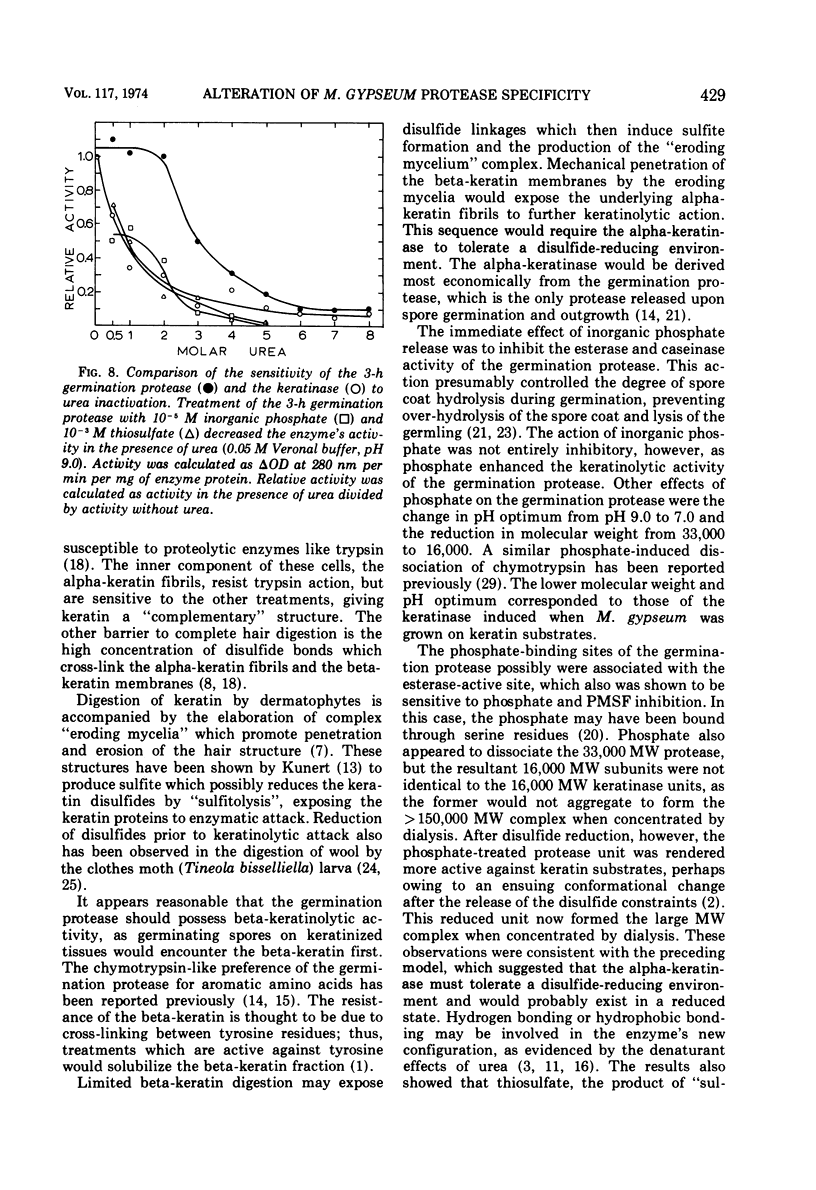
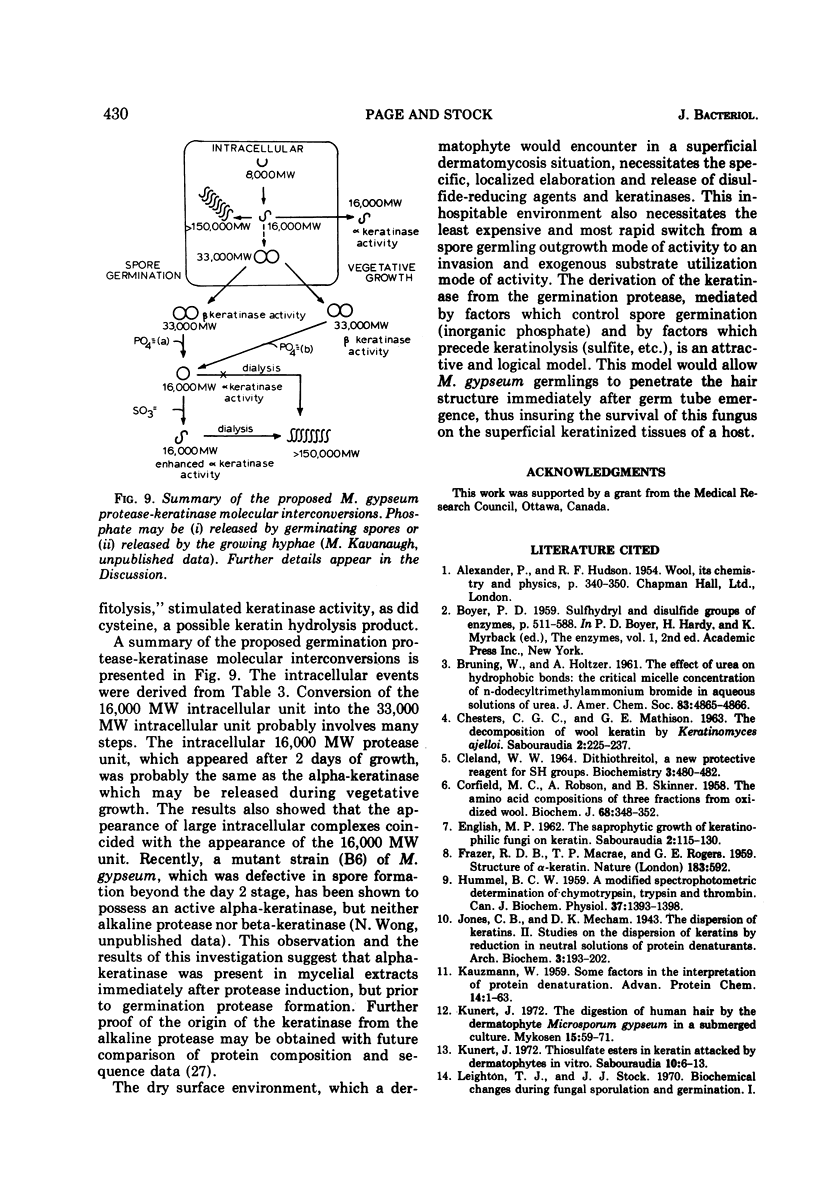
Abstract
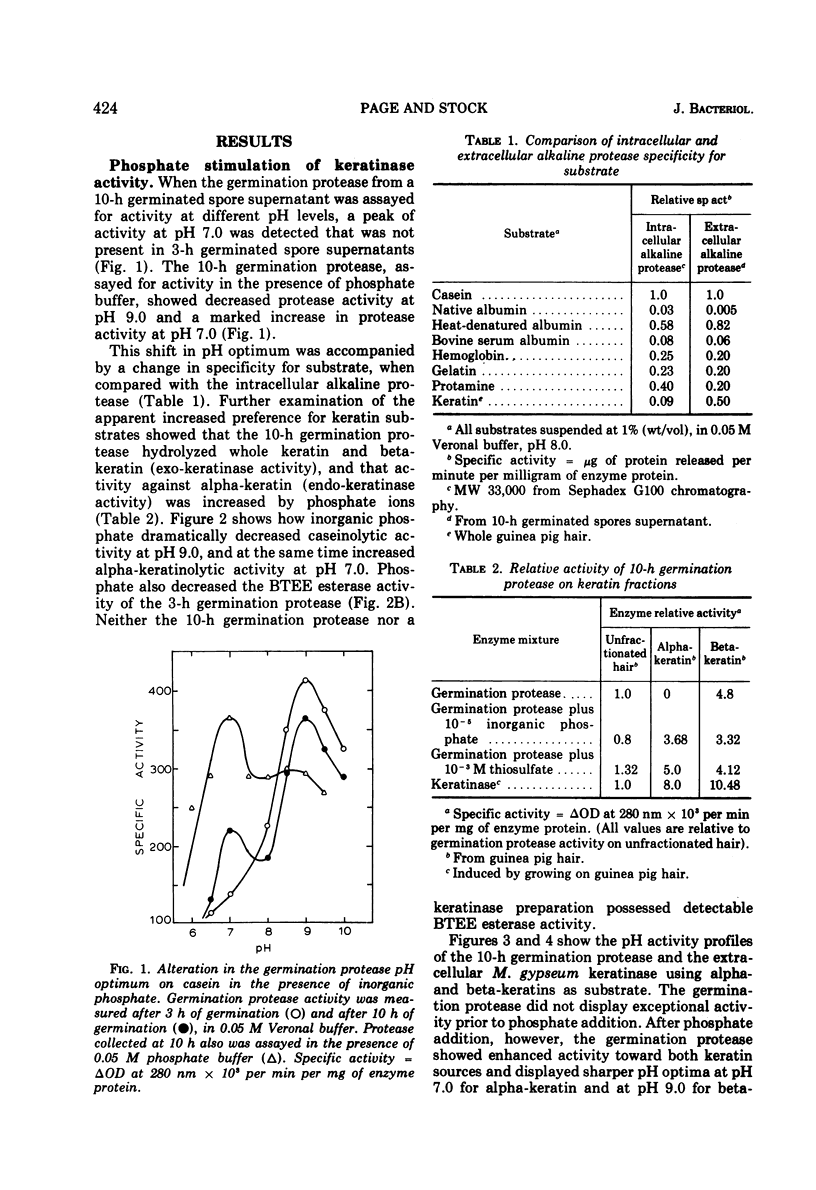
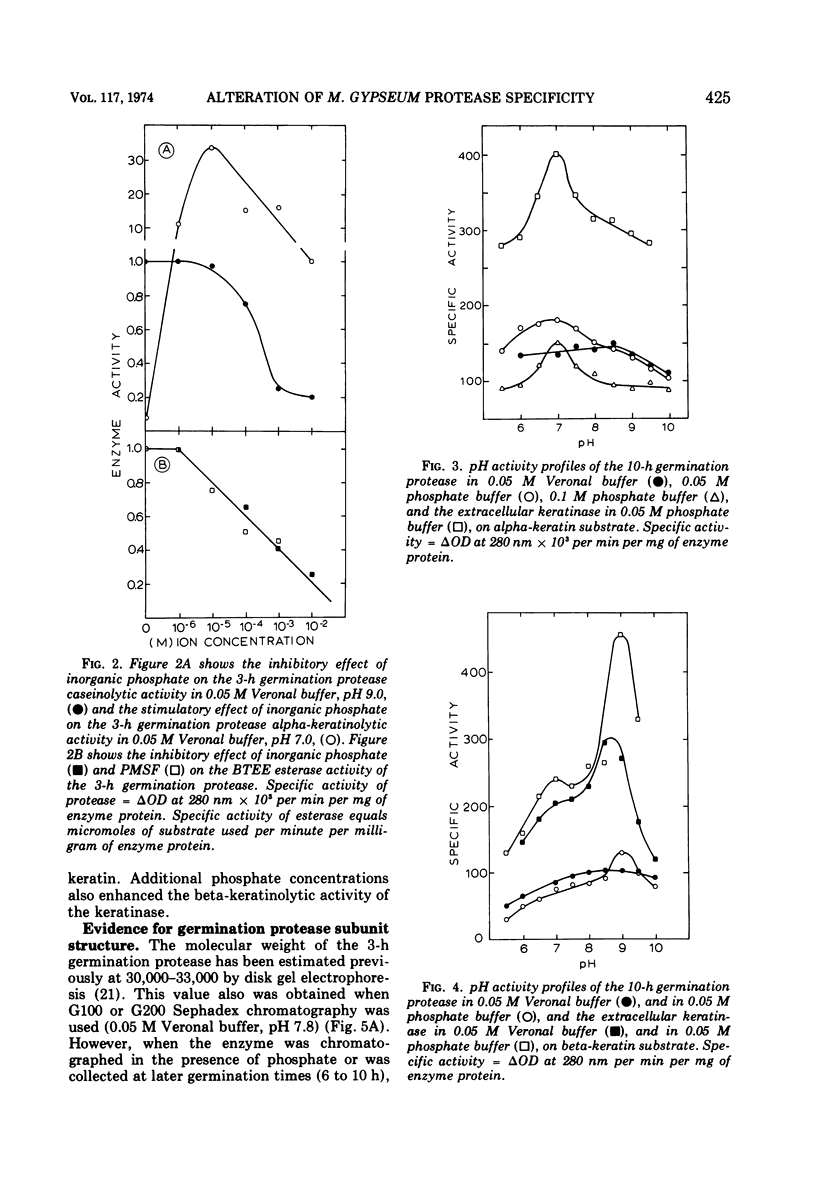
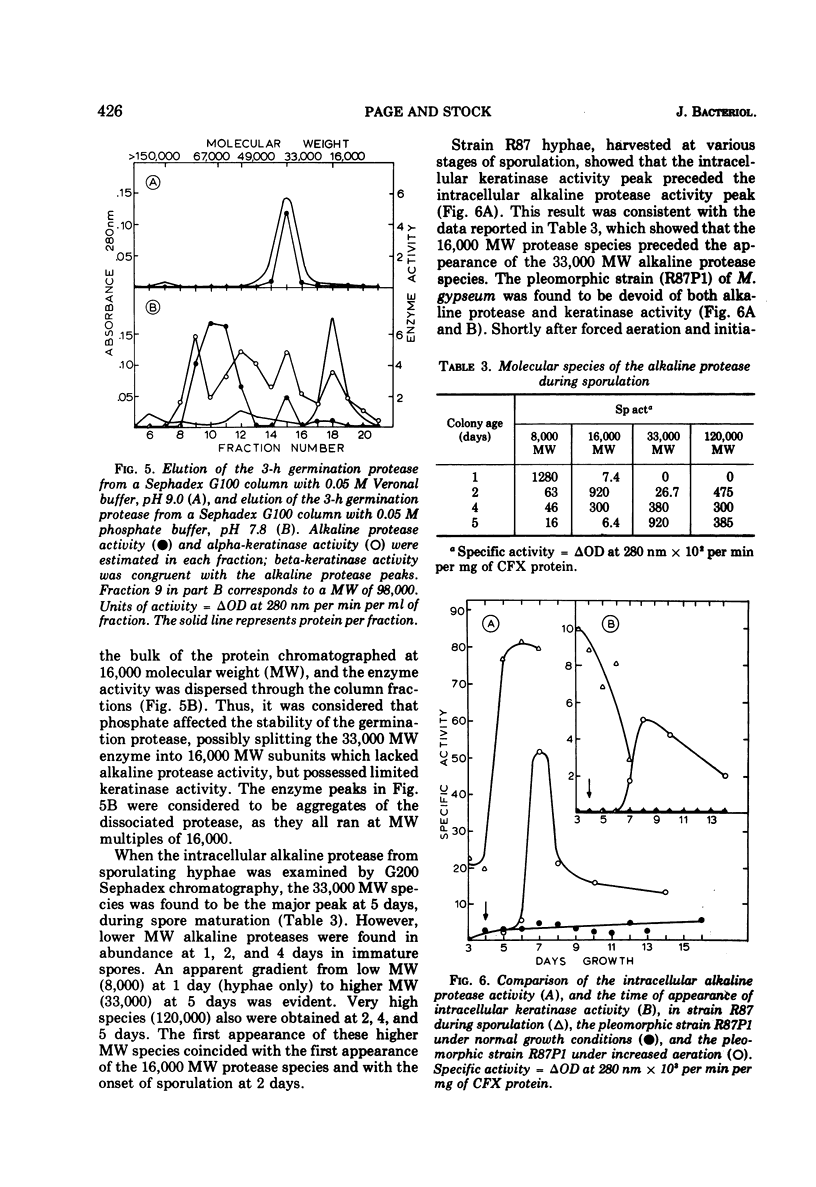
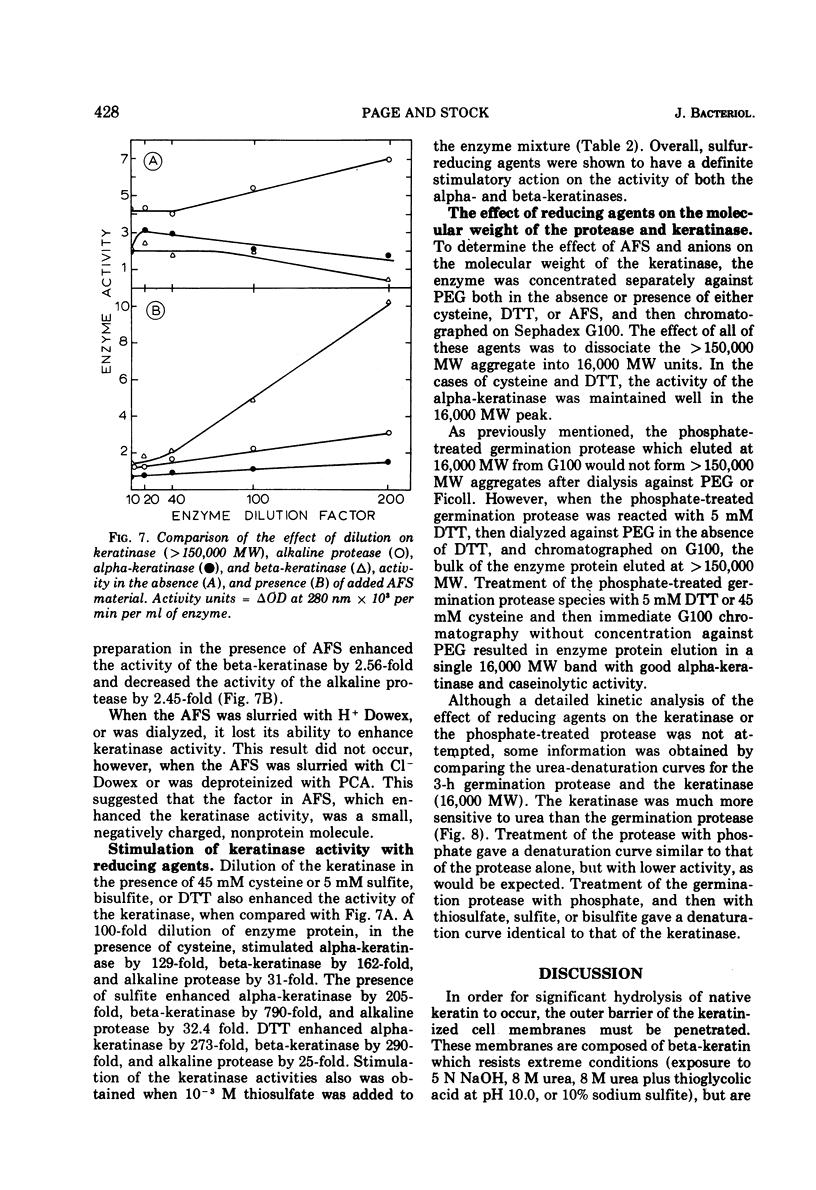
Inorganic phosphate was found to decrease the caseinolytic and ethyl-esterase activities of the Microsporum gypseum germination protease. The germination protease possessed exokeratinase (beta-keratinase) activity immediately after release from the fungal spore. After phosphate treatment of the enzyme, the germination protease also possessed endo-keratinase (alpha-keratinase) activity. Phosphate altered the protease's pH optimum from 9.0 to 7.0 and decreased the molecular weight from 33,000 to 16,000. These values were identical to those found for the keratinase. Alpha- and beta-keratinase activities were stimulated in excess of 200-fold by disulfide reducing agents. Natural and suspected keratin degradation products also enhanced keratinase activity. Cell fractionation and in vitro conversion of the alkaline germination protease into a functional keratinase suggested that the subunits comprising the germination protease and the keratinase were of a common origin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- CORFIELD M. C., ROBSON A., SKINNER B. The amino acid compositions of three fractions from oxidized wool. Biochem J. 1958 Feb;68(2):348–352. doi: 10.1042/bj0680348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER R. D., MACRAE T. P., ROGERS G. E. Structure of alpha-keratin. Nature. 1959 Feb 28;183(4661):592–594. doi: 10.1038/183592a0. [DOI] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Kunert J. The digestion of human hair by the dermatophyte Microsporum gypseum in a submerged culture. Mykosen. 1972 Feb 1;15(2):59–71. doi: 10.1111/j.1439-0507.1972.tb02451.x. [DOI] [PubMed] [Google Scholar]
- Kunert J. Thiosulphate esters in keratin attacked by dermatophytes in vitro. Sabouraudia. 1972 Mar;10(1):6–13. doi: 10.1080/00362177285190031. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leighton T. J., Stock J. J. Isolation and preliminary characterization of developmental mutants from Microsporum gypseum. J Bacteriol. 1970 Nov;104(2):834–838. doi: 10.1128/jb.104.2.834-838.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICKERSON W. J., DURAND S. C. KERATINASE. II. PROPERTIES OF THE CRYSTALLINE ENZYME. Biochim Biophys Acta. 1963 Sep 3;77:87–99. doi: 10.1016/0006-3002(63)90471-2. [DOI] [PubMed] [Google Scholar]
- POWNING R. F. Studies on the digestion of wool by insects. VIII. The significance of certain excretory products of the clothes moth, Tineola bisselliella, and the carpet beetle, Attagenus piceus. Aust J Biol Sci. 1953 Feb;6(1):109–117. [PubMed] [Google Scholar]
- Page W. J., Stock J. J. Initiation of dermatophyte pleomorphic strain sporulation by increased aeration. Appl Microbiol. 1972 Oct;24(4):650–657. doi: 10.1128/am.24.4.650-657.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Stock J. J. Isolation and characterization of Microsporum gypseum lysosomes: role of lysosomes in macroconidia germination. J Bacteriol. 1972 Apr;110(1):354–362. doi: 10.1128/jb.110.1.354-362.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Stock J. J. Regulation and self-inhibition of Microsporum gypseum Macroconidia germination. J Bacteriol. 1971 Oct;108(1):276–281. doi: 10.1128/jb.108.1.276-281.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAUBITSCHEK F. Mechanical versus chemical keratolysis by dermatophytes. Sabouraudia. 1961 Jun;1:87–90. doi: 10.1080/00362176285190191. [DOI] [PubMed] [Google Scholar]
- Shoer R., Rappaport H. P. Analysis of a Bacillus subtilis proteinase mutant. J Bacteriol. 1972 Feb;109(2):575–583. doi: 10.1128/jb.109.2.575-583.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TINOCO I., Jr The polymerization of enzymically active alpha-chymotrypsin. Arch Biochem Biophys. 1957 Jun;68(2):367–372. doi: 10.1016/0003-9861(57)90369-7. [DOI] [PubMed] [Google Scholar]
- Yu R. J., Harmon S. R., Blank F. Isolation and purification of an extracellular keratinase of Trichophyton mentagrophytes. J Bacteriol. 1968 Oct;96(4):1435–1436. doi: 10.1128/jb.96.4.1435-1436.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



