Abstract
Sex determination in the American alligator depends on the incubation temperature experienced during a thermo-sensitive period (TSP), although sex determination can be ‘reversed’ by embryonic exposure to an estrogenic compound. Thus, temperature and estrogenic signals play essential roles during temperature-dependent sex determination (TSD). The genetic basis for TSD is poorly understood, although previous studies observed that many of the genes associated with genetic sex determination (GSD) are expressed in species with TSD. Heat shock proteins (HSPs), good candidates because of their temperature-sensitive expression, have not been examined in regard to TSD but HSPs have the ability to modify steroid receptor function. A number of HSP cDNAs (HSP27, DNAJ, HSP40, HSP47, HSP60, HSP70A, HSP70B, HSP70C, HSP75, HSP90α, HSP90β, and HSP108) as well as cold-inducible RNA binding protein (CIRBP) and HSP-binding protein (HSPBP) were cloned, and expression of their mRNA in the gonadal-adrenal-mesonephros complex (GAM) was investigated. Embryonic and neonatal GAMs exhibited mRNA for all of the HSPs examined during and after the TSP. One-month-old GAMs were separated into 3 portions (gonad, adrenal gland, and mesonephros), and sexual dimorphism in the mRNA expression of gonadal HSP27 (male > female), gonadal HSP70A (male < female), and adrenal HSP90α (male > female) was observed. These findings provide new insights on TSD and suggest that further studies examining the role of HSPs during gonadal development are needed.
Key Words: Alligator, Heat shock protein, mRNA expression, Quantitative real-time PCR, Temperature-dependent sex determination
In crocodilians, sex is determined by incubation temperature of the egg during a specific period of embryonic development, the thermo-sensitive period (TSP), rather than by critical sex-determining genes (e.g. SRY) as in mammals [Lang and Andrews, 1994; Morrish and Sinclair, 2002]. Egg incubations at 33.0–33.5°C produce males, whereas egg incubations at 30°C produce females in American alligator [Lang and Andrews, 1994; Milnes et al., 2002]. This system of sex determination is called temperature-dependent sex determination (TSD), and a TSP for TSD has been defined for the American alligator as stages 21–24 [Lang and Andrews, 1994]. Unlike in mammals, estrogens play a central role in the sex determination of fish, birds, crocodilians, and turtles [Crews, 1996; Devlin and Nagahama, 2002]. Indeed, treatment with estrogens or estrogenic compounds during the TSP produces sex reversal in crocodilians or turtles, thus females at male-producing temperatures [Bull et al., 1988; Lance and Bogart, 1994; Crain et al., 1997; Milnes et al., 2002]. Gonadal aromatase (CYP19A1) expression is elevated during and after TSP in alligators and turtles [Smith et al., 1995; Pieau et al., 1999; Gabriel et al., 2001; Murdock and Wibbels, 2003; Pieau and Dorizzi, 2004]. Estrogen receptors (ERs) exist prior to TSP in the genital ridge of the developing red-eared slider turtle and are expressed at a higher density in the future ovary rather than in the testis [Bergeron et al., 1998]. Thus, estrogens are involved in differentiation of an ovary; however, the precise mechanisms and reciprocal actions of estrogens and TSD in reptiles have not been clarified so far.
To date, the expression of several genes known to be important for sex determination in mammals, such as SOX9, anti-müllerian hormone (AMH), WT1, SF1, DAX1, and DMRT1, has been analyzed in alligator gonadal tissue during the TSP [Morrish and Sinclair, 2002]. Endogenous or exogenous estrogen would generate molecular and cellular action through ERs which belong to the nuclear receptor super-family and act as transcription factors. Like birds and mammals, alligators exhibit 2 forms of the nuclear ER, ERα (ESR1) and ERβ (ESR2), which show high sequence similarity to ERs found in other crocodilians, birds, and mammals [Katsu et al., 2004, 2006; Naidoo et al., 2008]. Extensive research on the molecular action of the steroid hormone receptors, including the ERs, indicates that these receptors are part of a transcription complex along with a number of chaperones and cofactors, including the heat shock proteins (HSPs) [Picard, 2006]. Moreover, HSPs were originally identified as the proteins whose expression is induced by heat and other stresses [Lindquist, 1986; Schlesinger, 1994; Ritossa, 1996]. The key factor with the thermo-sensitivity needed to initiate the suite of genetic factors during TSD has not been identified. Therefore, HSPs are interesting candidates to play important roles during the interaction of temperature and estrogen signaling that is known to exist during the TSP. It is also important to recognize that spatially ubiquitous gonadal gene expressions and hormonal signaling would not necessarily drive the morphologically correct differentiation of the gonad; that is, normal morphogenesis requires spatially defined gene expression, thus changes in gonadal morphogenesis, such as the development of basement membranes which would compartmentalize the developing gonad, should also be important in reptilian TSD. For example, germ cells are found in the cortex of undifferentiated gonads, but testis and ovary, respectively, will have germ cells in the medulla and cortex after TSP [Smith and Joss, 1993; Smith and Sinclair, 2004; Yao et al., 2004]. The distribution of germ cells should be one of the critical points for the morphological differentiation in the gonad.
The HSP genes are highly conserved in all eukaryotes and prokaryotes studied to date [Morimoto et al., 1990; Feder and Hofmann, 1999]. Based on sequence homology and the molecular weight of the proteins, the genes have been divided into families such as HSP110, HSP100, HSP90, HSP70, HSP60, HSP40, and the small heat shock proteins, a family of proteins with a molecular weight generally less than 30 kDa [Nover and Scharf, 1997; Gething, 1998; van den Ijssel et al., 1999]. These gene families consist of stress-inducible genes and constitutively expressed genes. In general, inducible genes are expressed at low levels under non-stress conditions, but their expression increases rapidly in response to various stressors. In contrast, basal levels of constitutive genes are high and show relatively little change in the response to stress. However, the inducible and constitutive expression of HSPs is changed during development and cellular differentiation [Craig et al., 1983; Rybczynski and Gilbert, 2000]. HSPs also are induced when a cell or organism undergoes various types of environmental stresses such as heat, cold, desiccation, and oxygen deprivation [Feder and Hofmann, 1999; Kregel, 2002]. The HSP families are grouped according to molecular size, and each family plays various roles in the cells. For example, the HSP90 family works as a chaperone protein for steroid hormone receptors [Pratt, 1997], whereas the HSP70 family is necessary for translocation and protein folding [Gething and Sambrook, 1992], and the HSP60 family is involved in protein stability and folding [Ostermann et al., 1989; Martin et al., 1992].
In the present study, we report the molecular cloning of HSP cDNAs encoding HSP27, DNAJ, HSP40, HSP47, HSP60, HSP70A, HSP70B, HSP70C, HSP75, HSP90α, HSP90β, and HSP108, as well as one cold-inducible RNA-binding protein (CIRBP), and one HSP-binding protein (HSPBP) from the American alligator. Using this sequence information, we built primers allowing us to perform RT-PCR or quantitative real-time PCR in whole GAMs (gonadal-adrenal-mesonephros complexes) or separated GAMs, so we could begin to identify key HSP activities during TSD.
Materials and Methods
Animals
All experiments in this study involving alligators were carried out under the guidelines specified by the Institutional Animal Care and Use Committee at the University of Florida. All fieldwork was performed under permits from the Florida Fish and Wildlife Conservation Commission and the U.S. Fish and Wildlife Service. Alligator eggs were collected from Lake Woodruff National Wildlife Refuge. At least one egg from the clutch was opened to determine embryonic stage based on criteria described by [Ferguson 1985]. At embryonic stages 19, 24, and 25, eggs incubated at a male-producing temperature (33.5°C) or at female-producing temperature (30.0°C) were opened and GAMs were dissected from the embryos. GAMs were also isolated from neonates (within 48 h after hatching) and one-month-old alligators of both sexes. One animal per each stage was used for RT-PCR analysis to indicate the absence or presence of HSPs in embryonic and neonatal GAM tissue. Embryonic and neonatal sexes were determined by the temperature at which the eggs were incubated and by gross anatomy. They were further confirmed by mRNA expression of AMH and CYP19A1 that are highly sexually dimorphic in the GAM of alligators. At one month of age, GAM tissue from 7 male and 13 female alligators was analyzed; these animals had a mean snout-vent length of 13.9 ± 0.20 cm for males and 13.9 ± 0.15 cm for females. One-month-old animals were sexed by histological analysis and confirmed with mRNA expression of AMH and CYP19A1. GAMs were either flash frozen, fixed in RNAlater (Ambion) or fixed in Bouin's fixative. Flash frozen GAMs were stored at −70°C, and GAMs fixed in RNAlater were stored at −20°C until analyzed.
Molecular Cloning of Heat Shock Proteins
Lambda ZAP II cDNA libraries were constructed from stage 25 embryos incubated at male and female-producing temperatures (Stratagene), and cDNAs were randomly sequenced from the 5′-terminal end. Twelve clones encoding the alligator homologs of HSPs, one clone encoding cold-inducible RNA binding protein (CIRBP), and another clone encoding HSP-binding protein (HSPBP) were identified from the cDNA library. In the case of a missing 5′-end of the cDNA it was amplified by 5′-rapid amplification of cDNA end (RACE) using a SMART RACE cDNA Amplification kit (BD Biosciences Clontech). The amplified 5′-end of the cDNA was sequenced and analyzed together with its 3′-side of the cDNA sequence.
Database and Sequence Analysis
All sequences generated were searched for similarity using BLASTN and BLASTP at web servers of the National Center of Biotechnology Information (http://blast.ncbi.nlm.nih.gov/Blast.cgi). All homologous amino acid sequences of Homo sapiens (human), Gallus gallus (chicken), Xenopus laevis (African clawed frog), Danio rerio (zebrafish), Drosophila melanogaster (fruit fly), and Tribolium castaneum (rust-red flour beetle) were used for phylogenetic analysis if the sequence had reference(s). The following sequences were used in the analysis of HSP70s: GenBank accession number were NP_001002012, NP_001006686, NP_001079632, NP_001080064, NP_001080068, NP_001081462, NP_001086039, NP_001091238, NP_001093532, NP_001103873, NP_001107061, NP_001120990, NP_001121147, NP_002146, NP_005336, NP_005337, NP_005338, NP_005518, NP_006588, NP_032327, NP_034608, NP_034609, NP_038586, NP_068814, NP_071705, NP_112442, NP_511132, NP_524063, NP_524339, NP_524356, NP_524474, NP_524798, NP_524927, NP_571472, NP_650209, NP_694881, NP_727563, NP_727564, NP_727565, NP_729940, NP_729941, NP_731651, NP_731716, NP_731987, NP_731988, NP_731989, NP_788663, NP_788679, NP_788680, NP_956908, NP_990334, NP_990822, NP_998223, XP_ 001341446, XP_001811933, XP_001814612, XP_001814746, XP_966611, XP_966842, XP_973521, XP_974442, NP_001006147, NP_001079627, NP_001080166, NP_004125, NP_034611, NP_523741, NP_958483, XP_975386.
GenBank accession numbers for the phylogenetic analysis of HSP90s were NP_001103255, NP_996842, NP_989620, NP_034610, NP_032328, NP_035761, NP_005339, NP_001017963, NP_031381, NP_001085598, NP_001086624, NP_001083114, NP_001084280, NP_571403, NP_001038538, NP_571385, NP_937853, NP_523899, NP_651601, NP_001094067, XP_971540.
Phylogenetic trees for HSP70s and HSP90s were estimated by a web program, Phylogeny.fr [Dereeper et al., 2008], which used the Muscle program for alignments, the Gblocks program for curation, and the PhyML program for phylogeny. The statistical confidence for each branch in the tree was evaluated by aLRT in the PhyML program. Phylogeny was edited on the MEGA4 program [Tamura et al., 2007].
RNA Isolation and RT-PCR of Embryonic and Hatchling GAMs
Total RNAs were isolated from GAM tissues using RNeasy (Qiagen). For RT-PCR, 2 μg of total RNA was reverse transcribed using SuperScript III transcriptase (Invitrogen) and oligo (dT) primers. Primer sets used for RT-PCR analyses are shown in table 1. Thirty cycles of amplification were carried out under the following conditions: denaturing at 94°C for 15 s, annealing at 57°C for 30 s, and extension at 72°C for 1 min. At completion, PCR fragments were run on 2% agarose gels. The gel was stained with SYBR Green I (Cambrex), and the gel image was analyzed using a Fluoro-Image Analyzer (FLA-3000G, Fuji Photo Film Co., Ltd.).
Table 1.
Primer sets for RT-PCR
| Gene | Sense (5′–3prime;) | Antisense (5prime;–3prime;) |
|---|---|---|
| EEF1A1 | AAC ATC GTT GTC ATC GGC CAC GTG G | TTT TGG CTG TAA GGT GGC TCA GTG G |
| HSP27 | TAC TTC CGC TTC ATG CCC AGC CAA G | TTG AGA AAC TCA GTG CAC GTG CTG G |
| DNAJ | GCC CTA TTG AGA ACT GTG CAG AGA C | GAC ATG GAC AAC TTT CTG GCT CAGC |
| HSP40 | GAG TGT CAG GGC CAT GGG GAG CGT ATC | TCC TGC CTT GCA GGC AGC AGC TTT TCC |
| HSP47 | CAG GAC TTT CTG CTT CCC CTA CCC CCC | CAA GCC CGG CTG CCT AGT AAG AGA CCC |
| HSP60 | GGG TGG CGC TGT ATT TGG AGA AGA GGG | AGG GAA GCA ACA CCT GCA GCA TCC ATC |
| HSP70A | AGT GTC TGC TGT GGA CAA GAG CAC TGG C | AAG TGC AAT GGT GTC CAC CCT CCC CTC |
| HSP70B | GTG TCT GCT GTG GAC AAG AGC ACT GGC | AAG TGC AAT GGT GTC CAC CCT CCC CTC |
| HSP70C | CTG AGC AGA AGT GGG CAG AAT TCA G | ACT AGG CTA GCT GCA AAA CTG AAC G |
| HSP75 | TAG CCA AGA CAA ATG AGG AGC GTG C | AGT TGG CAC TGT AAG CTA AGC TGA C |
| HSP90α | CTG GTA CTC TGT CTG CAT TCC CTC | TAG GGT TGA ACT GCA CAT GCA GAG |
| HSP90β | GTG TGG GAA AGG TTT TCC AGC TCC | ATA CAA CAT CCT ACC CCA GGG AGG |
| HSP108 | AGT CTC CGT GTG CGC TTG TGG CTA GCC | CTG CCC ATA ATC CCA CAC CAC CCC CTC |
| CIRBP | TGG AGA CAG AGG CTA TGG TGG AAG C | TAC CCA GGC TGC ATT CCT ACC TTG C |
| HSPBP | ATC ATC AGT GGG TGC ACT TGC TTT C | TGC ACT GTT CCA AGA ATA CCC ATG C |
| CYP17A1 | GAG CAC GTG GAC TTT GCA ACA CAA CTG | TAG GCC TCT TCC TTT CTG TGT GTAC |
| AMH | CAG CCA CTA CAA GTT CAT TGC C | TGC GAT CCA TAC AGG TTC AAG A |
Histological Analysis of One-Month-Old GAMs
After fixation of GAM tissues in Bouin's fixative, they were dehydrated in a series of graded alcohols (70%, 95%, and 100%), cleared in Citrisol (Fisher Scientific), and embedded in paraffin (Tissue prep 2: Fisher Scientific). GAMs were cross-sectioned at 6 μm and double stained with Alcian Blue and Periodic Acid Schiff (AB-PAS) according to standard methods [Bancroft and Gamble, 2008].
RNA Isolation and Quantitative Real-Time PCR of One-Month-Old GAMs
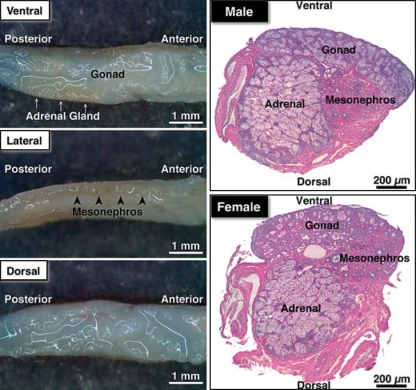
GAMs fixed in RNAlater were carefully partitioned into 3 pieces (gonad, adrenal gland, and mesonephros) under a stereomicroscope. The mesonephros, which had a pigmented line on the lateral surface of the GAM (fig. 1), was isolated first. Second, the gonadal tissue on the ventral surface was isolated, and then the adrenal gland, which was creamy in color, was isolated from the connective tissue of the dorsal side.
Fig. 1.
Anatomy and histology of the gonad-adrenal-mesonephros complex (GAM) isolated from 1-month-old alligators after stabilization or fixation in RNAlater or Bouin's fixative. The gonad was found on the ventral side and was approximately one fourth of the thickness of the GAM, whereas the adrenal gland was creamy in color lying underneath the gonad and the mesonephros was pigmented by yellow ocher on the lateral surface. As seen in the histology in the right panel, it was impossible to cleanly and completely separate 1-month-old GAM into 3 portions of the 3 tissue types; however, the GAMs were carefully dissected and separated into unique portions largely composed of gonad, adrenal, and mesonephros tissue.
Total RNAs were isolated from tissues using a total RNA isolation system (Promega) with a treatment of DNaseI. RNA quantity and quality was verified by measuring its optical density and then running it on an agarose gel. For quantitative real-time PCR (Q-PCR), 1 μg of total RNA was reverse-transcribed in 20 μl of a reaction mix using an iScript cDNA synthesis kit (Bio-Rad). Before starting the analysis, the cDNA was diluted 20-fold with TE buffer, and 1/25 volume of diluted cDNA was used in the Q-PCR analyses as a template. SYBR green-based Q-PCR was performed on a MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad) with AmpliTaq Gold (Applied Biosystems) based homebrew SYBR green reaction mix and the primers for Q-PCR (table 2).
Table 2.
Primers for quantitative real-time PCR of HSPs in American alligator gonadal, adrenal, and mesonephric tissue
| Gene | Sense (5′–3′) | Antisense (5′–3′) | Annealing C) |
|---|---|---|---|
| EEF1A1 | CGT TCT GGT AAG AAG CTG GA | TGA CAC CAA CAG CAA CAG TC | 63.6 |
| HSP27 | AGA CCA AGG ATG GCA TTG TA | GCA AGG TGT ATT TCC TGG TG | 63.6 |
| DNAJ | AGT TTG AGT TCC CAG CCT CT | TTG GCA GGG TTT GAT GTA TT | 62.0 |
| HSP40 | CTG GCT CTG CAA AAG AAT GT | TCC TGG TCC TAT CTG GTG AA | 62.0 |
| HSP47 | AGG ACT TTC TGC TTC CCC TA | CTT GCT GTG ATG CTG AAT TG | 63.6 |
| HSP60 | TGA TGC TGT CAT TGC TGA GT | ATG CCT TCA ATG ATT TCC AA | 62.0 |
| HSP70A | AGA AGA GCT GCA ATC ATG TCG G | GTT GGC AAT GAT CTC CAC CTT G | 62.9 |
| HSP70B | CGC AAT ACC ACA ATC CCC A | CAT GGC TCT CTC CCC TTC ATA C | 65.0 |
| HSP70C | ATT GGT GCT GCC ATT CAA GG | CAC CTC CCA GTG TCT CAA TTC C | 66.5 |
| HSP75 | TGC AGA ACA CCT GAC AGA AA | CTA GGC GTG GAG TCA CCT TA | 63.6 |
| HSP90_ | GTG AGA GAA AAT GCC ACT GAG G | TGC AGC CAG CTA ATA CAG ACA A | 66.5 |
| HSP90_ | ACA GGC ATT GGG ATG ACT AA | TTC TCA GCC ACC AGA TAA GC | 62.9 |
| HSP108 | GAA GGC ACT GAA GGA CAA GA | CTT TGC CAG TCT GGT ATG CT | 66.2 |
| CIRBP | TGA GGG AAA GCT TTT TGT TG | CTG AAC CCC CTC TGT ATC CT | 63.6 |
| HSPBP | TTA AGG ATG AAG CGG ACA AG | TGG TTC AAT CAC TCC CTC AT | 62.0 |
| CYP17A1 | CAG CCA GTT GTG GAC TTG ATC A | TTG TCC CCT TTT TCA CAG GAT AG | 62.0 |
| AMH | AGC AGC TCA ACC TCT CTG AGG A | TAG CAG AAA GCC AGA AGG TGC | 68.1 |
The expression value from each sample was calculated by comparison to standard samples which contained a known concentration of plasmid (copies/μl) ligated for each target. All mRNA expressions were weighted by expression of EEF1A1 mRNA, which was an internal control gene in this study; expressions are presented as weighted mean ± SE. Two-way ANOVA followed by Tukey's HSD test were used to evaluate differences among tissues and sex and were calculated using JMP version 7.0.2 (SAS Institute Inc.).
Results
Anatomy and Histology of GAMs from One-Month-Old Alligators
After stabilization or fixation of one-month-old GAMs in RNAlater or Bouin's fixative, the appearance of the GAM was examined (fig. 1). Gonadal tissue was found on the ventral side and was a white structure approximately one fourth of the thickness of the total GAM, whereas the adrenal gland was a creamy, off-white colored tissue lying underneath the gonad. The mesonephros was pigmented by yellow ocher and was found on the lateral surface (fig. 1). As seen in the histology on the right panel of figure 1, it was impossible to cleanly and completely separate one-month-old GAM into 3 portions. The GAMs isolated from one-month-old alligators were, however, carefully dissected under a stereomicroscope and separated into 3 unique portions of tissue largely representative of gonad, adrenal gland, and mesonephros, from which total RNAs were separately isolated, reverse-transcribed, and analyzed in Q-PCR as described in the Methods section.
Molecular Cloning of Alligator HSPs
Fourteen clones encoding alligator homologs of HSPs (HSP27, DNAJ, HSP40, HSP47, HSP60, HSP70A, HSP70B, HSP70C, HSP75, HSP90α, HSP90β, and HSP108), cold-inducible RNA-binding protein (CIRBP) and HSP-binding protein (HSPBP) were identified following BLAST searches, and their full-length sequences were obtained using the 5′-RACE technique. GenBank Accession numbers were obtained for these American alligator transcripts: HSP27 (AB306274), DNAJ (AB306275), HSP40 (AB306276), HSP47 (AB306277), HSP60 (AB306278), HSP70A (AB306279), HSP70B (AB306280), HSP70C (AB306281), HSP75 (AB306282), HSP90α (AB306283), HSP90β (AB306284), HSP108 (AB306285), CIRBP (AB306286), and HSPBP (AB306287). The cDNA and predicted amino acid sequences of each clone can be found in the GenBank database.
The sequence identity of the majority of the clones, except for the HSP70s and the HSP90s, were analyzed against orthologs from several other species (table 3). Of the clones examined, all were more similar in sequence to chicken than human, Xenopus, or zebrafish, except for CIRBP. Interestingly, HSP40 and HSP60 were highly conserved among alligator, chicken, and human, with percent similarities over 90% in their predicted amino acid sequences. Our data indicate that the alligator CIRBP reported here was more similar to human CIRBP than chicken or Xenopus CIRBP with a similarity to human CIRBP of 91% in amino acid sequence (table 3). Alligator CIRBPwas very similar to dog CIRBP isoform 3, but not isoform 2 (Canis familiaris isoform 2: XM_863507, isoform 3: XM_868601). The overall identities of alligator CIRBP with isoform 2 and isoform 3 of dog CIRBP were 88 and 97%, respectively. Likewise, dog CIRBP isoform 2 revealed a high similarity to chicken CIRBP (94%). These results indicate that alligator CIRBP is likely the ortholog of dog CIRBP isoform 3 and suggests that the alligator could possess another type of CIRBP that is similar to the CIRBP isoform 2 of the dog.
Table 3.
Percent similarities in amino acid sequences between American alligator HSPs and those of other vertebrate species
| Human | Chicken | Xenopus | Zebrafish | |
|---|---|---|---|---|
| HSP27 | 69 | 71 | 60 | 62 |
| DNAJ | 73 | 76 | 64 | 57 |
| HSP40 | 93 | 93 | 85 | 71 |
| HSP47 | 75 | 91 | 79 | 5 |
| HSP60 | 91 | 94 | 87 | 86 |
| HSP75 | 82 | 86 | – | – |
| HSP108 | 89 | 92 | 85 | 84 |
| CIRBP | 91 | 81 | 83 | 61 |
| HSPBP | 81 | 88 | 73 | 69 |
GenBank accession numbers.
HSP27: human: NM_001540, chicken: NM_205290, Xenopus: BC078509, zebrafish: NM_001008615; DNAJ: human: NM_015190, chicken: XM_421524, Xenopus: BC090203, zebrafish: NM_001002433; HSP40: human: XM_531970, chicken: NM_001012945, Xeno-pus: BC044329, zebrafish: NM_199662; HSP47: human: BC036298, chicken: X57157, Xenopus: BC044329, zebrafish: BC071301; HSP60: human: BC002676, chicken: NM_001012916, Xeno-pus: BC072058, zebrafish: NM_18133; HSP75: human: AF154108, chicken: NM_001006175; HSP108: human: NM_003299, chicken: AF387865, Xenopus: AY187545, zebrafish: NM_198210; CIRBP: human: NM_001280, chicken: NM_001031347, Xeno-pus: AF278702, zebrafish: NM_200017; HSPBP: human: NM_003932, chicken: NM_001030757, Xeno-pus: BC077246, zebrafish: NM_199769.
Alligator HSP70s
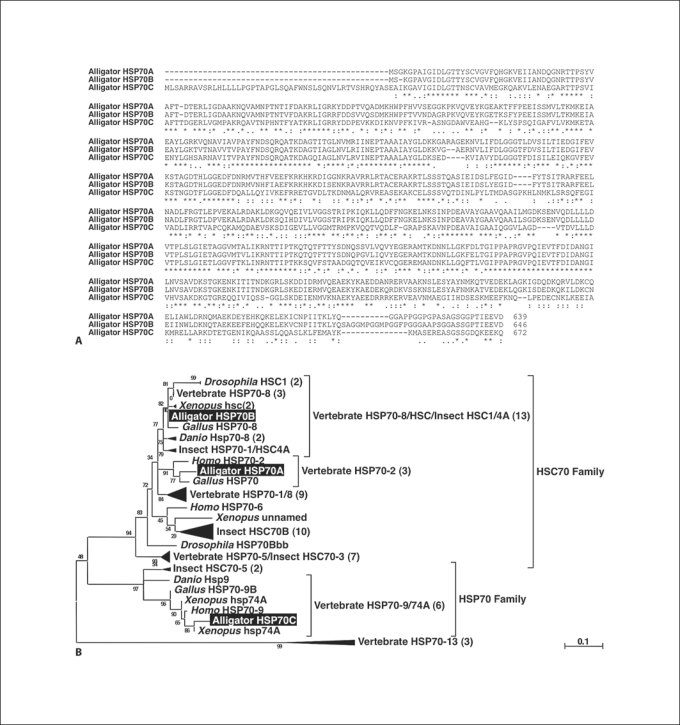
We isolated 3 HSP70 homologs, designated HSP70A, 70B, and 70C. The cDNA encoding the alligator HSP70A homolog consisted of 2,317 bp with an open reading frame (ORF) encoding a 639-amino-acid protein whereas HSP70B has 2,269 bp encoding a protein of 646 amino acids, and HSP70C has 2,788 bp encoding a protein of 672 amino acids. Analysis of sequence similarity indicated that HSP70A was more similar to HSP70B than HSP70C (70A vs. 70B: 86%, 70A vs. 70C: 48%, 70B vs. 70C: 49%; fig. 2A), which was expected as HSP70A and 70B have been reported to belong to the same multigene family. Based on BLAST searches and phylogenetic analysis we found that HSP70A, 70B, and 70C were similar to vertebrate HSP70 protein 2, HSP70 protein 8, and HSP70 protein 9B, respectively (fig. 2B).
Fig. 2.
Sequence comparison of the predicted amino acid sequences for HSP70s of the American alligator. A Alignment of the predicted amino acid sequence of HSP70A, 70B, and 70C using ClustalW. Identical amino acids are marked by asterisks under the sequence. Colons and periods indicate the fully conserved residues with ‘strong’ groups and ‘weaker’ groups, respectively. B Phylogenetic tree of selective amino acid sequences of the vertebrate HSP70 family (see Materials and Methods for software used to generate this tree). The statistical confidence for each branch in the tree was evaluated by the aLRT in the PhyML program and is indicated by the small number on each branch. A scale bar indicates 0.1 expected amino acid substitutions per site.
Alligator HSP90s
The cDNA encoding the alligator HSP90α homolog consisted of 2,957 bp with an ORF that encoded a 728-amino-acid protein, whereas the cDNA encoding the alligator HSP90β homolog had 2,748 bp that encoded a 729-amino-acid protein. In comparison of the amino acid sequences with those of HSP90s from other vertebrate species, alligator HSP90α was more similar to the α-type of HSP90 (93.6%: mouse, 99.0%: chicken, 91.6%: frog) than the β-type (84.5%: mouse, 83.5%: chicken, 83.5%: frog) (table 4). However, the sequence for alligator HSP90α was more closely related to Hsp90β of the zebrafish than the Hsp90α form (Hsp90α: 82.8%, Hsp90β: 84.1%).
Table 4.
Comparison of American alligator HSP90α and HSP90β with known HSP90s from other vertebrate species
| Alligator |
Mouse |
Chicken |
Xenopus |
Zebrafish |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 90α | 90β | 90α | 90β | 90α | 90β | 90α | 90β | 90α | 90β | |
| HSP90α | 100 | 82 | 64 | 85 | 99 | 83 | 92 | 84 | 83 | 84 |
| HSP90β | 82 | 100 | 82 | 94 | 81 | 92 | 81 | 89 | 77 | 87 |
The amino acid sequence of alligator HSP90α was very similar to alligator HSP90β (81.8%) (fig. 3A; table 4). The predicted amino acid sequences of alligator HSP90 proteins were similar to each other (81.8%), and the nucleic acid sequences of the coding regions also showed high homology (73.4%, data not shown). However, the sequences of the 3′-untranslated region (3′-UTR) were quite different (fig. 3B; 21.7%). These results were similar to data we obtained following an examination of chicken HSP90 sequence analyses. The sequences of the predicted amino acids and nucleic acids for chicken HSP90α and HSP90β were 82.8 and 73.9% similar to each other, respectively. However, like the alligator, the sequence similarity of the 2 HSP90s of the chicken 3′-UTR was only 23.5%.
Fig. 3.
Sequence comparison of the predicted amino acids and nucleic acids for the HSP90S of the American alligator. A Alignment of the predicted amino acid sequence of American alligator HSP90α and HSP90β by ClustalW. Identical amino acids are marked by asterisks under the sequence. Colons and periods indicate the fully conserved residues with ‘strong’ groups and ‘weaker’ groups, respectively. B Alignment of nucleotide sequences of the 3′-untranslated region of American alligator HSP90α and HSP90β. Identical nucleic acids are marked by asterisks under the sequence. TAA in the black box indicates the stop codon. C Phylogenetic tree of the amino acid sequences for the HSP90 family, estimated by Phylogeny.fr (see Materials and Methods for software used to generate this tree). The statistical confidence for each branch in the tree was evaluated by the aLRT in the PhyML program and is indicated by the small number on each branch. A scale bar indicates 0.1 expected amino acid substitutions per site.
HSP90 genes have been analyzed in several vertebrate groups, including mammals, avian, amphibian, and fishes. Further, fly (X03810), nematode (AF461150 for Hetrodera glycines, AJ005784 for Brugia pahangi, Q18688 for Caenorhabditis elegans), scallop (AY362761), and Schistosoma (AY815927) HSP90 genes have also been cloned and their complete sequences determined. Phylogenetic analyses of HSP90 amino acid sequences were generally consistent with existing phylogenetic hypotheses regarding vertebrate and invertebrate relationships, and 2 types of HSP90s were not observed in invertebrates. To better understand the position of alligator HSP90α and β proteins and their reciprocal relationship, a phylogeny was constructed using numerous HSP90 proteins from various vertebrate and invertebrate species (fig. 3C). The results show that alligator HSP90α and HSP90β are similar to vertebrate HSP90α and vertebrate HSP90β, respectively, as would be predicted (fig. 3C).
Analyses of HSP Expression in Embryonic, Neonatal, and One-Month-Old GAMs
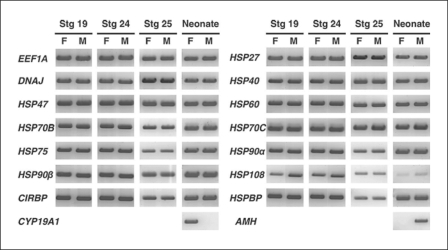
We hypothesized that temperature-induced sex determination in the alligator could involve temperature-regulated HSP gene expression. This hypothesis, in part, recognizes the role of HSPs as steroid receptor chaperones and cofactors. Steroids, and thus their receptors, appear to play an important role during TSD in the American alligator and other reptiles with this mode of sex determination. To test this hypothesis, we examined the existence of mRNAs of various HSPs. Total RNA was isolated from male and female embryonic GAMs at embryonic stages 19, 24, and 25 and from neonates. All HSPs we cloned were expressed in embryonic and neonatal GAMs isolated from both sexes (fig. 4), although the expression of HSP70A was detectable, but too low to show a band on the gel (data not shown). In female neonatal GAM CYP19A1 mRNA was obviously expressed at a high level, whereas we detected little or no CYP19A1 in male GAM tissue. In contrast, anti-müllerian hormone (AMH) expression was robust in male neonatal GAM but not in female GAM tissue (fig. 4). The expression of these 2 genes, CYP19A1 and AMH, provided robust positive control genes for ovarian and testicular differentiation, respectively.
Fig. 4.
Expressions of various HSP mRNAs by RT-PCR in GAMs from the American alligator. The mRNA expression of alligator EEF1A1, HSP27, DNAJ, HSP40, HSP47, HSP60, HSP70B, HSP70C, HSP75, HSP90α, HSP90β, HSP108, CIRBP, and HSPBP were detected in GAMs at embryonic stages 19, 24, 25, and in neonates.
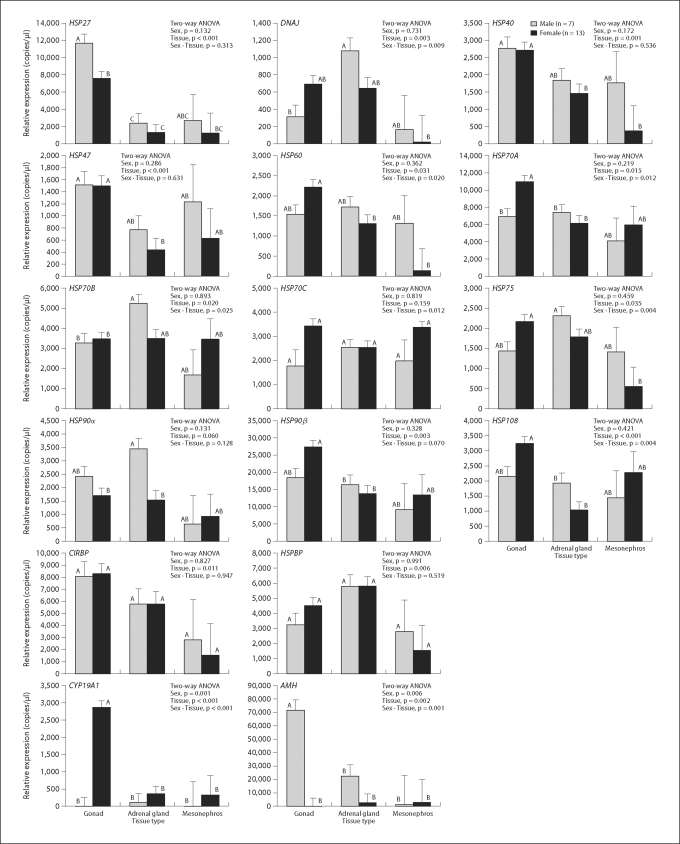
AMH or CYP19A1 mRNA in one-month-old alligator tissues revealed significant effects of both sex and tissue differences on their expressions levels (fig. 5; table 5). Tissue differences within the GAM were shown for most of the HSP mRNAs examined except for HSP70C and 90α (fig. 5; table 5). The 2 primary factors in this study, sex and tissue type, affected the expression of HSP60, HSP70A, HSP70B, HSP70C, HSP75 and HSP108 mRNA (fig. 5; table 5). Sexually dimorphic patterns were observed in mRNA expression of gonadal HSP27, gonadal HSP70A, and adrenal HSP90α (fig. 5; table 5).
Fig. 5.
Quantitative expression of HSP mRNAs in gonad, adrenal gland, and mesonephros isolated from one-month-old alligators. Each expression value was calculated by comparing individual HSP mRNA expression to standard samples which contained a known concentration of plasmid (copies/μl) ligated for each target cDNA. All mRNA expressions were weighted by expression of EEF1A1 mRNA, which was an internal control gene in this study, and are shown as weighted mean ± SE. Two-way ANOVA followed by Tukey's HSD test were calculated, and levels with differing superscripts are significantly different (p < 0.05). Grey columns indicate male animals and black columns females. For each gene the left 2 columns show the results of the gonad tissue, the middle columns the adrenal gland, and the right columns the mesonephros results.
Table 5.
Statistic summary of quantitative real time-PCR on 3 portions of the gonadal-adrenal-mesonephros complexes (GAMs) obtained from one-month old American alligators
| Gene | Two-way ANOVA |
Sexual dimorphism |
||||
|---|---|---|---|---|---|---|
| Sex | Tissue | Sex∗ tissue | Gonad | Adrenal | Meso-nephros | |
| HSP27 | ns | ∗∗ | ns | m > f | ns | ns |
| DNAJ | ns | ∗∗ | ∗∗ | ns | ns | ns |
| HSP40 | ns | ∗∗ | ns | ns | ns | ns |
| HSP47 | ns | ∗∗ | ns | ns | ns | ns |
| HSP60 | ns | ∗ | ∗ | ns | ns | ns |
| HSP70A | ns | ∗ | ∗ | m < f | ns | ns |
| HSP70B | ns | ∗ | ∗ | ns | ns | ns |
| HSP70C | ns | ns | ∗∗ | ns | ns | ns |
| HSP75 | ns | ∗∗ | ∗ | ns | ns | ns |
| HSP90α | ns | ns | ns | ns | m > f | ns |
| HSP90β | ns | ∗∗ | ns | ns | ns | ns |
| HSP108 | ns | ∗∗ | ∗∗ | ns | ns | ns |
| CIRBP | ns | ∗ | ns | ns | ns | ns |
| HSPBP | ns | ∗∗ | ns | ns | ns | ns |
| CYP19A1 | ∗∗ | ∗∗ | ∗∗ | m < f | ns | ns |
| AMH | ∗∗ | ∗∗ | ∗∗ | m > f | ns | ns |
p < 0.01
p < 0.05. ns = Not significant.
Discussion
The TSP for TSD is during embryonic stages 21–24 in the American alligator [Ferguson, 1985; Lang and Andrews, 1994]. Although we have not quantified expression levels of HSPs in the GAM during the TSP, the expression of mRNA for various HSPs was verified throughout this period in embryonic GAMs of both sexes.
Heat shock proteins provide a variety of functions in vertebrates, with various classes of HSPs exhibiting different actions [Picard, 2006]. Most of the data collected to date suggest that the steroid hormone receptors are associated with HSP90s in the absence of their specific ligand [Picard, 2006]. The receptor-HSP90 complex is associated with a number of chaperones and cofactors that generate different complexes; i.e., no single form is used to construct the transcription machinery needed for gene expression. Many of the studies of HSP action are based on in vitro biochemical studies of yeast extracts, yet all suggest that 5 components are necessary for the ATP-driven maturation of a steroid receptor-HSP90 complex, those being P23, HSP40, HSP70, HOP, and HSP90 [for review, see Picard, 2006]. Despite observations of HSPs in loggerhead turtles [Harry et al., 1990], no other investigation of the molecular biology of HSPs in reptilian TSD has been reported. Harry et al. [1990] found 42K and 46K proteins in embryonic urinogenital tissue of females, but the proteins were not identified. Our data indicate that all of the components of such a functional complex are present in the developing GAM of the American alligator at TSP. Enticingly, HSP27, HSP70A, and HSP90α revealed sexual dimorphism in mRNA expressions in gonadal or adrenal tissue isolated from one-month-old alligators. These data suggest that an in-depth quantitative analysis of HSP expression and action in embryonic GAM during the TSP is needed.
For example, HSP27 is inducible by environmental stress and developmental changes in mammals [Garrido et al., 2006]. It is involved in the resistance to stress, the organization of cytoplasmic actin, and is translocated from the cytoplasm to the nucleus upon stress induction in mammals [Garrido et al., 2006]. We observed that in one-month-old gonadal tissue from alligators HSP27 mRNA was significantly elevated in testicular tissue when compared to ovarian tissue of the same age. HSP27 can function as a suppressor of estrogen response element (ERE)-mediated transcription by competing with the estrogen receptor (ER) [Chen et al., 2004, 2008]; i.e., estrogenic signaling requires that the ligand (under normal conditions an endogenous estrogen) binds the ER which then dimerizes and translocates to the DNA where it binds to an ERE in the promoter of an estrogen-inducible gene. This process can be modified by various intracellular proteins as they can either complex with the ER machinery to augment or inhibit ERE binding or interact directly with the ERE thus altering estrogenic signaling. HSP27 is capable of suppressing ERE-mediated transcription at the ERE by acting as an intracellular estradiol-binding protein in mammals [Chen et al., 2004, 2008].
The HSP70 family can be divided into 2 subfamilies, a cognate or constitutive type (HSC70) and an inducible form (HSP70). Both of these subfamilies encode proteins that play key roles in the cell as molecular chaperones [Gething and Sambrook, 1992]. In mammalian cells, HSC70 remains unchanged or slightly upregulated upon exposure to environmental and pathological stressors. In contrast, HSP70 is highly induced from low levels, with transcriptional control mediated via heat shock factor 1 that binds in a trimeric form to an HSP70 promoter [Westwood et al., 1991; Kiang and Tsokos, 1998]. The constitutive HSC70 genes are slightly larger than the HSP70 genes, and the carboxyl region of these genes is highly heterogeneous as HSC70 displays a series of unique repeat GGXP motifs and a signature nonapeptide SGPTIEEVD at the N-terminus [Ali et al., 2003; Deane and Woo, 2005]. In the American alligator, HSP70A has 1 GGXP motif and a GGPTIEEVD region, whereas HSP70B has 2 GGXP motifs and a SGPTIEEV region. Alligator HSP70C has neither the GGXP motif nor the N-terminal SGPTIEEVD sequence. These results suggest that alligator HSP70A and 70B belong to the HSC70 family, whereas alligator HSP70C belongs to the HSP70 family. Further characterization of alligator HSP70s should be done using in vivo heat shock or stress experiments to determine whether these HSPs display similar constitutive or inducible patterns as seen in mammals. Our results appear to suggest that a drastic change in HSP70A expression occurs between neonatal and one-month-old time periods, but it is important to note that different primers were used for RT-PCR and Q-PCR as shown in tables 1 and 2. Thus, these results are not directly comparable with each other, and further analyses will be required to understand the potential details of such a change.
HSP70/HSC70 act in various ways including enhancing vitamin D-mediated transcription and its metabolism by working as an intracellular vitamin-D-binding protein [Gacad et al., 1997; Wu et al., 2002]. Vitamin D is an important factor in the synthesis of estrogen in both male and female gonads, as it induces CYP19A1 expression through the vitamin D receptor in mice [Kinuta et al., 2000; Bouillon et al., 2008]. In this study, we observed that neonatal ovarian tissue expressed significantly higher HSP70A and CYP19A1 mRNA levels when compared to testicular tissue of the same age. The enzyme CYP19A1 generates endogenous estrogens, thus being responsible for the transformation of androstenedione or testosterone into estrone or estradiol-17β, respectively. Although it is unlikely that HSP70A is the primary regulatory agent behind the very large difference in CYP19A1 expression when ovarian and testicular tissue is compared, it is possible that this HSP could augment the observed sexual dimorphism by maintaining differentiation of an ovary versus a testis by helping in the upregulation of CYP19A1 expression via vitamin D signaling.
HSP90 proteins are well-conserved molecular chaperones that are involved in signal transduction, protein folding, protein degradation, and morphologic evolution [Chen et al., 2005]. They usually associate with other co-chaperones and play important roles in folding newly synthesized proteins or stabilizing and refolding denatured proteins after stress [Chen et al., 2005]. There are 2 types of HSP90 proteins: HSP90α is an inducible form and HSP90β is a constitutive form in mammals [Chen et al., 2005]. Generally, HSP90 maintains the steroid hormone receptor in an inactive state but keeps it ready to bind its endogenous hormone [Pratt and Toft, 1997]. On the other hand, one isoform of HSP90, HSP90α, has been described to be extracellularly located where it interacts with the matrix metalloproteinase 2 (MMP2) in mammalian cancer cells [Eustace and Jay, 2004; Eustace et al., 2004]. Extracellular HSP90α enhances the invasiveness of cancer cells via regulating MMP2 activities [Eustace et al., 2004]. The degradation of the extracellular matrix by MMPs enhances cell migration that enables carcinoma formation [Nyberg et al., 2006]. The secretion of HSP90α and activation of MMPs does not occur only in cancer cells but also in normal tissues and cells. Prior to the TSP in turtles and crocodilians, when primordial germ cells migrate into the gonad, the germ cells are found at the cortex of undifferentiated gonads [Smith and Joss, 1993; Smith and Sinclair, 2004; Yao et al., 2004]. However, after the TSP and morphogenesis of the testis or ovary in turtles and crocodilians, germ cells lie inside of sex cords (medulla) in males or are outside of sex cords (cortex) in females [Smith and Joss, 1993; Smith and Sinclair, 2004; Yao et al., 2004; Yao and Capel, 2005]. Hence, the location of germ cells changes with gonadal differentiation and potentially is very important for sex determination itself. In our study, male adrenal glands in one-month-old alligators revealed higher expression of HSP90α than adrenals obtained from females. We hypothesize that this could lead to more secretion of HSP90α in the region of the male adrenal gland and the adjacent gonad in male GAMs when compared to females. If this elevated extracellular HSP90α of adrenal origin enhanced MMP2 in the male gonad, it could alter the basement membrane of the sex cords in male gonads providing access for the primordial germ cells. As the result of expressing more adrenal HSP90α in the male, germ cells could migrate into the sex cord through the basement membrane. Although hypothetical, this proposal is testable and helps provide one potential mechanism for the restructuring of the bipotential gonad that occurs during the TSP.
Dax1-deficient mice showed less or weaker basement membranes of seminiferous tubules in testis, whereas the females had multi-oocyte follicles in the ovary [Yu et al., 1998; Jeffs et al., 2001]. These data suggest that one of the functions of DAX1 during sex determination is via the regulation of the structural proteins in the basement membrane. Indeed, the structural protein laminin and its receptor (integrin) play important roles in the migration of primordial germ cells in Drosophila and mice [Jaglarz and Howard, 1995; Anderson et al., 1999]. Structural changes in the extracellular matrix are necessary for cell migration during tissue remodeling, differentiation, and also the TSD. During differentiation, MMP2 could induce cell migration by cleavage of laminin, which is a principal component of the basement membrane [Giannelli et al., 1997]. Additionally, it is well known that the migration of myoid cells into the gonad from the mesonephros is essential for testicular morphogenesis in mammals [Martineau et al., 1997; Tilmann and Capel, 1999; Ross et al., 2003]. Although Yao et al. [2004] could not detect male-specific mesonephric cell migration in the red-eared slider turtle, the structure of the sex cords and the migration of germ cells and/or mesonephric cells is likely important during sex determination in all vertebrates.
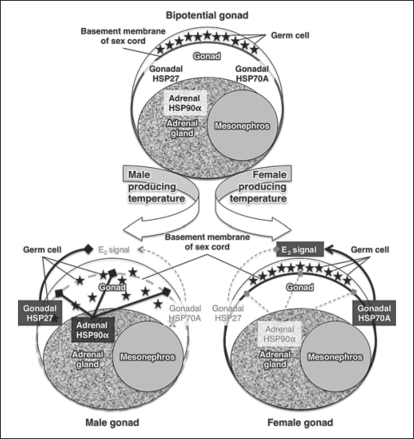
In conclusion, our finding that sexual dimorphism exists in mRNA expression of gonadal HSP27, gonadal HSP70A, and adrenal HSP90α suggests that further study is warranted to determine if such factors could be involved in TSD. Like all vertebrates, the alligator gonad is a bipotential gonad prior to sex determination, and the germ cells lie in the cortex. We have highlighted 2 important factors associated with temperature-dependent sex determination, those being (a) estrogen signaling (E2 signal) which is important in the formation of the ovary in most non-mammalian vertebrate species studied to date and (b) the distribution and movement of the germ cells and mesonephric cells in the developing gonad. We observed sexually dimorphic mRNA expression of gonadal HSP27 (male > female), gonadal HSP70A (male < female), and adrenal HSP90α (male > female) in one-month-old alligator tissues. Using these data, we have proposed a hypothesis that needs to be examined in detail in the future (fig. 6). Our hypothesis suggests that gonadal HSP27 can reduce the E2 signal directly at trans-activation, whereas gonadal HSP70A could induce E2 signaling by stimulating vitamin D signaling that leads to the induction of augmented CYP19A1 expression. By working as antagonists, HSP27 and HSP70A could modify E2 signaling which appears to play such a central role in driving gonadal differentiation in alligators (fig. 6). Further, sex determination is not gene expression alone but leads to dramatic changes in gonadal morphology including the location of the germ cells. We hypothesize that adrenal HSP90α is secreted into the extracellular matrix of the adrenal gland and the adjacent gonad altering the basement membrane of the sex cords via activation of the matrix metalloproteinase and thus facilitating the movement of the germ cells and mesonephric cells into the sex cords of the developing testis (fig. 6). Our current findings provide several new observations related to sexually dimorphic gene expression in the alligator gonad and provide insights into TSD, while suggesting a new, albeit simplistic, integrated theory of gene expression, hormonal regulation, and morphological alterations. Future studies must begin to integrate environmental as well as endocrine signaling into a theory that can explain the regulation of the gene networks responsible for the remarkable morphological and physiological transformation associated with gonadal determination and differentiation.
Fig. 6.
A hypothesis of the potential contribution of HSP27, 70A, and 90α during temperature-dependent sex determination in the American alligator. Alligator gonads are bipotential before sex determination, and germ cells are located in the cortex just below the surface epithelium. Our hypothesis suggests that gonadal HSP27 can reduce the E2 signal directly at transactivation, whereas gonadal HSP70A could induce E2 signaling by stimulating vitamin D signaling that leads to the induction of aromatase expression. By working as antagonists, HSP27 and HSP70A could modify E2 signaling which appears to play such a central role in driving gonadal differentiation in alligators. Further, sex determination is not gene expression alone but leads to dramatic changes in gonadal morphology including the location of the germ cells. We hypothesize that adrenal HSP90α is secreted into the extracellular matrix of the adrenal gland and the adjacent gonad altering the basement membrane of the sex cords via activation of the matrix metalloproteinase and thus facilitating the movement of the germ cells into the sex cords of the developing testis. An induction or a reduction is indicated by arrow end (→) or blunt end (→), respectively; an induced signal or a reduced signal is indicated by inverted bold black or narrow gray typeface, respectively.
Acknowledgements
We thank Ms. Rie Ichikawa and Ms. Megumi Hinago for their excellent technical assistance in cDNA cloning. The authors are grateful to the members of the Iguchi and Guillette laboratories for helpful discussions and comments. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (Y.K., Y.O., and T.I.), grants from the Ministry of Environment, Japan (Y.K. and T.I.), and grants from UF Opportunity Fund, Howard Hughes Medical Institute Professors program and U.S. NIH (NIEHS R21 ES014053-01) to L.J.G.
Footnotes
S.K. and Y.K. contributed equally to this work.
References
- Ali KS, Dorgai L, Abraham M, Hermesz E. Tissue- and stressor-specific differential expression of two hsc70 genes in carp. Biochem Biophys Res Commun. 2003;307:503–509. doi: 10.1016/s0006-291x(03)01206-3. [DOI] [PubMed] [Google Scholar]
- Anderson R, Fassler R, Georges-Labouesse E, Hynes RO, Bader BL, et al. Mouse primordial germ cells lacking β1 integrins enter the germline but fail to migrate normally to the gonads. Development. 1999;126:1655–1664. doi: 10.1242/dev.126.8.1655. [DOI] [PubMed] [Google Scholar]
- Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th ed. Philadelphia: Elsevier; 2008. [Google Scholar]
- Bergeron JM, Gahr M, Horan K, Wibbels T, Crews D. Cloning and in situ hybridization analysis of estrogen receptor in the developing gonad of the red-eared slider turtle, a species with temperature-dependent sex determination. Dev Growth Differ. 1998;40:243–254. doi: 10.1046/j.1440-169x.1998.00013.x. [DOI] [PubMed] [Google Scholar]
- Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull JJ, Gutzke WH, Crews D. Sex reversal by estradiol in 3 reptilian orders. Gen Comp Endocrinol. 1988;70:425–428. doi: 10.1016/0016-6480(88)90117-7. [DOI] [PubMed] [Google Scholar]
- Chen B, Piel WH, Gui LM, Bruford E, Monteiro A. The Hsp90 family of genes in the human genome: insights into their divergence and evolution. Genomics. 2005;86:627–637. doi: 10.1016/j.ygeno.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Chen H, Hewison M, Hu B, Sharma M, Sun ZJ, Adams JS. An Hsp27-related, dominant-negative-acting intracellular estradiol-binding protein. J Biol Chem. 2004;279:29944–29951. doi: 10.1074/jbc.M401317200. [DOI] [PubMed] [Google Scholar]
- Chen H, Hewison M, Adams JS. Control of estradiol-directed gene transactivation by an intracellular estrogen-binding protein and an estrogen response element-binding protein. Mol Endocrinol. 2008;22:559–569. doi: 10.1210/me.2007-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EA, Ingolia TD, Manseau LJ. Expression of Drosophila heat-shock cognate genes during heat-shock and development. Dev Biol. 1983;99:418–426. doi: 10.1016/0012-1606(83)90291-9. [DOI] [PubMed] [Google Scholar]
- Crain DA, Guillette LJ, Jr, Rooney AA, Pickford DB. Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ Health Perspect. 1997;105:528–533. doi: 10.1289/ehp.97105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D. Temperature-dependent sex determination: the interplay of steroid hormones and temperature. Zoolog Sci. 1996;13:1–13. doi: 10.2108/zsj.13.1. [DOI] [PubMed] [Google Scholar]
- Deane EE, Woo NYS. Cloning and characterization of the hsp70 multigene family from silver sea bream: modulated gene expression between warm and cold temperature acclimation. Biochem Biophys Res Commun. 2005;330:776–783. doi: 10.1016/j.bbrc.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36:W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin RH, Nagahama Y. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208:191–364. [Google Scholar]
- Eustace BK, Jay DG. Extracellular roles for the molecular chaperone, Hsp90. Cell Cycle. 2004;3:1098–1100. [PubMed] [Google Scholar]
- Eustace BK, Sakurai T, Stewart JK, Yimlamai D, Unger C, et al. Functional proteomic screens reveal an essential extracellular role for Hsp90 alpha in cancer cell invasiveness. Nat Cell Biol. 2004;6:507–514. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Ferguson MWJ. Reproductive biology and embryology of the crocodilians. In: Gans C, Maderson P, Billet F, editors. Biology of the Reptilia. Vol 14. New York: Wiley and Sons; 1985. pp. 329–491. [Google Scholar]
- Gabriel WN, Blumberg B, Sutton S, Place AR, Lance VA. Alligator aromatase cDNA sequence and its expression in embryos at male and female incubation temperatures. J Exp Zool. 2001;290:439–448. doi: 10.1002/jez.1087. [DOI] [PubMed] [Google Scholar]
- Gacad MA, Chen H, Arbelle JE, LeBon T, Adams JS. Functional characterization and purification of all intracellular vitamin D-binding protein in vitamin D-resistant new world primate cells – amino acid sequence homology with proteins in the Hsp-70 family. J Biol Chem. 1997;272:8433–8440. doi: 10.1074/jbc.272.13.8433. [DOI] [PubMed] [Google Scholar]
- Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- Gething M. Guidebook to Molecular Chaperones and Protein Folding Catalysts. Oxford: Oxford University Press; 1998. [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Harry JL, Williams KL, Briscoe DA. Sex determination in loggerhead turtles – differential expression of 2 hnRNP proteins. Development. 1990;109:305–312. doi: 10.1242/dev.109.2.305. [DOI] [PubMed] [Google Scholar]
- Jaglarz MK, Howard KR. The active migration of Drosophila primordial germ-cells. Development. 1995;121:3495–3503. doi: 10.1242/dev.121.11.3495. [DOI] [PubMed] [Google Scholar]
- Jeffs B, Meeks JJ, Ito M, Martinson FA, Matzuk MM, et al. Blockage of the rete testis and efferent ductules by ectopic Sertoli and Leydig cells causes infertility in Dax1-deficient male mice. Endocrinology. 2001;142:4486–4495. doi: 10.1210/endo.142.10.8447. [DOI] [PubMed] [Google Scholar]
- Katsu Y, Bermudez DS, Braun EL, Helbing C, Miyagawa S, et al. Molecular cloning of the estrogen and progesterone receptors of the American alligator. Gen Comp Endocrinol. 2004;136:122–133. doi: 10.1016/j.ygcen.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Katsu Y, Myburgh J, Kohno S, Swan GE, Guillette LJ, Jr, Iguchi T. Molecular cloning of estrogen receptor alpha of the Nile crocodile. Comp Biochem Physiol A Mol Integr Physiol. 2006;143:340–346. doi: 10.1016/j.cbpa.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 2000;141:1317–1324. doi: 10.1210/endo.141.4.7403. [DOI] [PubMed] [Google Scholar]
- Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Lance VA, Bogart MH. Studies on sex determination in the American alligator Alligator-mississippiensis. J Exp Zool. 1994;270:79–85. [Google Scholar]
- Lang JW, Andrews HV. Temperature-dependent sex determination in crocodilians. J Exp Zool. 1994;270:28–44. [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Martin J, Horwich AL, Hartl FU. Prevention of protein denaturation under heat-stress by the chaperonin Hsp60. Science. 1992;258:995–998. doi: 10.1126/science.1359644. [DOI] [PubMed] [Google Scholar]
- Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B. Male-specific cell migration into the developing gonad. Curr Biol. 1997;7:958–968. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- Milnes MR, Roberts RN, Guillette LJ., Jr Effects of incubation temperature and estrogen exposure on aromatase activity in the brain and gonads of embryonic alligators. Environ Health Perspect. 2002;110:393–396. doi: 10.1289/ehp.02110s3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Tissieres A, Georgopoulos C. Stress Proteins in Biology and Medicine. Cold Spring Harbor: Cold Spring Harbor Press; 1990. [Google Scholar]
- Morrish BC, Sinclair AH. Vertebrate sex determination: many means to an end. Reproduction. 2002;124:447–457. doi: 10.1530/rep.0.1240447. [DOI] [PubMed] [Google Scholar]
- Murdock C, Wibbels T. Cloning and expression of aromatase in a turtle with temperature-dependent sex determination. Gen Comp Endocrinol. 2003;130:109–119. doi: 10.1016/s0016-6480(02)00573-7. [DOI] [PubMed] [Google Scholar]
- Naidoo V, Katsu Y, Iguchi T. The influence of non-toxic concentrations of DDT and DDE on the old world vulture estrogen receptor alpha. Gen Comp Endocrinol. 2008;159:188–195. doi: 10.1016/j.ygcen.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Nover L, Scharf KD. Heat stress proteins and transcription factors. Cell Mol Life Sci. 1997;53:80–103. doi: 10.1007/pl00000583. [DOI] [PubMed] [Google Scholar]
- Nyberg P, Ylipalosaari M, Sorsa T, Salo T. Trypsins and their role in carcinoma growth. Exp Cell Res. 2006;312:1219–1228. doi: 10.1016/j.yexcr.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Ostermann J, Horwich AL, Neupert W, Hartl FU. Protein folding in mitochondria requires complex-formation with Hsp60 and ATP hydrolysis. Nature. 1989;341:125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Picard D. Chaperoning steroid hormone action. Trends Endocrinol Metab. 2006;17:229–235. doi: 10.1016/j.tem.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Pieau C, Dorizzi M. Oestrogens and temperature-dependent sex determination in reptiles: all is in the gonads. J Endocrinol. 2004;181:367–377. doi: 10.1677/joe.0.1810367. [DOI] [PubMed] [Google Scholar]
- Pieau C, Dorizzi M, Richard-Mercier N. Temperature-dependent sex determination and gonadal differentiation in reptiles. Cell Mol Life Sci. 1999;55:887–900. doi: 10.1007/s000180050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB. The role of the Hsp90-based chaperone system in signal transduction by nuclear receptors and receptors signaling via map kinase. Annu Rev Pharmacol Toxicol. 1997;37:297–326. doi: 10.1146/annurev.pharmtox.37.1.297. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- Ritossa F. Discovery of the heat shock response. Cell Stress Chaperones. 1996;1:97–98. doi: 10.1379/1466-1268(1996)001<0097:dothsr>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, Tilman C, Yao H, MacLaughlin D, Capel B. AMH induces mesonephric cell migration in XX gonads. Mol Cell Endocrinol. 2003;211:1–7. doi: 10.1016/j.mce.2003.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybczynski R, Gilbert LI. cDNA cloning and expression of a hormone-regulated heat shock protein (hsc 70) from the prothoracic gland of Manduca sexta. Insect Biochem Mol Biol. 2000;30:579–589. doi: 10.1016/s0965-1748(00)00031-x. [DOI] [PubMed] [Google Scholar]
- Schlesinger MJ. How the cell copes with stress and the function of heat-shock proteins. Pediatr Res. 1994;36:1–6. doi: 10.1203/00006450-199407001-00001. [DOI] [PubMed] [Google Scholar]
- Smith CA, Joss JMP. Gonadal sex-differentiation in Alligator mississippiensis, a species with temperature-dependent sex determination. Cell Tissue Res. 1993;273:149–162. [Google Scholar]
- Smith CA, Sinclair AH. Sex determination: insights from the chicken. Bioessays. 2004;26:120–132. doi: 10.1002/bies.10400. [DOI] [PubMed] [Google Scholar]
- Smith CA, Elf PK, Lang JW, Joss JMP. Aromatase enzyme-activity during gonadal sex-differentiation in alligator embryos. Differentiation. 1995;58:281–290. [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. Mega4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tilmann C, Capel B. Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad. Development. 1999;126:2883–2890. doi: 10.1242/dev.126.13.2883. [DOI] [PubMed] [Google Scholar]
- van den Ijssel P, Norman DG, Quinlan RA. Molecular chaperones: small heat shock proteins in the limelight. Curr Biol. 1999;9:R103–R105. doi: 10.1016/s0960-9822(99)80061-x. [DOI] [PubMed] [Google Scholar]
- Westwood JT, Clos J, Wu C. Stress-induced oligomerization and chromosomal relocalization of heat-shock factor. Nature. 1991;353:822–823. doi: 10.1038/353822a0. [DOI] [PubMed] [Google Scholar]
- Wu SX, Chun R, Gacad MA, Ren SY, Chen H, Adams JS. Regulation of 1,25-dihydroxyvitamin D synthesis by intracellular vitamin D binding protein-1. Endocrinology. 2002;143:4135–4138. doi: 10.1210/en.2002-220568. [DOI] [PubMed] [Google Scholar]
- Yao HHC, Capel B. Temperature, genes, and sex: a comparative view of sex determination in Trachemys scripta and Mus musculus. J Biochem. 2005;138:5–12. doi: 10.1093/jb/mvi097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HHC, DiNapoli L, Capel B. Cellular mechanisms of sex determination in the red-eared slider turtle, Trachemys scripta. Mech Dev. 2004;121:1393–1401. doi: 10.1016/j.mod.2004.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RN, Ito M, Saunders TL, Camper SA, Jameson JL. Role of Ahch in gonadal development and gametogenesis. Nat Genet. 1998;20:353–357. doi: 10.1038/3822. [DOI] [PubMed] [Google Scholar]