized β-coefficient.
References
- 1.Traber MG, Kayden HJ. Tocopherol distribution and intracellular localization in human adipose tissue. Am J Clin Nutr. 1987;46:488–95. [DOI] [PubMed] [Google Scholar]
- 2.Virtanen SM, van't Veer P, Kok F, Kardinaal AF, Aro A. Predictors of adipose tissue carotenoid and retinol levels in nine countries. The EURAMIC Study. Am J Epidemiol. 1996;144:968–79. [DOI] [PubMed] [Google Scholar]
- 3.Parker RS. Carotenoids in human blood and tissues. J Nutr. 1989;119:101–4. [DOI] [PubMed] [Google Scholar]
- 4.Blum M, Dolnikowski G, Seyoum E, Harris SS, Booth SL, Peterson J, Saltzman E, Dawson-Hughes B. Vitamin D (3) in fat tissue. Endocrine. 2008;33:90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimmons JE, Blanck HM, Tohill BC, Zhang J, Khan LK. Associations between body mass index and the prevalence of low micronutrient levels among US adults. MedGenMed. 2006;8:59. [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen LF, Jacobs DR Jr, Gross MD, Schreiner PJ, Dale WO, Lee DH. Longitudinal associations between body mass index and serum carotenoids: the CARDIA study. Br J Nutr. 2006;95:358–65. [DOI] [PubMed] [Google Scholar]
- 7.Vilarrasa N, Maravall J, Estepa A, Sanchez R, Masdevall C, Navarro MA, Alia P, Soler J, Gomez JM. Low 25-hydroxyvitamin D concentrations in obese women: their clinical significance and relationship with anthropometric and body composition variables. J Endocrinol Invest. 2007;30:653–8. [DOI] [PubMed] [Google Scholar]
- 8.Aasheim ET, Hofso D, Hjelmesaeth J, Birkeland KI, Bohmer T. Vitamin status in morbidly obese patients: a cross-sectional study. Am J Clin Nutr. 2008;87:362–9. [DOI] [PubMed] [Google Scholar]
- 9.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. [DOI] [PubMed] [Google Scholar]
- 10.Harris SS, Dawson-Hughes B. Reduced sun exposure does not explain the inverse association of 25-hydroxyvitamin D with percent body fat in older adults. J Clin Endocrinol Metab. 2007;92:3155–7. [DOI] [PubMed] [Google Scholar]
- 11.Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, Nakagawa K. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem. 2008;283:11270–9. [DOI] [PubMed] [Google Scholar]
- 12.Thijssen HH, Drittij-Reijnders MJ. Vitamin K status in human tissues: tissue-specific accumulation of phylloquinone and menaquinone-4. Br J Nutr. 1996;75:121–7. [DOI] [PubMed] [Google Scholar]
- 13.Lambert WE, Vanneste L, De Leenheer AP. Enzymatic sample hydrolysis and HPLC in a study of phylloquinone concentration in human milk. Clin Chem. 1992;38:1743–8. [PubMed] [Google Scholar]
- 14.Davidson KW, Sadowski JA. Determination of vitamin K compounds in plasma or serum by high-performance liquid chromatography using postcolumn chemical reduction and fluorimetric detection. Methods Enzymol. 1997;282:408–21. [DOI] [PubMed] [Google Scholar]
- 15.Sadowski JA, Hood SJ, Dallal GE, Garry PJ. Phylloquinone in plasma from elderly and young adults: factors influencing its concentration. Am J Clin Nutr. 1989;50:100–8. [DOI] [PubMed] [Google Scholar]
- 16.Booth SL, Dallal G, Shea MK, Gundberg C, Peterson JW, Dawson-Hughes B. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab. 2008;93:1217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gundberg CM, Nieman SD, Abrams S, Rosen H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab. 1998;83:3258–66. [DOI] [PubMed] [Google Scholar]
- 18.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 19.Booth SL, Suttie JW. Dietary intake and adequacy of vitamin K. J Nutr. 1998;128:785–8. [DOI] [PubMed] [Google Scholar]
- 20.El-Sohemy A, Baylin A, Kabagambe E, Ascherio A, Spiegelman D, Campos H. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. Am J Clin Nutr. 2002;76:172–9. [DOI] [PubMed] [Google Scholar]
- 21.Booth SL, O'Brien-Morse ME, Dallal GE, Davidson KW, Gundberg CM. Response of vitamin K status to different intakes and sources of phylloquinone-rich foods: comparison of younger and older adults. Am J Clin Nutr. 1999;70:368–77. [DOI] [PubMed] [Google Scholar]
- 22.Thijssen HH, Drittij-Reijnders MJ, Fischer MA. Phylloquinone and menaquinone-4 distribution in rats: synthesis rather than uptake determines menaquinone-4 organ concentrations. J Nutr. 1996;126:537–43. [DOI] [PubMed] [Google Scholar]
- 23.Elder SJ, Haytowitz DB, Howe J, Peterson JW, Booth SL. Vitamin K contents of meat, dairy, and fast food in the u.s. Diet. J Agric Food Chem. 2006;54:463–7. [DOI] [PubMed] [Google Scholar]
- 24.Erkkila AT, Lichtenstein AH, Jacques PF, Hu FB, Wilson PW, Booth SL. Determinants of plasma dihydrophylloquinone in men and women. Br J Nutr. 2005;93:701–8. [DOI] [PubMed] [Google Scholar]
- 25.Booth SL, Martini L, Peterson JW, Saltzman E, Dallal GE, Wood RJ. Dietary phylloquinone depletion and repletion in older women. J Nutr. 2003;133:2565–9. [DOI] [PubMed] [Google Scholar]
- 26.Sokoll LJ, Sadowski JA. Comparison of biochemical indexes for assessing vitamin K nutritional status in a healthy adult population. Am J Clin Nutr. 1996;63:566–73. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida M, Jacques PF, Meigs JB, Saltzman E, Shea MK, Gundberg C, Dawson-Hughes B, Dallal G, Booth SL. Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care. 2008;31:2092–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamon-Fava S, Sadowski JA, Davidson KW, O'Brien ME, McNamara JR, Schaefer EJ. Plasma lipoproteins as carriers of phylloquinone (vitamin K1) in humans. Am J Clin Nutr. 1998;67:1226–31. [DOI] [PubMed] [Google Scholar]
- 29.Shea MK, Booth SL, Massaro JM, Jacques PF, D'Agostino RB Sr, Dawson-Hughes B, Ordovas JM, O'Donnell CJ, Kathiresan S, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2008;167:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]