Abstract
The human ortholog of the gene responsible for audiogenic seizure susceptibility in Frings and BUB/BnJ mice (mouse gene symbol Mass1) recently was shown to underlie Usher syndrome type IIC (USH2C). Here we report that the Mass1frings mutation is responsible for the early onset hearing impairment of BUB/BnJ mice. We found highly significant linkage of Mass1 with ABR threshold variation among mice from two backcrosses involving BUB/BnJ mice with mice of strains CAST/EiJ and MOLD/RkJ. We also show an additive effect of the Cdh23 locus in modulating the progression of hearing loss in backcross mice. Together, these two loci account for more than 70% of the total ABR threshold variation among the backcross mice at all ages. The modifying effect of the strain-specific Cdh23ahl variant may account for the hearing and audiogenic seizure differences observed between Frings and BUB/BnJ mice, which share the Mass1frings mutation. During postnatal cochlear development in BUB/BnJ mice, stereocilia bundles develop abnormally and remain immature and splayed into adulthood, corresponding with the early onset hearing impairment associated with Mass1frings. Progressive base–apex hair cell degeneration occurs at older ages, corresponding with the age-related hearing loss associated with Cdh23ahl. The molecular basis and pathophysiology of hearing loss suggest BUB/BnJ and Frings mice as models to study cellular and molecular mechanisms underlying USH2C auditory pathology.
Keywords: Hearing loss, Mouse inbred strain, Frings, BUB/BnJ, VLGR1, USH2C, Mass1, Cdh23
Mice of the BUB/BnJ inbred strain exhibit impaired hearing at the youngest age that can be reliably assessed by auditory brain-stem response (ABR) thresholds (3 weeks), and they become deaf by 20 weeks of age [1]. We previously showed that the Ahl locus on mouse chromosome (Chr) 10 contributes to age-related hearing loss (AHL) in many inbred strains, including BUB/BnJ [2,3]. Recent evidence demonstrates that the effects of Ahl are caused by an inbred strain-specific 753A→G dimorphism of the Cdh23 gene [4]. The 753A variant corresponds with the recessive allele (Cdh23ahl) that confers susceptibility to AHL. The modifying effect of Cdh23ahl on hearing loss in BUB/BnJ mice, however, is much less than that seen in other inbred strains with AHL. For example, at 6 months of age, Cdh23ahl can account for 60–80% of the ABR threshold variation among NOD/LtJ, DBA/2J, or A/J backcross mice, but only 10–20% of the variation among BUB/BnJ backcross mice [3]. An additional genetic factor or factors, therefore, must underlie the early onset hearing impairment of BUB/BnJ mice.
The BUB/BnJ inbred strain is genetically similar to the Frings strain; both were derived from Swiss albino mice. The Frings strain was developed as a model of reflex epilepsy by selecting for mice that were highly susceptible to audiogenic seizures [5]. The trait subsequently was shown to be monogenic and was mapped to a locus on Chr 13 named “monogenic audiogenic seizure susceptibility 1,” symbol mass1 [6]. The molecular basis of the mass1 phenotype of Frings mice later was shown to be a single base pair deletion in a novel gene, designated Mass1, that leads to premature termination of the encoded protein [7]. The same mutation was found in BUB/BnJ mice but not in 11 other strains of Swiss albino mice that were subsequently examined. In support of the causative nature of this mutation, BUB/BnJ mice were shown to be susceptible to audiogenic seizures, whereas mice of a genetically very similar strain, SWR/Bm, were resistant [7].
The Mass1 gene was identified as an ~-kb cDNA with multiple Cal×-β repeat motifs [7] that later were shown to overlap with a portion of another cDNA encoded by a gene named “very large G-protein-coupled receptor 1” [8]. The entire gene comprises 90 exons and encodes multiple alternatively spliced variants that are expressed in the embryonic central nervous system and eye of mice [9]. Mice homozygous for targeted mutations of the extracellular, transmembrane, and cytoplasmic domains of Mass1 exhibit audiogenic seizures, similar to mice with the Mass1frings mutation [10,11]. Mutations of the homologous human gene (MASS1 or VLGR1) recently have been implicated in the pathogenesis of Usher syndrome type IIC (USH2C), a disorder characterized by sensorineural hearing loss and retinitis pigmentosa [12].
The association of MASS1 mutations with the human hearing disorder USH2C prompted us to examine the possibility that the Mass1frings mutation underlies hearing loss in BUB/BnJ mice. Here, we present backcross linkage data, ABR threshold results, and cochlear pathology demonstrating that the Mass1frings mutation is indeed responsible for the early onset hearing impairment of BUB/BnJ mice and that the additive effect of Cdh23ahl causes an age-related progression of this hearing loss.
Results
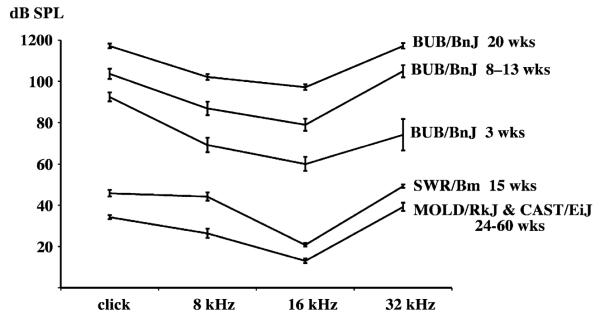
By 3 weeks of age, BUB/BnJ mice exhibit ABR thresholds about 40 dB higher than the normal thresholds exhibited by SWR/Bm mice tested at 15 weeks of age, and this hearing loss progresses to deafness by 20 weeks of age (Fig. 1). The SWR/Bm strain is genetically very similar to BUB/BnJ [13] but does not have the Mass1frings mutation[7] and thus can serve as a control strain for the effects of this mutation. Also shown in Fig. 1 are the combined ABR results for nine mice of the CAST/EiJ strain and nine mice of the MOLD/RkJ strain that were tested at 24–60 weeks of age. Because these wild-derived mice retain normal hearing well beyond 1 year of age [1], they were used to make F1 hybrids with hearing-impaired BUB/BnJ mice for the linkage backcross analysis.
Fig. 1.
Average ABR thresholds (dB SPL, with standard error bars) of BUB/BnJ, SWR/Bm, CAST/EiJ, and MOLD/RkJ inbred strain mice. BUB/BnJ mice were tested at ages 3 weeks (N = 6), 8 – 13 weeks (N = 17), and 20 weeks (N = 5). SWR/Bm mice (N = 6) were tested at 15 weeks of age. CAST/EiJ (N =9) and MOLD/RkJ (N = 9) mice were tested at 24 – 60 weeks of age.
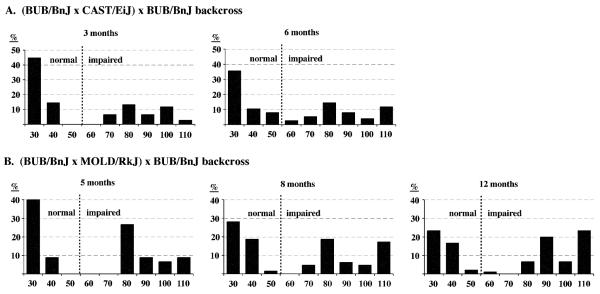
The (CAST/EiJ × BUB/BnJ) × BUB/BnJ backcross mice (N = 76) were tested twice for ABR thresholds, at 3 and 6 months of age. Mice of the (MOLD/RkJ × BUB/BnJ) × BUB/BnJ backcross were tested three times, at 5 (N = 44), 8 (N = 64), and 12 (N = 89) months of age. Frequency distributions of backcross mice with different ABR thresholds are shown in Fig. 2. The distributions are strongly bimodal at the youngest ages tested, suggesting the major influence of a single gene locus. The overall progression of hearing loss can be seen as increasing percentages of mice with higher ABR thresholds at older ages. For example, only 9% of (MOLD/RkJ × BUB/BnJ) × BUB/BnJ backcross mice have click thresholds greater than 100 dB sound pressure level (SPL) at 5 months of age compared with 23% at 12 months.
Fig. 2.
Frequency distributions of backcross mice ABR thresholds. The percentages of N2 backcross mice exhibiting click thresholds within each 10-dB SPL increment are shown as histograms. The vertical dotted line demarcates the border between normal and hearing impaired values as previously defined [1]. (CAST/EiJ × BUB/BnJ) × BUB/BnJ backcross mice were tested at 3 and 6 months of age. (MOLD/RkJ × BUB/BnJ) × BUB/BnJ backcross mice were tested at 5, 8, and 12 months of age.
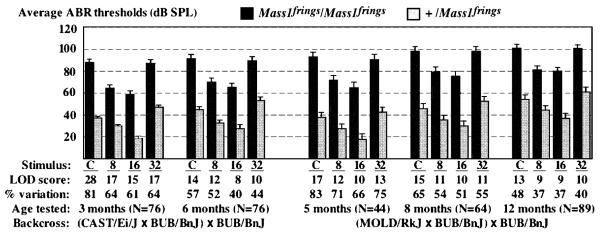
Quantitative trait loci (QTL) linkage analysis of ABR thresholds of backcross mice revealed highly significant associations with D13Mit9, a marker located in IVS89 within the Mass1gene (Fig. 3). To test formally if the maximum linkage association occurs at this locus, we typed six additional Chr 13 markers: D13Mit19, D13Mit157, D13Mit255, D13Mit159, D13Mit228,and D13Mit77. As expected, the peak association was with D13Mit9 and strongly supports the Mass1frings mutation as the likely molecular basis for the early onset hearing loss of BUB/BnJ mice. D13Mit9 variation is most closely linked with click thresholds of backcross mice that are younger than 6 months of age (Fig. 3). In contrast, D10Mit138, a marker near the Cdh23 locus, is most highly associated with high-frequency pure tones in mice older than 10 months of age [3].At 5 months of age, the Mass1 locus (marked by D13Mit9) can account for about 80% of the total ABR threshold variation, whereas the Cdh23 locus (marked by D10Mit138) accounts for less than 15% (Fig. 4). At 12 months of age, each of the two loci contributes about 40% of the total variation. Together, the two loci can account for 75–85% of the total ABR threshold variation among the backcross mice at all ages tested, and they likely account for more than 90% of the genetic variation, considering that variation in ABR measurements and other environmental variations are also included in the total variance estimate.
Fig. 3.
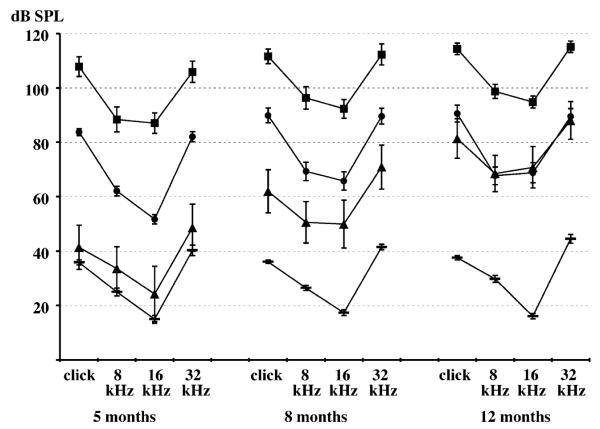
Linkage of the Mass1 locus with ABR thresholds. For each of the two backcrosses, average ABR thresholds in dB SPL (and their standard errors) are shown for Mass1frings/Mass1frings mice and for +/Mass1frings mice. Lod scores of linkage associations and percentages of total threshold variation attributable to the Mass1 locus are given for each auditory stimulus (C, click; 8, 8 kHz; 16, 16 kHz; 32, 32 kHz) and for each test age.
Fig. 4.
Percentage of total ABR threshold variation among (MOLD/RkJ ×BUB/BnJ) × BUB/BnJ backcross mice that can be explained by a QTL at the Mass1 locus (circles), a QTL at the Cdh23 locus (triangles), or both QTLs combined(squares). D13Mit9 was used to mark Mass1 and D10Mit138 to mark Cdh23.Mice were tested at 5, 8, and 12 months of age.
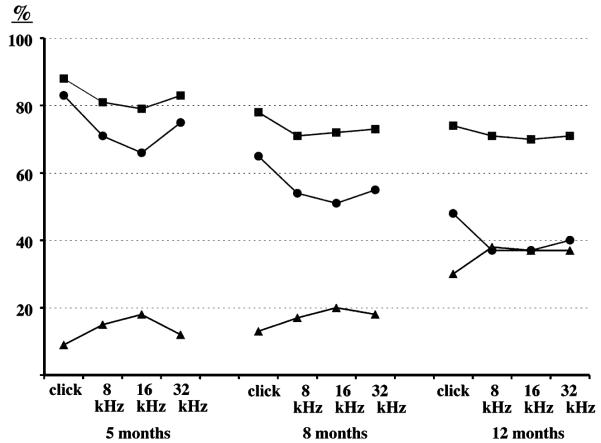
Hearing loss susceptibility alleles of both loci are recessive and originate from the BUB/BnJ strain, and resistance to hearing loss is conferred by dominant alleles (+) inherited from the wild-derived strains CAST/EiJ and MOLD/RkJ. The effects of the two loci on ABR thresholds of backcross mice are primarily additive, as shown in Fig. 5. No statistically significant interactive effects were detected between D13Mit9 and D10Mit138 with the Map Manager QTX computer program [14] for any of the auditory stimuli or ages that were tested. For example, analysis of the click threshold variation for 12-month-old backcross mice gave a lod score of only 1.5 for interaction, whereas the main effect for D13Mit9 is 12.6 and the main effect for D10Mit138 is 6.9. Compound homozygotes for susceptibility alleles (Mass1frings/Mass1frings Cdh23ahl/Cdh23ahl) exhibit the highest ABR thresholds and are already nearly deaf at the youngest ages tested (thresholds >60 dB above normal by 5 months of age). Compound heterozygotes (+/Mass1frings +/Cdh23ahl) retain normal hearing levels at the oldest ages tested(12 months), and singly homozygous mice (Mass1frings/Mass1frings +/Cdh23ahl and +/Mass1frings Cdh23ahl/Cdh23ahl) have intermediate ABR thresholds. The hearing loss of +/Mass1frings Cdh23ahl/Cdh23ahl mice is progressive and does not reach the level of Mass1frings/Mass1frings +/Cdh23ahl mice (about 40 dB above normal)until 12 months of age.
Fig. 5.
Average ABR thresholds (dB SPL, with standard error bars) of (MOLD/RkJ × BUB/BnJ) × BUB/BnJ backcross mice sorted by their two-locus Mass1 and Cdh23 genotypes. ABR thresholds of Mass1frings/Mass1frings cdh23ahl/cdh23ahl mice are designated by squares, Mass1frings/Mass1frings +/cdh23ahl miceby circles, +/Mass1frings cdh23ahl/cdh23ahl mice by triangles, and +/Mass1frings +/cdh23ahl mice by horizontal lines. D13Mit9 was used to mark Mass1 and D10Mit138 to mark Cdh23. Mice were tested at 5, 8, and 12 months of age.
Retinitis pigmentosa, as well as deafness or hearing impairment, is a characteristic of the Usher syndromes. Mouse tissue sources for ESTs indicate that the Mass1 gene is expressed in the retina and expression has been detected in eyes of mouse embryos [9]. However, the effect of the Mass1frings mutation on retinal function could not be evaluated with certainty because BUB/BnJ is homozygous for the retinal degeneration mutation on Chr 5 (Pde6brd1).Because all of the Usher syndrome type IIc patients with MASS1 mutations that have been identified so far are female [12], we also examined the effect of sex on hearing loss in the BUB/BnJ backcross mice. We sorted backcross mice by sex and Mass1 (D13Mit9) genotypes but found no statistically significant ABR threshold differences between male and female mice at any age in either backcross.
BUB/BnJ mice show audiogenic seizures only when tested at young ages (<25 days of age), whereas Frings mice retain seizure sensitivity well into adulthood [6]. Because both BUB/BnJ and Frings mice share the Mass1frings mutation [7], we reasoned that a modifier locus, affecting progressive hearing loss, might explain this difference. To test this hypothesis, Frings mice were crossed with BUB/BnJ mice to generate F1 hybrids, and these were then intercrossed to generate F2 animals. All (50/50) F1 hybrids retained audiogenic seizure susceptibility well after 25 days of age. All F2 intercross mice seized at 19 days of age (n = 161); however, 43/161 (26.7%) lost seizure susceptibility after 25 days of age. When tested for ABR thresholds at 42 days of age, seizing (n = 118) and nonseizing (n = 43) F2 mice had mean 16-kHz thresholds of 50.0 and 66.0 dB SPL, respectively ( p < 0.0001). The 3:1 segregation ratio of seizing to nonseizing F2 mice and the elevated ABR thresholds of nonseizing mice are consistent with a single recessive gene that contributes to progressive hearing loss in homozygous mice and that consequently eliminates the audiogenic seizure susceptibility of these mice at older ages.
The Chr 10 Ahl gene (Cdh23ahl) previously was shown to influence hearing loss progression in BUB/BnJ backcross mice [3,4]. To see if Cdh23ahl could be responsible for the differences in audiogenic seizure susceptibility and hearing thresholds between BUB/BnJ and Frings mice, we sequenced the region of Cdh23 containing the exon 7 splicing variant thought to underlie the Ahl phenotype.Sequence analysis showed that Frings and the closely related SWR/BmJ strains have the 753G variant of Cdh23, which confers resistance to hearing loss, whereas the BUB/BnJ strain has the 753A variant, which increases hearing loss susceptibility. Taken together, seizure segregation and sequence data are consistent with a causative role for Cdh23 in the audiogenic seizure differences observed between Frings and BUB/BnJ mice.
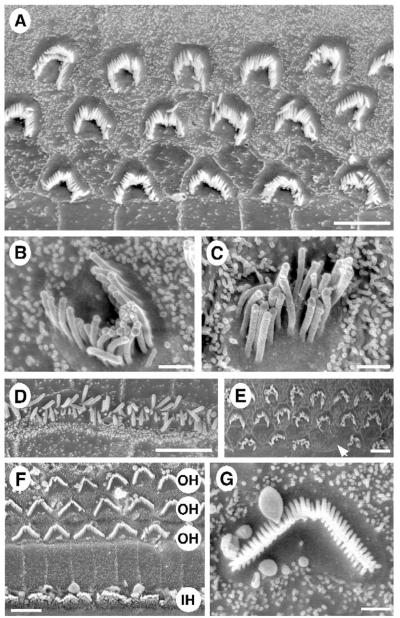
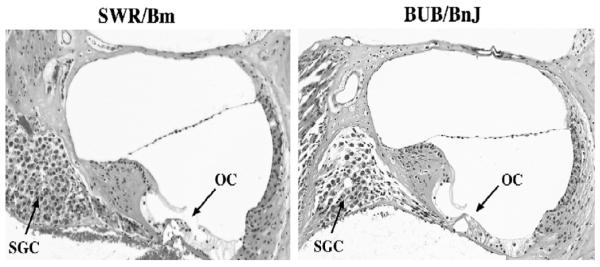
To ascertain the cellular pathology associated with the hearing loss of BUB/BnJ mice we examined surface preparations of organs of Corti by scanning electron microscopy. At postnatal day 14 (P14) the stereocilia bundle of outer hair cells along the length of the neuroepithelium is largely immature and underdeveloped (Fig. 6A). Lateral and apical aspects of the bundle appear rounded and deformed, and the bundle fails to form its characteristic V-shaped structure. In addition, stereocilia are disconnected and detached, are sometimes found outside their unit, and in some instances are bent (Fig. 6B). The most severely affected bundles have entirely lost their polarity and graded height (Fig. 6C), although the pointing direction of the majority of bundles is toward the nonneural side of the organ of Corti. Hair bundles of inner hair cells show a similar phenotype: stereocilia are splayed, disorganized, and of irregular height (Fig. 6D). Interestingly, with increasing age the morphology of the bundle changes little: at 7 weeks of age (P41) stereocilia maintain their overall height, location, and immature appearance although sporadically there are gaps in the cellular array (Fig. 6E). At 15 weeks of age, degeneration of hair cells and spiral ganglion cells is pronounced in the basal turn, as seen in cochlear cross sections of BUB/BnJ mice but not SWR/Bm mice (Fig. 7).
Fig. 6.
Stereocilia pathology associated with early onset hearing loss of BUB/BnJ mice. (A – C) Taken from P14 BUB/BnJ mice from an area located 1 – 3 mm distal to the apex, which corresponds to a frequency spectrum of approximately 2 – 14 kHz [17]. (A) Representative surface view of organ of Corti showing three rows of outer hair cells; magnification bar corresponds to 4 μm. (B, C) High magnification (original magnification 20,000 ; 3 keV) of individual stereocilia bundles; magnification bar, 1 Am. (D) Stereocilia bundles from two inner hair cells are shown; magnification bar, 1 μm. (E) Shown is an organ of Corti surface view characteristic of a 41-day-old BUB/BnJ mouse showing three rows of outer hair cells; arrow points to a missing hair cell; magnification bar, 4 μm. (F) A surface view of the organ of Corti of a normal hearing C3HeBe/FeJ mouse at P10; rows of outer (OH) and inner (IH) hair cells are indicated; magnification bar, 5 μm. (G) The structure of a normal stereocilia hair bundle from a C3HeB/FeJ mouse.
Fig. 7.
Cochlear pathology associated with age-related hearing loss of BUB/BnJ mice. Both cross sections are from the basal turn of cochleae from 15-week-old mice. The genetically related SWR/Bm strain is shown for comparison to illustrate normal cochlear structure. Arrows indicate the organ of Corti (OC) and spiral ganglion cells (SGC).
Discussion
We present several lines of evidence supporting the hypothesis that the Mass1frings mutation is the major underlying cause of early hearing loss in BUB/BnJ mice: (i) BUB/BnJ mice exhibit an early onset hearing impairment, but mice of genetically similar strains that lack the Mass1frings mutation (such as SWR/Bm) do not. (ii) The major genetic factor associated with the early hearing loss of BUB/BnJ mice maps to the same Chr 13 locus as the Mass1 gene. (iii) Homozygosity of Mass1frings is entirely sufficient to cause hearing impairment in young backcross mice. (iv) Mutations of the orthologous human gene (MASS1) underlie Usher syndrome type IIc, which includes congenital moderate to severe hearing impairment and normal vestibular responses as defining inner ear characteristics, similar to the phenotype of BUB/BnJ mice.
In mice of both backcrosses, the early onset ABR threshold elevations associated with Mass1frings are greater for broad-band clicks than for the higher frequency pure tones (Fig. 3), whereas the opposite is the case for the agerelated threshold elevations associated with Cdh23ahl. These different physiological responses may indicate different mechanisms of pathology. Hearing loss associated with Cdh23ahl in several strains of mice is accompanied by a progressive base–apex hair cell degeneration starting at old ages [15,16]. In young Cdh23ahl homozygotes, such as C57BL/6J mice, the hair bundle shows a normal appearance [17]. Coinciding with the dissimilar profile in ABR responses between BUB/BnJ and C57BL/6J mice, we also observe a specific cochlear pathology in young BUB/BnJ mice that likely is a consequence of the Mass1frings mutation. Hair cells along the entire length of the organ of Corti never form a mature hair bundle; instead they remain immature until at least 7 weeks of age (Fig. 6). In addition, during this time there is no obvious base-to-apex increase in the severity of the phenotype; occasional hair cell degeneration occurs randomly along the duct. In older mice (Fig. 7), however, hair cell degeneration occurs in the base-to-apex direction typically associated with the age-related hearing loss of Cdh23ahl homozygotes.
In mice and rats, susceptibility to audiogenic seizures is closely tied to postnatal auditory development: hearing attenuation caused by intense acoustic stimulation or tympanic membrane obstruction during a critical period of postnatal development (“priming”) can induce audiogenic seizure susceptibility in resistant strains [18]. Most seizure-prone strains (those that do not require priming) are genetically hearing impaired at young ages, including DBA/2J [19] and Black Swiss [20] strains, as well as the Frings and BUB/BnJ strains. Audiogenic seizures require activation of brain-stem auditory pathways and are primarily initiated through the midbrain inferior colliculus [18]. Consistent with results obtained from other rodent strains, Frings mice display significant neuronal hyperresponsiveness in the inferior colliculus [21]. Early onset hearing impairment may thus be the primary effect of the Mass1frings mutation and audiogenic seizure susceptibility a secondary effect of disturbed auditory development.
The Mass1frings mutation is the cause of audiogenic seizures in both Frings and BUB/BnJ mice, but Frings mice retain their seizure susceptibility well into adulthood, whereas BUB/BnJ mice do not exhibit seizures beyond 25 days of age [6,7]. The “modifier effect” of the Cdh23 dimorphism can explain this difference. Mice homozygous for the Mass1frings mutation but with the Cdh23+ resistance allele (like Frings mice) retain sufficient hearing even at old ages to elicit audiogenic responses, whereas mice homozygous for both Mass1frings and Cdh23ahl (like BUB/BnJ mice) exhibit a rapid hearing loss and thus may fail to respond to audiogenic stimuli by 25 days of age. An alternative explanation for the modifying effect of Cdh23 on seizure phenotype may relate to its expression in the brain. A function of cadherin 23 in the brain stem is consistent with ultrastructural changes observed in the posterio- and anteroventral cochlear nucleus in strains with age-related hearing loss [22,23].
In conclusion, we identified the Mass1frings allele as the cause of early onset hearing loss in BUB/BnJ mice and showed an association of this hearing loss with immature stereocilia hair bundle formation. The additive age-related effect of cdh23ahl modulates progression of hearing loss and likely underlies the age-related cessation of audiogenic seizure susceptibility in BUB/BnJ mice. BUB/BnJ and Frings mice provide models to study the molecular mechanism and cellular function of the Mass1 gene and may give clues to the cochlear pathology underlying Usher syndrome type IIc.
Materials and methods
Mice and linkage crosses
To map loci associated with the hearing loss of BUB/BnJ mice, male and female mice from the CAST/EiJ and MOLD/RkJ inbred strains were first mated with BUB/BnJ mice. CAST/EiJ is an inbred strain derived from a wild population of Mus musculus castaneus, and MOLD/RkJ is an inbred strain derived from a wild population of M. m. molossinus. The F1 hybrids (both males and females) produced from these matings were then backcrossed to BUB/BnJ mice to produce N2 generation progeny for linkage analysis. All of the inbred strain mice, F1 hybrids, and backcross mice used in these studies were reared and tested under modified barrier conditions in the Mouse Mutant Resource at The Jackson Laboratory (TJL). The Animal Care and Use Committees of TJL, the Howard Hughes Medical Institute, and the National Institutes of Health (ASP 997/01 and 1139-03) approved the care and use of the animals reported in this study.
ABR threshold measurements
The inbred strains, F1 hybrids, and backcross mice were assessed for hearing by ABR thresholds as previously described [1]. Briefly, mice are anesthetized and body temperature is maintained at 37–38°C by placing them on an isothermal pad in a sound-attenuating chamber. Subdermal needle electrodes are inserted at the vertex and ventrolaterally to each ear. Stimulus presentation, ABR acquisition, equipment control, and data management are coordinated using a computerized system (Intelligent Hearing Systems, Miami, FL, USA). High-frequency transducers generate specific acoustic stimuli— broad-band clicks and 8-, 16-, and 32-kHz pure tone bursts— that are channeled into the animal’s ear canals. The amplified brain-stem responses are averaged and displayed on a computer screen. Auditory thresholds are obtained for each stimulus by varying the SPL at 5-dB steps to identify the lowest level at which an ABR pattern can be recognized.
Linkage analysis
ABR thresholds for click, 8-kHz, 16-kHz, and 32-kHz stimuli were treated as quantitative traits to map loci affecting AHL in populations of backcross mice, as previously described [3]. Microsatellite linkage markers were typed by standard PCR methods from DNA obtained from tail tips of backcross mice. PCRs were carried out for 30 cycles and products were separated on 3% agarose gels (Metaphor; FMC BioProducts, Rockland, ME, USA) and visualized by ethidium bromide staining. QTL linkage analyses were performed using the computer program Map Manager QTX [14]. This program uses a fast regression method to detect and localize quantitative trait loci within intervals defined by genetic markers. The QTX program also performs a pair-wise locus analysis to search for QTL interactive effects.
Seizure susceptibility and hearing assessment in BUB/BnJ Frings hybrids
(BUB/BnJ × Frings) F1 and F2 hybrid animals were tested for audiogenic seizure susceptibility as previously described [6]. Mice were exposed to a 110-dB, 11-kHz stimulus and scored for seizure susceptibility at 19 and 42 days of age. Hearing thresholds were assessed in F2 mice by ABR with 10-kHz click and 16-kHz pure tone stimuli before (19 days of age) and after (42 days of age) seizure-susceptible F2 mice were expected to lose seizure susceptibility.
Identification of the 753A and 753G variants of Cdh23
PCR primers that flank the variant exon 7 region of Cdh23 [4] were designed: forward 5′-ATCATCACGGACATGCAAGA and reverse 5′-AGCTACCAGGAACAGCTTGG. These primers were used with genomic DNA from BUB/BnJ, SWR/BmJ, and Frings mice to produce 315-bp PCR products. The PCR products were then purified with the QIAquick PCR Purification Kit (Qiagen, Inc., Valencia, CA, USA) and sequenced using an Applied Biosystems 373A DNA sequencer and an optimized DyeDeoxy Terminator cycle sequencing method. The same primers used for PCR amplification were also used for cycle sequencing.
Scanning electron microscopy
Inner ears were dissected in Leibovitz’s medium and fixed for 2 h at room temperature in 4% glutaraldehyde, 3 mM CaCl2, 0.1 M Hepes, pH 7.4. Temporal bone, stria vascularis, Reissner’s membrane, and tectorial membrane were removed to expose the sensory epithelium. Samples were washed in PBS, postfixed in 1% OsO4 for 30 min, dehydrated through a graded series of ethanol, critical point dried against liquid CO2, sputter coated with platinum, and imaged in a field emission scanning electron microscope (Hitachi, S-4500). Images were captured using Quartz PCI software.
Microscopic analysis of cochlear cross sections
Inner ears from mutant and control mice were dissected, perfused with Bouin’s fixative, immersed in same for 24–48 h, decalcified with Cal-EX solution for 6 h, and embedded in paraffin. Sections (7 μm) were cut, mounted on glass slides, and counterstained in hematoxylin/eosin.
Acknowledgments
We thank Larry Erway, University of Cincinnati, for his help in initiating studies at The Jackson Laboratory (TJL) to assess hearing loss in inbred strains of mice. The (MOLD/RkJ × BUB/BnJ) × BUB/BnJ backcross mice were kindly provided by Bo Chang and Norm Hawes of TJL’s Eye Research Program. We thank Matt Neddo and Gavin P. Riordan for technical assistance and Agnieszka Rzadzinska and Bechara Kachar for help with SEM. We thank Bo Chang and Verity Letts for their careful review of the manuscript. This work is supported by grants from the National Institutes of Health: DC005827 (NIDCD), RR01183 (NCRR), and CA34196 (NCI). L.P. is an Investigator of the Howard Hughes Medical Institute. M.D.W. is supported by a grant from the Deafness Research Foundation.
References
- [1].Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear. Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear. Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- [3].Johnson KR, Zheng QY, Erway LC. A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics. 2000;70:171–180. doi: 10.1006/geno.2000.6377. [DOI] [PubMed] [Google Scholar]
- [4].Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat. Genet. 2003;35:21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Frings H, Frings M. The production of stocks of albino mice with predictable susceptibilities to audiogenic seizures. Behaviour. 1953;5:305–319. doi: 10.1126/science.117.3037.283. [DOI] [PubMed] [Google Scholar]
- [6].Skradski SL, White HS, Ptacek LJ. Genetic mapping of a locus (mass1) causing audiogenic seizures in mice. Genomics. 1998;49:188–192. doi: 10.1006/geno.1998.5229. [DOI] [PubMed] [Google Scholar]
- [7].Skradski SL, Clark AM, Jiang H, White HS, Fu YH, Ptacek LJ. A novel gene causing a Mendelian audiogenic mouse epilepsy. Neuron. 2001;31:537–544. doi: 10.1016/s0896-6273(01)00397-x. [DOI] [PubMed] [Google Scholar]
- [8].Nikkila H, McMillan DR, Nunez BS, Pascoe L, Curnow KM, White PC. Sequence similarities between a novel putative G proteincoupled receptor and Na+/Ca2+ exchangers define a cation binding domain. Mol. Endocrinol. 2000;14:1351–1364. doi: 10.1210/mend.14.9.0511. [DOI] [PubMed] [Google Scholar]
- [9].McMillan DR, Kayes-Wandover KM, Richardson JA, White PC. Very large G protein-coupled receptor-1, the largest known cell surface protein, is highly expressed in the developing central nervous system. J. Biol. Chem. 2002;277:785–792. doi: 10.1074/jbc.M108929200. [DOI] [PubMed] [Google Scholar]
- [10].McMillan DR, White PC. Loss of the transmembrane and cytoplasmic domains of the very large G-protein-coupled receptor-1 (VLGR1 or Mass1) causes audiogenic seizures in mice. Mol. Cell. Neurosci. 2004;26:322–329. doi: 10.1016/j.mcn.2004.02.005. [DOI] [PubMed] [Google Scholar]
- [11].Yagi H, Takamura Y, Yoneda T, Konno D, Akagi Y, Yoshida K, Sato M. Vlgr1 knockout mice show audiogenic seizure susceptibility. J. Neurochem. 2005;92:191–202. doi: 10.1111/j.1471-4159.2004.02875.x. [DOI] [PubMed] [Google Scholar]
- [12].Weston MD, Luijendijk MW, Humphrey KD, Moller C, Kimberling WJ. Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of Usher syndrome type II. Am. J. Hum. Genet. 2004;74:357–366. doi: 10.1086/381685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Atchley WR, Fitch W. Genetic affinities of inbred mouse strains of uncertain origin. Mol. Biol. Evol. 1993;10:1150–1169. doi: 10.1093/oxfordjournals.molbev.a040070. [DOI] [PubMed] [Google Scholar]
- [14].Manly KF, Cudmore RH, Jr., Meer JM. Map Manager QTX, crossplatform software for genetic mapping. Mamm. Genome. 2001;12:930–932. doi: 10.1007/s00335-001-1016-3. [DOI] [PubMed] [Google Scholar]
- [15].Ohlemiller KK, Gagnon PM. Cellular correlates of progressive hearing loss in 129S6/SvEv mice. J. Comp. Neurol. 2004;469:377–390. doi: 10.1002/cne.11011. [DOI] [PubMed] [Google Scholar]
- [16].Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. J. Acoust. Soc. Am. 1997;101:3546–3553. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- [17].Garfinkle TJ, Saunders JC. Morphology of inner hair cell stereocilia in C57BL/6J mice as studied by scanning electron microscopy, Otolaryngol. Head Neck Surg. 1983;91:421–426. doi: 10.1177/019459988309100415. [DOI] [PubMed] [Google Scholar]
- [18].Ross KC, Coleman JR. Developmental and genetic audiogenic seizure models: behavior and biological substrates. Neurosci. Biobehav. Rev. 2000;24:639–653. doi: 10.1016/s0149-7634(00)00029-4. [DOI] [PubMed] [Google Scholar]
- [19].Neumann PE, Seyfried TN. Mapping of two genes that influence susceptibility to audiogenic seizures in crosses of C57BL/6J and DBA/2J mice. Behav. Genet. 1990;20:307–323. doi: 10.1007/BF01067798. [DOI] [PubMed] [Google Scholar]
- [20].Misawa H, Sherr EH, Lee DJ, Chetkovich DM, Tan A, Schreiner CE, Bredt DS. Identification of a monogenic locus (jams1) causing juvenile audiogenic seizures in mice. J. Neurosci. 2002;22:10088–10093. doi: 10.1523/JNEUROSCI.22-23-10088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Klein BD, Fu HY, Ptacek LJ, White HS. c-Fos immunohistochemical mapping of the audiogenic seizure network and tonotopic neuronal hyperexcitability in the inferior colliculus of the Frings mouse. Epilepsy Res. 2004;64:13–25. doi: 10.1016/j.eplepsyres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- [22].Willott JF, Bross LS. Morphological changes in the anteroventral cochlear nucleus that accompany sensorineural hearing loss in DBA/2J and C57BL/6J mice. Brain Res. Dev. Brain Res. 1996;91:218–226. doi: 10.1016/0165-3806(95)00188-3. [DOI] [PubMed] [Google Scholar]
- [23].Schwartz IR, Keh A, Hsu G. Morphology, GluR1 and GRIP-C localization differ in octopus cells of C57BL6 and B6Cast mice. Hear. Res. 2002;171:1–12. doi: 10.1016/s0378-5955(01)00396-3. [DOI] [PubMed] [Google Scholar]