Abstract
Background/Aims
Endothelium-dependent dilation of skeletal muscle arterioles is mediated by unknown factors in very young rats. We assessed the possible contribution of carbon monoxide (CO) to this dilation and to dilation in older animals.
Methods
The effects of de-endothelialization or various pharmacological inhibitors on responses to CO or endothelium-dependent dilators were studied in gracilis muscle arterioles from rats at 3–4 weeks (‘weanlings’) and 6–7 weeks (‘juveniles’).
Results
Exogenous CO constricted, rather than dilated, arterioles from both age groups. This constriction was reduced by endothelial removal or NOS inhibition in juvenile, but not weanling, arterioles. In contrast, this constriction was abolished by K+ channel inhibition in weanling, but not juvenile, arterioles. The heme precursor δ-aminolevulinic acid constricted juvenile arterioles but did not affect weanling arterioles. The heme oxygenase inhibitor chromium (III) mesoporphyrin IX abolished the endothelium-dependent dilation of juvenile arterioles to simvastatin, and reduced ACh- and simvastatin-induced dilations of weanling arterioles.
Conclusion
These findings suggest that relatively high concentrations of exogenous CO can cause constriction by inhibiting endothelium-derived NO in juvenile arterioles and inhibiting K+ channels in weanling arterioles. Endogenous CO produced at lower concentrations can contribute to endothelium-dependent dilation in both age groups.
Key Words: Microcirculation, Endothelium, Postnatal growth, Carbon monoxide
Introduction
Studies from our laboratory have documented that postnatal growth of the microvasculature is accompanied by progressive changes in a number of factors that can influence arteriolar tone and blood flow [1,2,3,4]. For example, skeletal muscle arterioles from young rats exhibit fully developed endothelium-dependent dilation, but these responses are not mediated by nitric oxide (NO), cyclooxygenase metabolites or hydrogen peroxide (H2O2), as they are in arterioles from more mature rats [1, 2]. The chemical mediator(s) of arteriolar endothelium-dependent dilation in these younger animals have not yet been identified.
Carbon monoxide (CO), a signaling molecule that can be important for regulation of vascular tone and blood flow under some conditions [5,6,7], is produced via metabolism of heme by heme oxygenase (HO), with biliverdin and free iron also being generated in the process [8]. The 2 isoforms of this enzyme, HO-1 and HO-2, have been found in endothelial [9, 10] and vascular smooth muscle [11, 12] cells. HO-1 is an inducible isoform; its expression can be dramatically increased by numerous stimuli including hypoxia, hypertension, endotoxic shock and shear stress [12,13,14]. In contrast, HO-2 is constitutive and therefore presumably responsible for moment-to-moment changes in vascular CO production in the absence of pathologic stimuli [15]. CO is generally considered to be a vasodilator molecule [16]; among other vessel types, it has been found to relax rabbit and rat aorta [17, 18], dog coronary arteries [17], rabbit pulmonary arteries [19], rat afferent arterioles [20] and pial arterioles in newborn pigs [21]. However, CO can also reduce NO production by inhibiting NO synthase (NOS) [22, 23], and at least 1 study has demonstrated that CO can induce arteriolar constriction [24]. The effect of CO on arteriolar tone in skeletal muscle is variable. Some investigators have found CO-dependent dilation of gracilis muscle arterioles [25], whereas others have found that both exogenously applied and endogenously formed CO constricts these arterioles after pretreatment with phenylephrine [24]. Such discrepancies may be due in part to differences in the concentration of CO reached at or within the vessel wall. For example, NO release in rat afferent arterioles is increased at low CO levels but suppressed at high CO levels [20].
Because CO has been identified as an important vasoactive factor in the cerebral microcirculation of newborn pigs [21], we undertook the current study to determine if CO could be playing a similar role as a mediator of endothelium-dependent dilation in skeletal muscle arterioles of young rats. A second aim of this study was to determine if there is a change in the mechanism(s) by which CO can influence arteriolar tone during rapid juvenile growth.
Materials and Methods
Animals
All surgical and experimental procedures were approved by the West Virginia University Animal Care and Use Committee. Experiments were performed on isolated gracilis muscle arterioles from male Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, Ind., USA) of 3 age groups: 3–4 weeks (‘weanlings’), 6–7 weeks (‘juveniles’) and 9–10 weeks (‘adults’). Arterioles from adult rats were used in only 1 subset of the experiments described below (see Results).
Rats were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg body weight), with supplemental anesthetic (10% of original dose) administered if needed. The right carotid artery was cannulated with polyethylene tubing for measurement of mean arterial pressure, which was assessed immediately before removal of the gracilis arteriole.
Preparation of Isolated Vessels
The small muscular branch of the femoral artery that directly supplies the gracilis muscle (a first-order arteriole) was removed, handling only the surrounding connective tissue to minimize vessel stretching or damage. The rat was sacrificed immediately after vessel removal by intracardiac injection of sodium pentobarbital. The excised vessel was placed in warmed physiological salt solution (PSS) equilibrated with 21% O2, 5% CO2 and 74% N2, and having the following composition (in mM): 119 NaCl, 4.7 KCl, 1.17 MgSO4, 1.6 CaCl2, 1.18 NaH2PO4, 24 NaHCO3, 0.026 EDTA and 5.5 glucose. After isolation, each vessel was prepared for in vitro video microscopy as previously described [26]. Briefly, the vessel was mounted in a heated (37°C) chamber that allowed for its lumen and exterior surface to be perfused and superfused, respectively, with PSS from separate reservoirs. The vessel was cannulated at both ends with glass micropipettes (tip diameters: 50 μm for weanling vessels, 70 μm for juvenile and adult vessels) and secured to the inflow and outflow pipettes using 9-0 nylon suture. Any side branches were ligated with a single strand teased from 6-0 silk suture. The inflow pipette was connected to a reservoir perfusion system for control of intralumenal pressure and flow. The vessel was then extended to its in situ length and equilibrated at 80% of the animal's mean arterial pressure to approximate its normal in vivo perfusion pressure [27].
Vessel diameter was measured using an onscreen video micrometer. Any vessel that did not demonstrate endothelial viability, as judged by pronounced dilation to 10–7M acetylcholine (ACh; Sigma Chemical, St. Louis, Mo., USA), was not used in this study. Changes in vessel diameter to all agonists and inhibitors (see below) were made under static, zero-flow conditions after a 30-min equilibration period with continuous perfusion. Resting vascular tone under zero-flow conditions was calculated as (ΔD/Dmax) × 100, where ΔD is the diameter increase from rest in response to Ca2+-free PSS (30- to 40-min equilibration with Ca2+-free bath solution), and Dmax is the maximum diameter measured under these conditions.
Agonists
Endothelium-dependent dilation was elicited by application of ACh or simvastatin (Merck Research Laboratories, Rahway, N.J., USA) at bath concentrations of 10–5 or 10–7M[3, 4]. Simvastatin was first activated by alkaline lysis (5.25 ml of 0.1 N NaOH per 140 mg, dissolved in 3.5 ml of ETOH) at 50°C for 2 h. The resulting solution was then diluted to a volume of 35 ml with PBS, and neutralized to pH 7.4 with HCl. One-milliliter aliquots of this solution were then serially diluted with PBS for addition to the vessel bath.
CO-saturated solution (CP grade 99.5%; Airgas Mid America, Bowling Green, Ky., USA) was prepared as described by Johnson and Johnson [24]. Briefly, ice-cold distilled H2O was vigorously bubbled with CO through a glass gas diffuser for 30 min to prepare a 10–2M solution. Increasing volumes of this solution were incrementally added to the vessel bath to produce final CO concentrations of 10–6, 10–5 or 10–4M. After each application, the vessel was allowed to return to its control diameter before the next CO application. Control experiments were performed using ice-cold H2O bubbled with N2 (Airgas Mid America). To stimulate endogenous CO production through HO activity, the heme precursor δ-aminolevulinic acid (δ-ALA; Frontier Scientific, Logan, Utah, USA) was added to the bath at a concentration of 10–6M[28].
Inhibitors
Some vessels were pretreated with chromium (III) mesoporphyrin IX chloride (CrMP; Frontier Scientific), a competitive inhibitor of HO that is less sensitive to photo degradation than other metalloporphyrins [29]. Although there are many commercially made metalloporphyrins that inhibit HO, CrMP was chosen because it is the most selective inhibitor of HO activity [30]. A 10–2M stock solution of CrMP in 0.1 N NaOH was diluted in the bath to produce a final concentration of 10–5M[28]. During exogenous CO application, endogenous CO production was also inhibited with CrMP to more directly assess intrinsic vascular responsiveness to CO.
To evaluate possible interactions of exogenous or endogenous CO with the NO system, the NOS inhibitor Nω-nitro-L-arginine methyl ester (L-NAME; Sigma) was added to the bath at a concentration of 10–4M[31], alone or in the presence of CrMP. Both CrMP and L-NAME were administered for 20 min before vessels were challenged with agonists.
The contribution of potassium (K+) channels to arteriolar responses was assessed by using iberiotoxin (Ibtx; Sigma) [32], and the antidiabetic sulphonylurea, glibenclamide (Glib; Sigma) [33]. We used a 10–7-M bath concentration of Ibtx to selectively block Ca2+-activated K+ (KCa) channels [31, 32], and 10–6M Glib to selectively block ATP-sensitive K+ (KATP) channels [34].
Endothelial Denudation
To determine the role of the endothelium in mediating arteriolar responses to exogenous CO, the endothelium was removed in some experiments by mechanical abrasion [35]. The pipette tip at each end of the vessel was gently advanced and then retracted through the vessel lumen 3 times to ensure elimination of the endothelium. We have previously verified that this method successfully denudes the endothelium of gracilis muscle arterioles without affecting the underlying smooth muscle [1]. To verify that smooth muscle function was intact following denudation in the current experiments, vasoconstrictor responses to 10–5M phenylephrine (Sigma) and vasodilator responses to 10–5M sodium nitroprusside (SNP; Sigma) were assessed before and after the denudation procedure. Only those vessels with unchanged responses to both agonists were included in the final data set.
HO-1 and HO-2 Protein Measurements
Gracilis artery/arteriole segments were harvested from weanling and juvenile rats, snap frozen in liquid N2, and stored at −80°C until analysis. Protein was harvested from vessels by repeated vortexing and boiling in a sample buffer containing 0.225 M Tris-Cl pH 6.8, 50% glycerol, 5% SDS, 0.25 M dithiothreitol and 5% 2-mercaptoethanol. Total protein concentration of each sample was determined using a Nano-Orange assay (Invitrogen, Carlsbad, Calif., USA) according to the manufacturer's protocol. For each gel, protein samples were diluted in sample buffer to an equal concentration, boiled for 10 min and spun for 10 min at 9,300 g prior to loading onto precast 10% Bis-Tris polyacrylamide gels (Invitrogen). Gels were loaded with 50 μg total protein per well. Electrophoresis was carried out at 150 V for 1.5 h and resolved proteins were transferred to an Immobilon PVDF membrane (Millipore, Billerica, Mass., USA) at 30 V for 1.25 h using Invitrogen's Sure Lock Mini-Cell electrophoresis system and associated X-Cell II Blot Module. Membranes were blocked overnight at 4°C in Superblock T-20 (Pierce Biotechnology, Rockford, Ill., USA) and then incubated with either HO-1 or HO-2 antibodies (Assay Designs; Stressgen, Ann Arbor, Mich., USA) for 1.5 h. Membranes were then washed and incubated with appropriate horseradish peroxidase-conjugated secondary antibody. To verify equal protein loading among lanes, membranes were washed and incubated with antibody to glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Fitzgerald Industries International Inc., Concord, Mass., USA) for 1 h, washed, and incubated with appropriate secondary antibody. All proteins were visualized using an Amersham ECL Western blotting kit.
Data and Statistical Analysis
For each arteriole, vascular tone at each pressure was calculated as follows: tone = [(Dmax – Drest)/Dmax] × 100, where Dmax is the passive diameter and Drest is the resting diameter. A tone of 100% would represent complete vessel closure, whereas 0% would represent a completely passive vessel. All data are presented as mean ± SE. For all analyses, a probability value of p < 0.05 was considered to be statistically significant. Dilation in response to Ca2+-free PSS is expressed as percent increase from control diameter. Differences among the means of individual experimental groups were evaluated by one- or two-way ANOVA (post hoc analysis: Newman-Keuls test), or by an unpaired Student's t test when 2 independent means were compared.
Results
General characteristics of all rats from which vessels were removed for in vitro study are reported in table 1. Most experiments were conducted on vessels from weanling and juvenile rats only, although the effects of CrMP on responses to ACh and simvastatin were also assessed in arterioles from adult rats (see below). Body weight increased with age from weanlings to adults, but mean arterial pressure increased only from weanling to juvenile rats, with no further increase from juvenile to adult rats. Table 1 also summarizes the general characteristics of all arterioles used in this study. Resting and passive arteriolar diameters progressively increased with age, but the level of spontaneous tone that developed upon vessel pressurization progressively decreased with age.
Table 1.
General characteristics of all rats and vessels used for functional studies
| Weanlings | Juveniles | Adults | |
|---|---|---|---|
| Animal characteristics | |||
| n | 47 | 52 | 6 |
| Age, days | 25.3 ± 0.3 | 43.8 ± 0.5∗ | 64.5 ± 0.7∗∗ |
| Body weight, g | 62 ± 1 | 168 ± 2∗ | 294 ± 7∗∗ |
| MAP, mm Hg | 76 ± 1 | 97 ± 2∗ | 98 ± 5∗ |
| Vessel characteristics | |||
| n | 47 | 52 | 6 |
| Resting diameter, μm | 34 ± 1 | 51 ± 2∗ | 77 ± 5∗∗ |
| Passive diameter, μm | 57 ± 1 | 79 ± 2∗ | 109 ± 6∗∗ |
| Resting vascular tone % max. | 41 ± 2 | 35 ± 2∗ | 29 ± 2∗∗ |
Values are given as means ± SE. MAP = Mean arterial pressure.
p < 0.05 vs. weanling group;
p < 0.05 vs. weanling and juvenile groups.
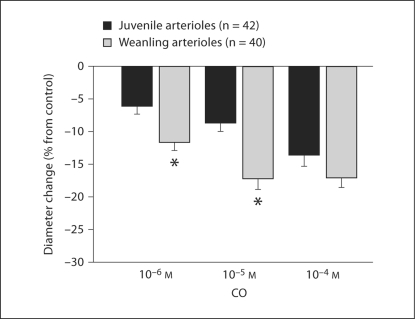
CrMP had no effect on resting arteriolar diameters in any age group (table 2). δ-ALA significantly reduced the resting diameter of juvenile arterioles (by 25 ± 1%), but not weanling arterioles. Exogenous CO caused significant arteriolar constriction in both groups, with weanling arterioles constricting significantly more than juvenile arterioles in response to 10–6 and 10–5M CO (fig. 1). The CO vehicle alone (equilibrated with N2) had no effect in either group (data not shown). CrMP was without effect on these responses to exogenous CO in either group, indicating that there were no acute changes in endogenous CO production that could influence arteriolar behavior under these conditions (data not shown).
Table 2.
Summary of resting arteriolar diameters under control conditions and after treatment with CrMP (10−5 M) or δ-ALA (10−6 M)
| n | Control | CrMP | n | Control | δ-ALA | |
|---|---|---|---|---|---|---|
| Adults | 6 | 77 ± 5 | 78 ± 3 | − | − | − |
| Juveniles | 16 | 47 ± 3 | 47 ± 3 | 10 | 52 ± 3 | 39 ± 6∗ |
| Weanlings | 17 | 32 ± 2 | 37 ± 3 | 7 | 35 ± 3 | 38 ± 4 |
Values are given as means ± SE.
p < 0.05 vs. control.
Fig. 1.
Responses of gracilis muscle arterioles from juvenile and weanling rats to increasing concentrations of exogenous CO. Values are given as means ± SE. ∗ p < 0.05 vs. juvenile response.
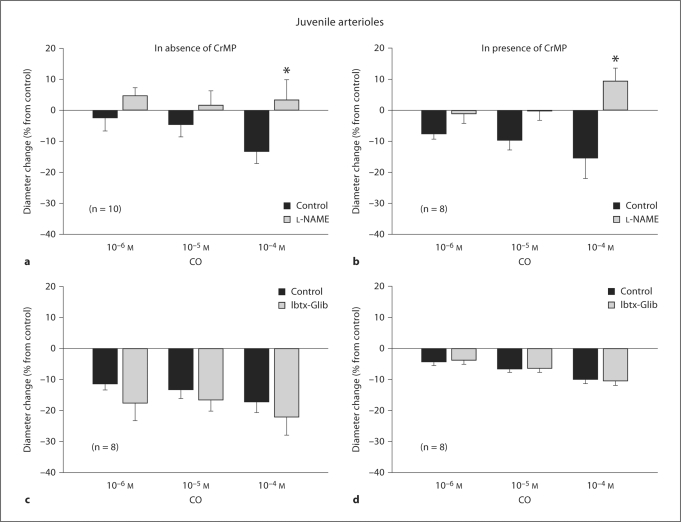
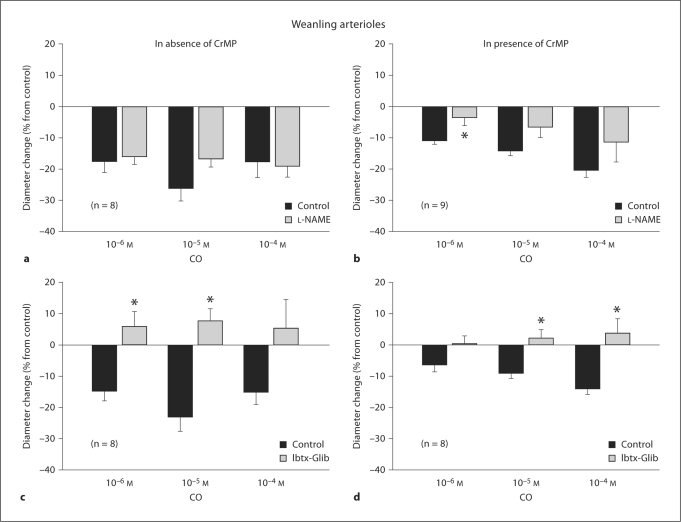
L-NAME treatment reduced the resting diameter of both juvenile arterioles (from 55 ± 5 to 40 ± 5 μm, a 27% average diameter reduction) and weanling arterioles (from 35 ± 4 to 25 ± 3 μm, a 29% average diameter reduction). In contrast, L-NAME had no effect on resting diameters in either group if CrMP was present (44 ± 6 before vs. 44 ± 4 μm after L-NAME in juveniles, 30 ± 3 before vs. 37 ± 4 μm after L-NAME in weanlings). However, L-NAME abolished the constrictor responses of juvenile arterioles to exogenous CO whether or not CrMP was present (fig. 2a, b). L-NAME had no effect on weanling arteriole responses to CO at any concentration (fig. 3a), although it tended to reduce these responses to CO in the presence of CrMP (significantly at 10–6M CO) (fig. 3b).
Fig. 2.
Responses of arterioles from juvenile rats to exogenous CO before and after NOS inhibition with L-NAME alone (a) or in the presence of CrMP (b), and before and after inhibition of KCa and KATP channels with Ibtx + Glib alone (c) or in the presence of CrMP (d). n = Number of vessels. Values are given as means ± SE. ∗ p < 0.05 vs. control.
Fig. 3.
Responses of arterioles from weanling rats to exogenous CO before and after NOS inhibition with L-NAME alone (a) or in the presence of CrMP (b), and before and after inhibition of KCa and KATP channels with Ibtx + Glib alone (c) or in the presence of CrMP (d). Values are given as means ± SE. ∗ p < 0.05 vs. control.
Treatment with Ibtx + Glib reduced the resting diameters of both juvenile arterioles (from 51 ± 6 to 35 ± 4 μm, a 31% average diameter reduction) and weanling arterioles (from 43 ± 2 to 29 ± 3 μm, a 33% average diameter reduction). For juvenile arterioles, Ibtx + Glib had no effect on CO-induced constriction, whether or not CrMP was present (fig. 2c, d). However, for weanling arterioles, Ibtx + Glib abolished the constrictor responses to exogenous CO at all concentrations, whether or not CrMP was present (fig. 3c, d).
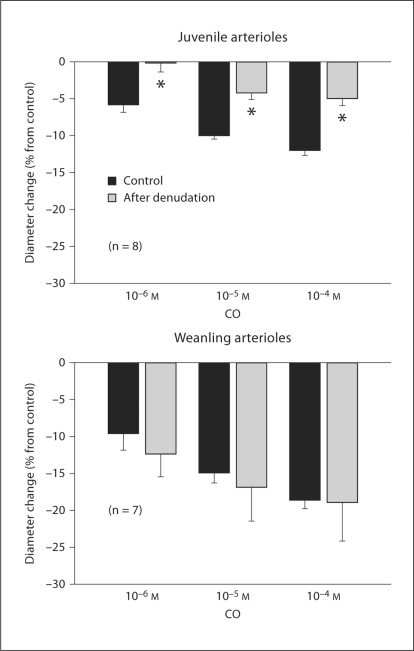
Endothelial removal completely abolished responses to 10–5M ACh in both juvenile and weanling arterioles (data not shown). After the denudation procedure, resting diameters tended to be smaller in juvenile arterioles (55 ± 2 before vs. 49 ± 2 μm after denudation) and weanling arterioles (26 ± 3 vs. 20 ± 2 μm), but these diameter reductions did not reach statistical significance. Endothelial removal had no effect on CO-induced constriction of weanling arterioles, but significantly reduced these responses in juvenile arterioles (fig. 4).
Fig. 4.
Effect of endothelial removal on responses of juvenile and weanling arterioles to exogenous CO. Values are given as means ± SE. ∗ p < 0.05 vs. control.
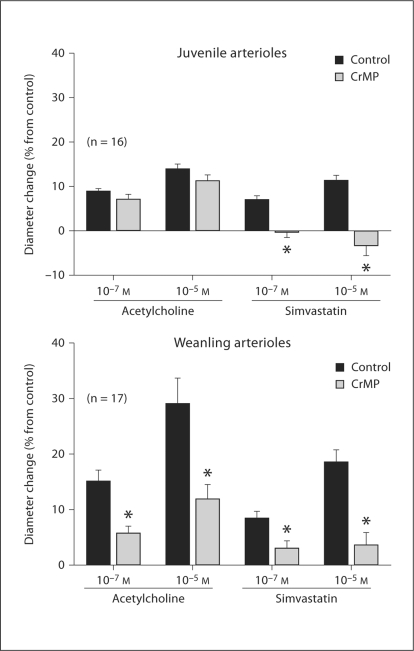
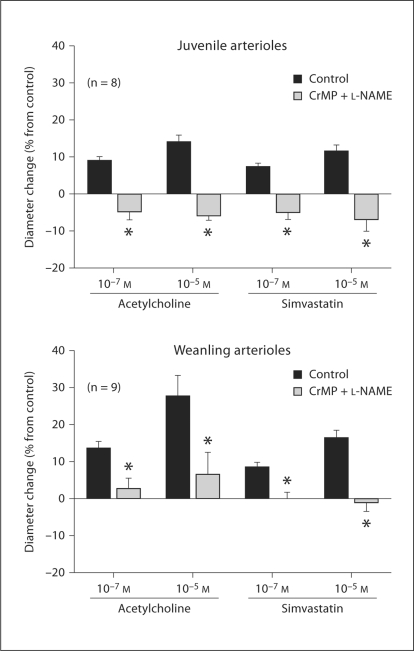
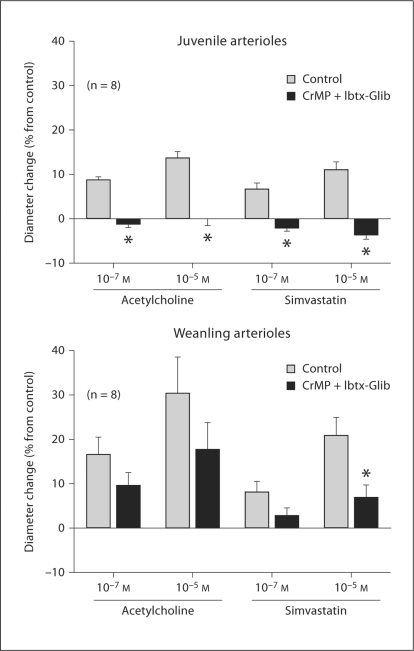
Although treatment with CrMP did not affect the dilation of juvenile arterioles to ACh, it abolished the dilation of these vessels to simvastatin (fig. 5). However, CrMP treatment reduced the dilation of weanling arterioles to both ACh and simvastatin (fig. 5). Addition of L-NAME after CrMP treatment abolished dilation to ACh and had no further effect on simvastatin responses in juvenile arterioles, and had no further effect on weanling arteriolar responses to ACh or simvastatin (fig. 6). In juvenile arterioles, treatment with Ibtx + Glib following CrMP abolished responses to ACh and had no further effect on responses to simvastatin (fig. 7). In weanling arterioles, treatment with Ibtx + Glib following CrMP had no effect on ACh responses, but reduced the dilation to 10–5M simvastatin (fig. 7).
Fig. 5.
Effect of CrMP (10–5M) on responses of juvenile and weanling arterioles to ACh and simvastatin. Values are given as means ± SE. ∗ p < 0.05 vs. control.
Fig. 6.
Responses of arterioles from juvenile and weanling rats to ACh and simvastatin before and after treatment with CrMP and L-NAME. Values are given as means ± SE. ∗ p < 0.05 vs. control.
Fig. 7.
Responses of arterioles from juvenile and weanling rats to ACh and simvastatin before and after treatment with CrMP and Ibtx-Glib. Values are given as means ± SE. ∗ p < 0.05 vs. control.
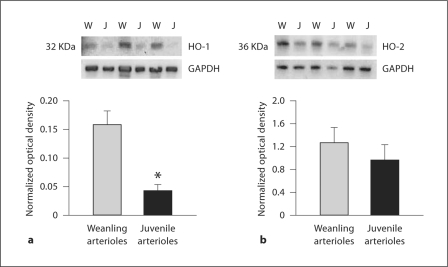
Western blot analysis of gracilis artery/arteriole homogenates is shown in figure 8. Vessels from juvenile rats had a significantly lower abundance of HO-1 protein than those from weanling rats. In both groups, the expression of HO-2 was approximately an order of magnitude higher than that of HO-1, but there was no difference in HO-2 protein abundance between weanling and juvenile rats (fig. 8).
Fig. 8.
Assessment of HO-1 (a) and HO-2 (b) protein abundance by Western blot in femoral artery/gracilis arteriole segments from both age groups. W = Weanling vessels; J = juvenile vessels. Densitometric values for HO-1 and HO-2 are normalized as a fraction of the corresponding value for GAPDH (loading control). Similar findings were observed in three separate experiments. ∗ p < 0.05 vs. weanling arterioles.
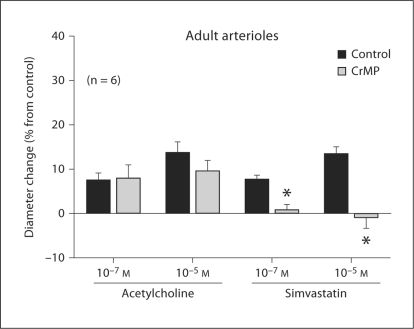
Treatment with CrMP had no effect on the dilation of adult arterioles to ACh, but it abolished the dilation of these vessels to simvastatin (fig. 9). In this regard, the behavior of arterioles from adult rats was virtually identical to that of arterioles from juvenile rats (fig. 5).
Fig. 9.
Effect of CrMP (10–5M) on responses of adult arterioles to ACh and simvastatin. Values are given as means ± SE. ∗ p < 0.05 vs. control.
Discussion
Growth-related changes in microvascular endothelial function have been reported by our laboratory and others [1, 4, 36]. In our recent investigations on gracilis muscle arterioles [1, 2], we ruled out NO, prostanoids, cytochrome P-450 metabolites and H2O2 as possible mediators of endothelium-dependent dilation to various agonists in young (weanling) rats. Since CO has been found to mediate endothelium-dependent dilation of cerebral arterioles in newborn pigs [21], we undertook the current study in part to determine if CO could be playing an analogous role in the skeletal muscle circulation of weanling rats.
Juvenile Arterioles
The HO inhibitor CrMP had no effect on the resting diameters of arterioles in any age group (table 2). This suggests that under the conditions of our study, basal CO release in these vessels, if present, is insufficient to influence resting smooth muscle tone. Similar findings have been reported for larger gracilis muscle arterioles located upstream from those studied here [37] and in various conduit arteries from the rat [38, 39]. However, CrMP does elicit constriction of gracilis muscle arterioles after these vessels have been pretreated with L-NAME or denuded of their endothelium [25, 37, 38], which suggests that the effects of basally released NO normally mask the influence of endogenous CO on resting arteriolar tone. This effect has also been reported in rat renal afferent arterioles [40]. Conversely, we found that treatment with L-NAME reduces the diameter of juvenile arterioles under normal conditions, but not if the vessels had been pretreated with CrMP. There have been similar reports for other vessel types [41], and collectively these findings suggest that although a continuous effect of low levels of NO on resting tone may obscure a similar effect of CO, some level of HO activity may actually be required for this tonic NO influence to be present in the first place. The exact mechanism of this synergistic effect is not known. CO is not a cofactor or cosubstrate for NOS, but the smooth muscle signaling pathways through which CO and NO act share common elements, including an increase in soluble guanyl cyclase activity and cGMP production [41]. Basally released CO, even at concentrations too low to exert a direct effect on vascular tone, could act to ‘prime’ the NO signaling pathway in vascular smooth muscle, thereby increasing smooth muscle sensitivity to basally released NO. Low levels of CO have also been found to induce NO release from resistance vessels, most likely from a preexisting pool of heme-bound NO [20]. Further studies will be necessary to clarify the complex interaction between CO and NO in regulating arteriolar tone.
The heme precursor δ-ALA increases arteriolar CO production in a dose-dependent manner [42], and our finding that 1 μM δ-ALA reduces juvenile arteriole diameters by approximately 25% (table 2) demonstrates that increased endogenous CO production can increase the resting tone of these vessels. δ-ALA-induced constriction has been reported in larger arterioles from rat gracilis muscle [24], although that effect was more modest (less than 10% constriction in response to 80 μM δ-ALA). The difference in responsiveness to δ-ALA between these 2 studies may reflect a difference in HO activity or substrate availability between different branch orders in the arteriolar network. In the current study, exogenous CO at concentrations from 10–6 to 10–4M also caused arteriolar constriction (fig. 1), which is consistent with the earlier study on larger gracilis muscle arterioles [24].
Relatively high concentrations of CO induce vascular constriction by inhibiting endothelial NO release [20,22,23,24, 41], which is consistent with our finding that CO-induced constriction of juvenile arterioles is abolished by L-NAME (fig. 2a, b) and greatly reduced by endothelial denudation (fig. 4). As also shown in figure 2, the highest concentration of CO elicited a dilation of these arterioles during L-NAME exposure. This is similar to recent findings in renal interlobular arteries [43] and presumably reflects a direct relaxing effect of the exogenous CO that should predominate once its ability to inhibit endothelial NO release becomes irrelevant. CO-induced vasodilation is mediated by an increase in smooth muscle cGMP levels (see above) and/or activation of KCa channels and membrane hyperpolarization [21, 39, 44].
Interestingly, a comparison of figures 2 and 4 reveals that endothelial denudation was less effective than L-NAME in reducing arteriolar responses to 10–5 and 10–4M CO. This may indicate that some of the NO inhibited by CO is of smooth muscle origin, or it may simply reflect an incomplete removal of the endothelium. However, the latter possibility appears less likely given that our denudation procedure completely abolished arteriolar responses to ACh (see Results). Simultaneous blockade of KCa and KATP channels had no effect on CO-induced constriction of juvenile arterioles (fig. 2c, d), indicating that a change in K+ channel activity normally does not mediate or attenuate the CO-induced constriction of these vessels.
Whereas CrMP had no effect on ACh-induced dilation of these vessels, it completely abolished dilator responses to simvastatin (fig. 5). This suggests that endogenous CO may mediate some forms of endothelium-dependent dilation, despite our finding that increasing HO substrate with δ-ALA produced arteriolar constriction. These divergent results may be due to differences in the concentration of CO within the arteriolar wall achieved by these two procedures. CO at lower concentrations (0.01–0.1 μM) triggers maximal NO release in rat arterioles, whereas CO at higher concentrations suppresses NO release in these vessels [20]. In the rat gracilis muscle arterioles studied here, simvastatin may have triggered a smaller increase in endogenous CO production than that achieved by δ-ALA, leading to lower arteriolar wall CO levels that could promote dilation. If this is correct, the constriction of these vessels to exogenous CO in the range of 10–6 to 10–4M (fig. 1) may reflect a relatively large increase in arteriolar wall CO levels, similar to that triggered by δ-ALA. Unfortunately, we were unable to reliably apply CO to these vessels at concentrations lower than 10–6M. It is difficult to maintain stable CO levels in aqueous solution, and in our hands, dilutions to concentrations below 10–6M introduced an unacceptable degree of uncertainty in the true concentration of CO in solution.
It is conceivable that the effect of CrMP on juvenile arteriole responses to simvastatin could be due to a reduction in smooth muscle responsiveness to the NO that is released, rather than inhibition of endothelial CO production. However, this seems unlikely, because such a nonspecific effect would also have reduced arteriolar responsiveness to ACh. In addition, control experiments for this study indicate that CrMP has no effect on the responses of juvenile arterioles to the endothelium-independent NO donor SNP. In these experiments, addition of 10–7, 10–6 and 10–5M SNP to the vessel bath elicited dilations of 5 ± 1, 7 ± 3 and 10 ± 1% under control conditions versus 4 ± 1, 7 ± 2 and 9 ± 1% in the presence of CrMP.
A comparison of figures 5 and 9 clearly shows that both the dilations to ACh and simvastatin and the effect (or absence thereof) of CrMP on these responses are identical in the juvenile and ‘adult’ arterioles we studied. Therefore, age-dependent changes in the contribution of CO to these responses appear to be complete by the time the animal reaches 6–7 weeks of age. Similarly, previous studies from our laboratory have demonstrated that the ability of the arteriolar endothelium to release NO in response to shear stress increases dramatically from the weanling to the juvenile stage, but does not change further from the juvenile to the adult stage [4]. Taken in aggregate, these findings suggest that with respect to the endothelium-dependent control of arteriolar tone, the adult phenotype appears to be established by 6–7 weeks of age.
Combined treatment with CrMP + L-NAME abolished the dilation of juvenile arterioles to ACh as well as simvastatin (fig. 6). We previously demonstrated that these responses are entirely endothelium-dependent, and that L-NAME treatment alone reduces juvenile arteriole responses to ACh and simvastatin by approximately 90 and 60%, respectively [1]. Our current findings, taken together with these previous findings, suggest that although simvastatin releases endothelium-derived CO in addition to NO, ACh does not. Combined KATP and KCa channel inhibition abolished ACh-induced dilations in the presence of CrMP (fig. 7). We previously demonstrated that inhibition of both channel types in juvenile arterioles reduces ACh responses by 60–70% [1]. Our current finding that the additional presence of CrMP leads to a complete abolition of ACh responses is surprising, since CrMP treatment alone had no effect on ACh-induced dilation (fig. 5). This finding may be attributable to some nonspecific effect of CrMP in these experiments.
Weanling Arterioles
As with juvenile arterioles, CrMP had no effect on the resting diameter of weanling arterioles (table 2), suggesting that basally produced CO also does not influence the resting tone of arterioles from younger rats. However, unlike juvenile arterioles, the diameter of weanling arterioles was also unaffected by δ-ALA, indicating that even when the substrate for HO is increased, CO is still not produced in quantities large enough to influence arteriolar tone at this earlier stage of growth.
Leffler et al. [21] have reported that exogenous CO dilates cerebral arterioles of newborn pigs in vivo. In contrast, exogenous CO constricted the weanling arterioles we studied, in some cases even more than that seen with juvenile arterioles (fig. 1). Although this could reflect normal differences between species or vascular beds, it is also possible, as discussed above, that differences in CO concentration reached within the arteriolar wall could explain these divergent observations. Whereas the CO-induced dilations in newborn pigs occurred at superfusate concentrations ranging from 10–11 to 10–7M, with maximal dilation at 10–9M[42], we applied CO at bath concentrations between 10–6 and 10–4M.
Although exogenous CO did not dilate our weanling arterioles, we found reduced dilations to ACh and simvastatin in the presence of CrMP (fig. 5), suggesting that a portion of this endothelium-dependent dilation is due to endogenous CO release. In these experiments, endogenously produced CO in the arteriolar wall may have been in the concentration range reached by Leffler et al. [21], which could produce dilation, rather than the higher concentrations we applied exogenously, which produced constriction. In contrast to juvenile arterioles, L-NAME did not affect CO-induced constriction of weanling arterioles (fig. 3a, b), suggesting that a decrease in NO production does not mediate these responses. Although L-NAME did reduce the resting diameter of the weanling arterioles, this did not happen after CrMP treatment, which is similar to our findings in juvenile arterioles and suggests that some level of HO activity is also required for basal NO to modulate resting arteriolar tone at this earlier stage of growth.
In contrast to our findings in the juvenile arterioles, K+ channel inhibitors abolished the constrictor effect of CO in weanling arterioles (fig. 3c, d). This finding that some initial level of K+ channel activity is required for this response suggests that CO may constrict weanling arterioles by inhibiting K+ channel activity in vascular smooth muscle. Endothelial denudation had no effect on this response (fig. 4), which is consistent with a decrease in smooth muscle K+ channel activity being the predominant mechanism of CO-induced constriction. However, this contrasts with other reports that CO increases the activity of smooth muscle KCa channels to induce dilation of gracilis muscle arterioles and other vessels from the rat [14, 44]. These previous studies involved rats that were older than the weanlings studied here, with ages corresponding to our juvenile or adult groups. This raises the possibility that in the rat, postnatal growth could be accompanied by a change in the overall effect of CO on vascular K+ channels. Our finding that K+ channel activity is not required for CO-induced constriction of juvenile arterioles (see above) is consistent with such a change, but further studies are required to critically test this hypothesis. It is also important to point out that there are vascular beds in which such a change clearly does not occur. For example, exogenous CO triggers a K+ channel-dependent dilation of cerebral arterioles in pigs at only 3 days of age [21], and does not elicit a constriction of these vessels at any age [45].
HO-1 and HO-2 are present in both the vascular smooth muscle and endothelium of gracilis muscle arterioles [37], and we found no difference between weanling and juvenile rats in vascular HO-2 protein abundance (fig. 8). HO-2 is the constitutive isoform of the protein and responsible for acute changes in CO production [15]. This suggests that changes in HO activity, rather than its expression, underlie the differences we found between weanling and juvenile arterioles. In contrast, we found a relatively large age-related reduction in vascular HO-1 abundance. The reason for this difference between age groups is not clear, but one possibility is that the lower HO-1 expression in juveniles reflects an age-related difference in the shear stresses to which these vessels are normally exposed. Shear stress is an effective inducer of HO-1 expression [14], and we have previously documented that hemodynamic shear stress in rat skeletal muscle arterioles undergoes a large reduction between the weanling and juvenile stages studied here [4]. Further studies would be required to critically test this hypothesis.
In summary, the role of CO in regulating arteriolar tone appears to change during juvenile growth. Exogenous CO at 10–6 to 10–4M produces arteriolar constriction mediated by K+ channel inhibition and smooth muscle depolarization in weanling vessels, but by NO inhibition in juvenile vessels. Endogenous CO contributes to the endothelium-dependent dilation of arterioles from both age groups, but this role for CO may be more widespread in weanling arterioles. Such differences underscore the importance of further studies to define the effect of postnatal growth on microvascular function to more fully understand the mechanisms of blood flow regulation during this critical period.
Acknowledgements
This work was funded by the American Heart Association (0330194N to J.C.F. and 0150199N to M.A.B.) and the National Institutes of Health (DK 64668 to J.C.F. and HL 44012 to M.A.B.).
References
- 1.Balch Samora J, Frisbee J, Boegehold M. Growth-dependent changes in endothelial factors regulating arteriolar tone. Am J Physiol. 2007;292:H207–H214. doi: 10.1152/ajpheart.00677.2006. [DOI] [PubMed] [Google Scholar]
- 2.Balch Samora J, Frisbee JC, Boegehold MA. Hydrogen peroxide emerges as a regulator of tone in skeletal muscle arterioles during juvenile growth. Microcirculation. 2008;15:151–161. doi: 10.1080/10739680701508497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linderman JR, Boegehold MA. Arteriolar network growth in rat striated muscle during juvenile maturation. Int J Microcirc Clin Exp. 1996;16:232–239. doi: 10.1159/000179179. [DOI] [PubMed] [Google Scholar]
- 4.Linderman JR, Boegehold MA. Growth-related changes in the influence of nitric oxide on arteriolar tone. Am J Physiol. 1999;277:H1570–H1578. doi: 10.1152/ajpheart.1999.277.4.H1570. [DOI] [PubMed] [Google Scholar]
- 5.Fiumana E, Parfenova H, Jaggar JH, Leffler CW. Carbon monoxide mediates vasodilator effects of glutamate in isolated pressurized cerebral arterioles of newborn pigs. Am J Physiol. 2003;284:H1073–H1079. doi: 10.1152/ajpheart.00881.2002. [DOI] [PubMed] [Google Scholar]
- 6.Gonzales RJ, Walker BR. Role of CO in attenuated vasoconstrictor reactivity of mesenteric resistance arteries after chronic hypoxia. Am J Physiol. 2002;282:H30–H37. doi: 10.1152/ajpheart.2002.282.1.H30. [DOI] [PubMed] [Google Scholar]
- 7.Kanu A, Whitfield J, Leffler CW. Carbon monoxide contributes to hypotension-induced cerebrovascular vasodilation in piglets. Am J Physiol. 2006;291:H2409–H2414. doi: 10.1152/ajpheart.01368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 9.Marks GS, McLaughlin BE, Vreman HJ, Stevenson DK, Nakatsu K, Brien JF, Pang SC. Heme oxygenase activity and immunohistochemical localization in bovine pulmonary artery and vein. J Cardiovasc Pharmacol. 1997;30:1–6. doi: 10.1097/00005344-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Parfenova H, Neff RA, 3rd, Alonso JS, Shlopov BV, Jamal CN, Sarkisova SA, Leffler CW: Cerebral vascular endothelial heme oxygenase: expression, localization, and activation by glutamate. Am J Physiol. 2001;281:C1954–C1963. doi: 10.1152/ajpcell.2001.281.6.C1954. [DOI] [PubMed] [Google Scholar]
- 11.Christodoulides N, Durante W, Kroll MH, Schafer AI. Vascular smooth muscle cell heme oxygenases generate guanylyl cyclase-stimulatory carbon monoxide. Circulation. 1995;91:2306–2309. doi: 10.1161/01.cir.91.9.2306. [DOI] [PubMed] [Google Scholar]
- 12.Yet SF, Pellacani A, Patterson C, Tan L, Folta SC, Foster L, Lee WS, Hsieh CM, Perrella MA. Induction of heme oxygenase-1 expression in vascular smooth muscle cells: a link to endotoxic shock. J Biol Chem. 1997;272:4295–4301. doi: 10.1074/jbc.272.7.4295. [DOI] [PubMed] [Google Scholar]
- 13.Ewing JF, Raju VS, Maines MD. Induction of heart heme oxygenase-1 (HSP32) by hyperthermia: possible role in stress-mediated elevation of cyclic 3′:5′-guanosine monophosphate. J Pharmacol Exp Ther. 1994;271:408–414. [PubMed] [Google Scholar]
- 14.Warabi E, Wada Y, Kajiwara H, Kobayashi M, Koshiba N, Hisada T, Shibata M, Ando J, Tsuchiya M, Kodama T, Noguchi N. Effect on endothelial cell gene expression of shear stress, oxygen concentration, and low-density lipoprotein as studied by a novel flow cell culture system. Free Radic Biol Med. 2004;37:682–694. doi: 10.1016/j.freeradbiomed.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Ding Y, McCoubrey WK, Jr, Maines MD: Interaction of heme oxygenase-2 with nitric oxide donors. Is the oxygenase an intracellular ‘sink' for NO? Eur J Biochem. 1999;264:854–861. doi: 10.1046/j.1432-1327.1999.00677.x. [DOI] [PubMed] [Google Scholar]
- 16.Johnson RA, Kozma F, Colombari E. Carbon monoxide: from toxin to endogenous modulator of cardiovascular functions. Braz J Med Biol Res. 1999;32:1–14. doi: 10.1590/s0100-879x1999000100001. [DOI] [PubMed] [Google Scholar]
- 17.Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28:52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- 18.Lin H, McGrath JJ. Vasodilating effects of carbon monoxide. Drug Chem Toxicol. 1988;11:371–385. doi: 10.3109/01480548809018109. [DOI] [PubMed] [Google Scholar]
- 19.Steinhorn RH, Morin FC, 3rd, Russell JA: The adventitia may be a barrier specific to nitric oxide in rabbit pulmonary artery. J Clin Invest. 1994;94:1883–1888. doi: 10.1172/JCI117538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am J Physiol. 1999;277:F882–F889. doi: 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- 21.Leffler CW, Nasjletti A, Yu C, Johnson RA, Fedinec AL, Walker N. Carbon monoxide and cerebral microvascular tone in newborn pigs. Am J Physiol. 1999;276:H1641–H1646. doi: 10.1152/ajpheart.1999.276.5.H1641. [DOI] [PubMed] [Google Scholar]
- 22.Pufahl RA, Marletta MA. Oxidation of NG-hydroxy-L-arginine by nitric oxide synthase: evidence for the involvement of the heme in catalysis. Biochem Biophys Res Commun. 1993;193:963–970. doi: 10.1006/bbrc.1993.1719. [DOI] [PubMed] [Google Scholar]
- 23.White KA, Marletta MA. Nitric oxide synthase is a cytochrome P-450 type hemoprotein. Biochemistry. 1992;31:6627–6631. doi: 10.1021/bi00144a001. [DOI] [PubMed] [Google Scholar]
- 24.Johnson FK, Johnson RA. Carbon monoxide promotes endothelium-dependent constriction of isolated gracilis muscle arterioles. Am J Physiol. 2003;285:R536–R541. doi: 10.1152/ajpregu.00624.2002. [DOI] [PubMed] [Google Scholar]
- 25.Kozma F, Johnson RA, Nasjletti A. Role of carbon monoxide in heme-induced vasodilation. Eur J Pharmacol. 1997;323:R1–R2. doi: 10.1016/s0014-2999(97)00145-3. [DOI] [PubMed] [Google Scholar]
- 26.Fredricks KT, Liu Y, Lombard JH. Response of extraparenchymal resistance arteries of rat skeletal muscle to reduced PO2. Am J Physiol. 1994;267:H706–H715. doi: 10.1152/ajpheart.1994.267.2.H706. [DOI] [PubMed] [Google Scholar]
- 27.DeLano FA, Schmid-Schonbein GW, Skalak TC, Zweifach BW. Penetration of the systemic blood pressure into the microvasculature of rat skeletal muscle. Microvasc Res. 1991;41:92–110. doi: 10.1016/0026-2862(91)90011-y. [DOI] [PubMed] [Google Scholar]
- 28.Andresen JJ, Shafi NI, Durante W, Bryan RM., Jr Effects of carbon monoxide and heme oxygenase inhibitors in cerebral vessels of rats and mice. Am J Physiol. 2006;291:H223–H230. doi: 10.1152/ajpheart.00058.2006. [DOI] [PubMed] [Google Scholar]
- 29.Vreman HJ, Ekstrand BC, Stevenson DK. Selection of metalloporphyrin heme oxygenase inhibitors based on potency and photoreactivity. Pediatr Res. 1993;33:195–200. doi: 10.1203/00006450-199302000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Appleton SD, Chretien ML, McLaughlin BE, Vreman HJ, Stevenson DK, Brien JF, Nakatsu K, Maurice DH, Marks GS. Selective inhibition of heme oxygenase, without inhibition of nitric oxide synthase or soluble guanylyl cyclase, by metalloporphyrins at low concentrations. Drug Metab Dispos. 1999;27:1214–1219. [PubMed] [Google Scholar]
- 31.Frisbee JC, Roman RJ, Murali Krishna U, Falck JR, Lombard JH. Altered mechanisms underlying hypoxic dilation of skeletal muscle resistance arteries of hypertensive versus normotensive Dahl rats. Microcirculation. 2001;8:115–127. [PubMed] [Google Scholar]
- 32.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 33.Lombard JH, Liu Y, Fredricks KT, Bizub DM, Roman RJ, Rusch NJ. Electrical and mechanical responses of rat middle cerebral arteries to reduced PO2 and prostacyclin. Am J Physiol. 1999;276:H509–H516. doi: 10.1152/ajpheart.1999.276.2.H509. [DOI] [PubMed] [Google Scholar]
- 34.Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- 35.Uluoglu C, Zengil H. Comparison of different de-endothelization procedures in the isolated rat thoracic aorta: a short communication. Res Commun Mol Pathol Pharmacol. 2003;113–114:289–297. [PubMed] [Google Scholar]
- 36.Willis AP, Leffler CW. Endothelial NO and prostanoid involvement in newborn and juvenile pig pial arteriolar vasomotor responses. Am J Physiol. 2001;281:H2366–H2377. doi: 10.1152/ajpheart.2001.281.6.H2366. [DOI] [PubMed] [Google Scholar]
- 37.Johnson FK, Teran FJ, Prieto-Carrasquero M, Johnson RA. Vascular effects of a heme oxygenase inhibitor are enhanced in the absence of nitric oxide. Am J Hypertens. 2002;15:1074–1080. doi: 10.1016/s0895-7061(02)03062-5. [DOI] [PubMed] [Google Scholar]
- 38.Kozma F, Johnson RA, Zhang F, Yu C, Tong X, Nasjletti A. Contribution of endogenous carbon monoxide to regulation of diameter in resistance vessels. Am J Physiol. 1999;276:R1087–R1094. doi: 10.1152/ajpregu.1999.276.4.R1087. [DOI] [PubMed] [Google Scholar]
- 39.Wang R, Wang Z, Wu L. Carbon monoxide-induced vasorelaxation and the underlying mechanisms. Br J Pharmacol. 1997;121:927–934. doi: 10.1038/sj.bjp.0701222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Botros FT, Navar LG. Interaction between endogenously produced carbon monoxide and nitric oxide in regulation of renal afferent arterioles. Am J Physiol. 2006;291:H2772–H2778. doi: 10.1152/ajpheart.00528.2006. [DOI] [PubMed] [Google Scholar]
- 41.Foresti R, Green CJ, Motterlini R. Nitric oxide and the heme oxygenase/carbon monoxide system: cooperation in the control of vascular function. In: Wang R, editor. Carbon Monoxide and Cardiovascular Functions. Boca Raton: CRC Press; 2002. pp. 111–124. [Google Scholar]
- 42.Leffler CW, Balabanova L, Sullivan CD, Wang X, Fedinec AL, Parfenova H. Regulation of CO production in cerebral microvessels of newborn pigs. Am J Physiol. 2003;285:H292–H297. doi: 10.1152/ajpheart.01059.2002. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez F, Zhang F, Dinocca S, Nasjletti A. Nitric oxide synthesis influences the renal vascular response to heme oxygenase inhibition. Am J Physiol. 2003;284:F1255–F1262. doi: 10.1152/ajprenal.00435.2002. [DOI] [PubMed] [Google Scholar]
- 44.Zhang F, Kaide J, Wei Y, Jiang H, Yu C, Balazy M, Abraham NG, Wang W, Nasjletti A. Carbon monoxide produced by isolated arterioles attenuates pressure-induced vasoconstriction. Am J Physiol. 2001;281:H350–H358. doi: 10.1152/ajpheart.2001.281.1.H350. [DOI] [PubMed] [Google Scholar]
- 45.Holt DC, Fedinec AL, Vaughn AN, Leffler CW. Age and species dependence of pial arteriolar responses to topical carbon monoxide in vivo. Exp Biol Med. 2007;232:1465–1469. doi: 10.3181/0705-BC-136. [DOI] [PMC free article] [PubMed] [Google Scholar]