Abstract
The common occurrence of hearing loss in both humans and mice, and the anatomical and functional similarities of their inner ears, attest to the potential of mice being used as models to study inherited hearing loss. A large-scale, auditory screening project is being undertaken at The Jackson Laboratory (TJL) to identify mice with inherited hearing disorders. To assess hearing sensitivity, at least five mice from each inbred strain had auditory brainstem response (ABR) thresholds determined. Thus far, we have screened 80 inbred strains of mice; 60 of them exhibited homogeneous ABR threshold values not significantly different from those of the control strain CBA/CaJ. This large database establishes a reliable reference for normal hearing mouse strains. The following 16 inbred strains exhibited significantly elevated ABR thresholds before the age of 3 months: 129/J, 129/ReJ, 129/SvJ, A/J, ALR/LtJ, ALS/LtJ, BUB/BnJ, C57BLKS/J, C57BR/cdJ, C57L/J, DBA/2J, I/LnJ, MA/MyJ, NOD/LtJ, NOR/LtJ, and SKH2/J. These hearing impaired strains may serve as models for some forms of human non-syndromic hearing loss and aid in the identification of the underlying genes.
Keywords: Hearing loss, Non-syndromic, Mouse, Inbred strain, Gene, Deafness model
1. Introduction
Genetic impairment of hearing affects about one of every 2000 children (Morton, 1991). About 70% of all genetic forms of human deafness appear to be non-syndromic and about 80% of those are expressed as recessive genes. Genes responsible for non-syndromic hearing impairment (NSHI) are difficult to identify because of their extreme genetic heterogeneity and absence of clinical criteria allowing for their differentiation (Petit, 1996). During the last decade, analyses of NSHI among small, isolated populations, together with current genetic mapping techniques, have resulted in the identification of more than 45 genetically distinct forms of NSHI; however, only eight underlying genes have been cloned (Van Camp and Smith, 1998).
The mouse is an excellent animal model for the study of human genetic deafness (Steel and Brown, 1996). The mouse cochlea is anatomically similar to that of humans (Steel and Bock, 1983), and hereditary abnormalities of the inner ear have been shown to be similar in both humans and mice (Brown and Steel, 1994). Most importantly, the homologies between the mouse and human genomes are well established. Defined regions of chromosomes are highly conserved for gene content and gene linkages between these two mammalian species. There are nearly 100 naturally occurring mouse mutations with hearing impairment that may serve as models for human deafness (Lyon et al., 1996; Steel, 1995). Genetic crosses of mice can produce thousands of progeny for mapping and positionally cloning spontaneous mutations. Genetically engineered mice with targeted gene disruptions can be used to examine and validate genes that may be candidates for any of the human deafness genes.
The genetic analysis of mouse deafness mutations already has proven instrumental in the identification of four human NSHI genes. Mice homozygous for the shaker-1 mutation (sh1) are characterized by hyperactivity, head-shaking and circling behaviors and also are deaf due to a neuroepithelial defect in the cochlea. sh1 was shown by positional cloning to be a mutation of the Myo7a gene, which encodes an unconventional myosin-type protein (Gibson et al., 1995). Subsequently, the homologous MYO7A gene in humans was shown to be responsible for both dominant (DFNA11) and recessive (DFNB2) forms of NSHI (Liu et al., 1997a,b), as well as for Usher syndrome type 1B (Weil et al., 1995). Mice homozygous for the shaker-2 mutation (sh2) have the same phenotype as sh1 mice. sh2 recently was shown to be a mutation of the Myo15 gene (Probst et al., 1998) which encodes another unconventional myosin-type protein. Concurrently, the homologous MYO15 gene in humans was shown to be responsible for a recessive form of NSHI, DFNB3 (Wang et al., 1998). A targeted inactivation of the Pou4f3 gene in the mouse causes severe deafness (Erkman et al., 1996). The chromosomal localization of the homologous human POU4F3 gene near a dominant form of NSHI (DFNA15) ultimately led to its identification as the responsible gene (Vahava et al., 1998). Additional mouse hearing-related genes will likely be found that help to identify genes for other forms of human deafness.
The deafness caused by most mouse mutations is congenital and usually associated with other phenotypic effects. To our knowledge, only two mouse mutations, deafness (dn) (Keats et al., 1995) and deaf (Vdf ) (Deol, 1956), have been reported that are not associated with balance or with other non-auditory defects. In contrast to the congenital, syndromic deafness caused by most mouse mutations, certain inbred strains of mice exhibit a progressive, non-syndromic hearing loss, with onset at more advanced ages. These strains have provided useful models for human age-related hearing loss (AHL), or presbycusis. Several inbred strains of mice have been shown to exhibit differing severity and onset of AHL (Henry, 1982; Willott, 1983). The genetic nature of AHL in the few inbred strains examined so far has been attributed to 1–3 major genes per strain (Erway et al., 1993); one of these genes has been mapped to chromosome 10 (Johnson et al., 1997). The combined effects of multiple AHL genes may contribute to the earlier onset and greater severity of hearing impairment observed in some strains. It is not known how many different AHL genes are present collectively in existing mouse strains. Some of the genes contributing to AHL may also exacerbate the effects of single gene mutations causing hearing impairment, as may be the case for a strain-specific modifier (mdfw) of the deaf waddler mutations (Noben-Trauth et al., 1997).
To begin to identify genes that contribute to hearing loss in both inbred and mutant strains of mice, a largescale, auditory screening project has been undertaken at The Jackson Laboratory (TJL). This project, supported by the Intramural Research Program of the National Institute on Deafness and Other Communication Disorders (NIDCD), was specifically designed to provide genetic models for non-syndromic forms of human deafness. More than three million mice are produced annually at TJL. These mice belong to nearly 1700 distinct inbred strains, including standard inbred strains, recombinant inbred strains, congenic inbred strains, and inbred strains carrying both spontaneous and induced mutations. Most of these inbred strains have not been tested for hearing ability prior to this screening effort.
Here, we report auditory brainstem response (ABR) thresholds for 80 inbred strains of mice and discuss the implications of these measurements in terms of establishing a reference for hearing ability of commonly used inbred strains of mice, identifying strains with significant hearing impairment, and defining the characteristic attributes of the impairment observed in these strains. This is the first publication resulting from this screening program, including results for 61 inbred strains of mice with normal hearing and 19 inbred strains or substrains with hearing impairment before 3 months of age. Genetic crosses are in progress to determine inheritance patterns for the hearing loss observed in some of these inbred strains and to map the responsible genes.
2. Materials and methods
2.1. Mice and animal care
All mice used in this study were produced within the production or research facilities of TJL. Prior to electrode placement, animals were anesthetized by intraperitoneal injection with Avertin (tribromoethanol stabilized in tertiary amyl hydrate) given at a dose of 5 mg tribromoethanol/10 g body weight. Body temperature was maintained at 37–38°C by placing anesthetized mice on an isothermal pad (Deltaphase, model 39dp, Braintree Scientific Inc., Massachusetts). The care and use of the animals described in this study were approved by the Animal Care and Use Committee of TJL (Grant 3 N01 DC62108). TJL is fully accredited by the Accreditation of Laboratory Animal Care.
2.2. Recording chamber, sound system, and acoustic stimuli
ABR testing was conducted in a sound-attenuating chamber (inner dimensions: 50×60×85 cm3, Acoustic Systems, Austin, TX). The stimulus presentation, ABR acquisition, equipment control and data management were coordinated using the computerized Intelligent Hearing Systems (IHS, Miami, FL) with the Smart-EP 10 version software. A pair of high frequency transducers (Mike Ravicz, Somerville, MA) was coupled with the IHS system to generate specific acoustic stimuli. The output from the high frequency transducers was channeled through plastic tubes (diameter: 3 mm, 10 mm long) into the animal’s ear canals.
Acoustic stimuli were calibrated by the IHS Corp., with a Bruel and Kjaer Nexus conditioning amplifier, Type 2690, and 1/4 inch calibrated condenser microphone, Type 4136, connected to a microphone preamplifier, Type 2670. A Bruel and Kjaer 2 cc coupler, Type DB0138, was used to standardize acoustic conditions between the transducer output tubes and the microphone and to approximate the actual stimulus delivered to the mouse ear. Calibrations were made with reference to the programmed output for 70 dB sound pressure levels (SPL re: 20 WPa). These calibrations were made using an external filter with the measuring amplifier, with 20 Hz–100 kHz settings.
Clicks, and 8, 16, and 32 kHz tone bursts were respectively routinely presented to mice. Waveforms for click and tone bursts were digitally created. A half cosine square window was used to create tone burst signals with a 3 ms duration (1.5 ms rise-fall with no plateau). Click stimuli were calibrated with the ‘impulse’ function and tone pips with ‘RMS fast’ function of the measuring amplifier. The click had substantial energy in the 2–8 kHz range (e.g., filtering frequencies above and below this decreased the SPL by less than 5 dB).
Samples of CBA/CaJ mice were tested periodically as references for normal hearing, and for monitoring the reliability of the equipment and testing procedures.
2.3. ABR procedure and establishment of ABR thresholds
One channel of ABR was recorded after binaural stimulation. Sub-dermal needles were used as electrodes (Model F-E2, Astro-Med Inc., Rhode Island). The active electrode was inserted at the vertex, the reference electrode ventrolateral to the left ear and the ground electrode ventrolateral to the right ear. Biological signals were bandpass filtered below 100 Hz and above 3000 Hz and amplified 200000 times; an A/D sampling rate of 25 kHz was employed. Artifact rejection level was set at 31.00 μV. The amplified responses were averaged by a computer and displayed on the computer screen. Analysis time was 10 ms.
Auditory thresholds were obtained for each stimulus by reducing the SPL at 10 dB steps and finally at 5 dB steps up and down to identify the lowest level at which an ABR could be recognized. This was done by comparing the ABR patterns with two or three suprathreshold ABRs displayed successively on the screen. The ABRs were typically identified with 128–512 stimuli presented at the rate of 19.1/s; low amplitude responses required more stimulus presentations to verify an ABR pattern just above its threshold.
The first 1000 mice were tested for ABR threshold differences between the left and right ears, but no consistent left-right asymmetries were found in any inbred strain. Thereafter, to increase the efficiency of screening, we tested ABR binaurally rather than testing each ear separately. The majority of human inherited hearing impairments are likewise bilaterally symmetrical (Martini et al., 1997) and little would be gained by testing ears separately. In fact, binaural stimulation is now being used for infant hearing screening with ABR (Hall and Rupp, 1997). For particular types of mouse mutations with melanocyte abnormalities causing chimeric coat color patterns (such as white spotting), ears should be individually examined because melanocytes also form an integral part of the stria vascularis (Steel, 1995).
2.4. Records and statistical analyses
When possible, a minimum of five age-matched mice were tested at least once approximately at 12 weeks of age. In some cases, fewer mice were tested because of their limited availability; more mice from these strains will be tested when they become available. All established ABR thresholds were recorded in a log; individual records were made for each mouse tested with each of the four stimuli. The log also included the strain, genotype, birth and test dates for each mouse tested. These records were subsequently entered and stored in an electronic database. All data were analyzed for group arithmetic means and standard deviations. T-tests were used for comparing means of ABR thresholds.
In addition to threshold values, a series of ABR waveforms also was obtained for the click stimulus and digitally stored on disk at the threshold (T), and at T+10, T+20 and T+30 dB. These extensive ABR data files are available for future analyses of intensitylatency functions among the ABR peaks of all the strains of mice tested.
3. Results
3.1. Inbred strains of mice with normal ABR thresholds
As a reference for normal hearing, we repeatedly tested CBA/CaJ mice at four ages from 9 to 39 weeks (Fig. 1, Table 1). The mean thresholds for CBA/CaJ mice were 36, 24, 15, and 39 dB SPL for the click, 8 kHz, 16 kHz, and 32 kHz stimuli, respectively. We then defined as hearing impaired those inbred strains that exhibited average ABR thresholds at least 15 dB SPL above these mean values, for any of the four acoustic stimuli at any age of testing. An increase of 15 dB is at least three standard deviations above the CBA/CaJ mean values for all four test stimuli (Table 1) and is, therefore, a conservative criterion for significance. Thus, we considered any strain with average ABR threshold values above 55 (for click stimulus), 40 (for 8 kHz), 35 (for 16 kHz), or 60 (for 32 kHz) dB SPL to be hearing impaired. Strains with average ABR values below these values for all four test stimuli were considered normal hearing.
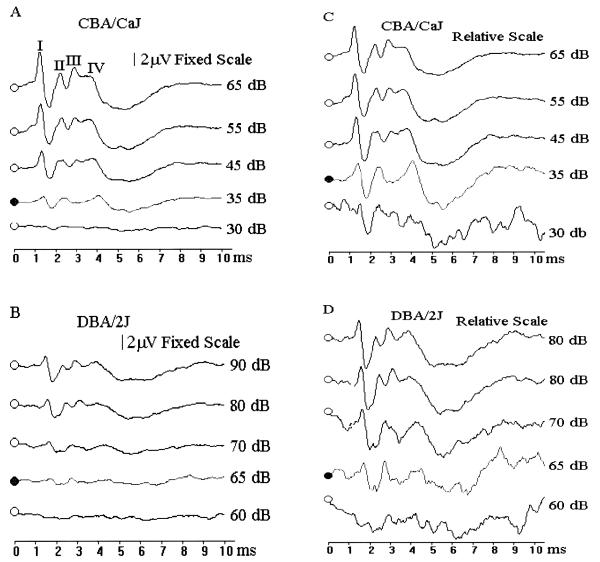
Fig. 1.
Determination of ABR thresholds. Normal ABR patterns for a 32 week old CBA/CaJ mouse (A) induced by click stimuli of 65, 55, 45, 35 and 30 dB SPL. The ABR threshold for this mouse was estimated to be 35 dB SPL. (There are usually 4 or 5 response peaks, labeled I, II, II, and IV respectively.) ABR patterns for a 5 week old DBA/2J mouse (B) induced by click stimuli of 90, 80, 70, 65 and 60dB SPL. The ABR threshold for this mouse was estimated to be 65 dB SPL. Response amplitudes for all four stimuli are shown on the same fixed scale in A and B to better compare the amplitude differences between normal and impaired mice. For routine threshold determinations, ABR were displayed in a relative scale adjusted to maximize the wave pattern for each stimulus as shown in C and D, because these wave patterns were more easily recognized thans when displayed in a fixed scale.
Table 1.
ABR thresholds (dB SPL), means (shown in boldface) and associated standard deviations (S.D.) of standard strain CBA/CaJ
| Strain | Number | Age (weeks) | Clicks |
8 kHz |
16 kHz |
32 kHz |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| mean | S.D. | mean | S.D. | mean | S.D. | mean | S.D. | |||
| CBA/CaJ | 8 | 9 | 34 | 3 | 230 | 3 | 15 | 3 | 40 | 7 |
| CBA/CaJ | 6 | 13 | 38 | 4 | 28 | 5 | 19 | 6 | 42 | 4 |
| CBA/CaJ | 5 | 30 | 37 | 3 | 26 | 4 | 15 | 0 | 41 | 4 |
| CBA/CaJ | 8 | 39 | 37 | 3 | 28 | 4 | 14 | 2 | 39 | 4 |
| CBA/CaJ | 6 | 47 | 33 | 3 | 17 | 3 | 12 | 3 | 33 | 3 |
| Total number tested | 33 | |||||||||
| Grand means/S.D.s for all ages | 36 | 3 | 24 | 5 | 15 | 4 | 39 | 5 | ||
Inbred strains with normal hearing are arranged alphabetically by their strain designations in Table 2. The mice tested were composed of two major age groups, one about 4–12 weeks of age, the other about 25–33 weeks of age. If the old retired breeders (25–33 weeks of age) did not exhibit elevated ABR thresholds, we assumed that they had normal hearing at younger ages. Mice from some strains were tested at more than one age. Arithmetic means and standard deviations were computed for each strain/age group.
Table 2.
Inbred mouse strains with normal ABR thresholds (dB SPL). A total of 430 mice from 60 strains or substrains were tested
| Strain | Number | Age (weeks) | Clicks |
8 kHz |
16 kHz |
32 kHz |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| mean | S.D. | mean | S.D. | mean | S.D. | mean | S.D. | |||
| 129/Sv−+p+Tyr–c+Mgf–SIJ/J | 6 | 13 | 43 | 10 | 33 | 9 | 14 | 8 | 52 | 10 |
| *129/SvEMS−+Ter?/J | 5 | 8 | 41 | 4 | 32 | 4 | 15 | 5 | 42 | 4 |
| A/HeJ | 5 | 27 | 40 | 4 | 30 | 5 | 21 | 4 | 45 | 5 |
| *A/WySnJ | 5 | 8 | 36 | 2 | 25 | 0 | 14 | 4 | 38 | 3 |
| AKR/J | 5 | 26 | 42 | 3 | 32 | 3 | 21 | 4 | 49 | 2 |
| BALB/cByJ | 6 | 34 | 41 | 5 | 33 | 4 | 23 | 5 | 48 | 6 |
| BALB/cJ | 6 | 34 | 44 | 6 | 39 | 4 | 28 | 5 | 53 | 7 |
| BDP/J | 5 | 14 | 35 | 0 | 24 | 2 | 11 | 2 | 45 | 4 |
| BXSB/MpJ | 5 | 7 | 37 | 3 | 24 | 4 | 18 | 4 | 40 | 4 |
| BXSB/MpJ | 4 | 34 | 40 | 0 | 36 | 3 | 18 | 3 | 48 | 5 |
| C3H/HeJ | 6 | 21 | 37 | 4 | 24 | 7 | 18 | 6 | 43 | 8 |
| C3H/HeOuJ | 5 | 10 | 35 | 0 | 26 | 2 | 16 | 2 | 41 | 2 |
| C3H/HeOuJ | 5 | 41 | 46 | 2 | 43 | 3 | 26 | 5 | 55 | 4 |
| *C3H/HeSnJ | 5 | 9 | 33 | 3 | 24 | 4 | 16 | 5 | 41 | 5 |
| C3HeB/FeJ | 5 | 32 | 37 | 3 | 27 | 3 | 17 | 3 | 42 | 3 |
| C57BL/10J | 5 | 32 | 37 | 3 | 28 | 3 | 19 | 4 | 41 | 2 |
| C57BL/10SnJ | 5 | 32 | 43 | 7 | 33 | 8 | 22 | 8 | 45 | 9 |
| C57BL/6ByJ | 5 | 7 | 33 | 3 | 24 | 2 | 14 | 4 | 40 | 4 |
| *C57BL/6J | 6 | 27 | 43 | 3 | 33 | 3 | 21 | 2 | 55 | 7 |
| *C57BL/6J | 6 | 33 | 39 | 4 | 33 | 6 | 17 | 3 | 45 | 8 |
| *C58/J | 5 | 9 | 40 | 0 | 31 | 2 | 22 | 6 | 49 | 4 |
| CASA/RK | 4 | 41 | 36 | 3 | 26 | 3 | 15 | 0 | 41 | 3 |
| CAST/Ei | 6 | 22 | 30 | 0 | 18 | 4 | 9 | 2 | 32 | 3 |
| CAST/Ei | 2 | 61 | 37 | 4 | 20 | 0 | 10 | 0 | 35 | 0 |
| CBA/J | 5 | 28 | 37 | 3 | 31 | 7 | 16 | 4 | 45 | 5 |
| *CE/J | 6 | 12 | 41 | 4 | 29 | 4 | 20 | 3 | 45 | 3 |
| CZECH II/Ei | 4 | 16 | 35 | 0 | 29 | 8 | 16 | 6 | 40 | 7 |
| *DBA/1J | 5 | 7 | 42 | 3 | 30 | 6 | 21 | 7 | 49 | 4 |
| *DBA/1J | 5 | 12 | 45 | 5 | 38 | 8 | 23 | 8 | 57 | 4 |
| *DBA/1LacJ | 5 | 9 | 39 | 2 | 33 | 7 | 18 | 7 | 49 | 7 |
| DBA/2HaSmn | 7 | 16 | 44 | 16 | 36 | 17 | 22 | 13 | 53 | 13 |
| FVB/NJ | 5 | 9 | 33 | 3 | 25 | 4 | 17 | 3 | 37 | 3 |
| FVB/NJ | 6 | 28 | 38 | 3 | 29 | 2 | 15 | 0 | 43 | 3 |
| HRS/J hrl+ | 10 | 26 | 42 | 5 | 35 | 4 | 26 | 5 | 54 | 7 |
| *KK/H1J | 5 | 8 | 38 | 3 | 28 | 4 | 21 | 4 | 46 | 5 |
| *LG/J | 5 | 8 | 37 | 3 | 30 | 4 | 20 | 4 | 44 | 4 |
| *LG/J | 2 | 22 | 40 | 0 | 28 | 4 | 20 | 0 | 40 | 0 |
| *LP/J | 10 | 11 | 48 | 5 | 30 | 6 | 22 | 9 | 54 | 7 |
| MOLD/Rk | 10 | 37 | 37 | 4 | 30 | 8 | 16 | 5 | 48 | 19 |
| MOLF/Ei | 4 | 28 | 35 | 4 | 28 | 9 | 13 | 3 | 38 | 5 |
| MOLG/Dn | 5 | 31 | 35 | 4 | 23 | 4 | 14 | 2 | 36 | 4 |
| *MRL/MpJ | 5 | 9 | 36 | 2 | 25 | 4 | 19 | 2 | 43 | 3 |
| NON/LtJ | 5 | 8 | 34 | 2 | 25 | 4 | 13 | 3 | 35 | 4 |
| NON/LtJ | 11 | 29 | 36 | 2 | 29 | 4 | 15 | 3 | 40 | 3 |
| NZB/B1NJ | 5 | 9 | 34 | 2 | 23 | 3 | 15 | 4 | 36 | 4 |
| NZB/B1NJ | 5 | 18 | 35 | 4 | 26 | 4 | 14 | 2 | 38 | 3 |
| NZO/NIJ | 4 | 22 | 35 | 0 | 26 | 3 | 16 | 3 | 41 | 3 |
| NZW/LacJ | 5 | 8 | 35 | 0 | 27 | 3 | 18 | 3 | 41 | 2 |
| *P/J | 8 | 10 | 41 | 7 | 30 | 12 | 17 | 5 | 46 | 9 |
| *P/J | 4 | 16 | 41 | 8 | 33 | 5 | 26 | 16 | 48 | 5 |
| PERA/camEi | 7 | 45 | 33 | 3 | 25 | 4 | 10 | 0 | 35 | 0 |
| PERC/Ei | 9 | 14 | 34 | 5 | 25 | 8 | 15 | 10 | 39 | 8 |
| PL/J | 10 | 13 | 41 | 5 | 35 | 7 | 24 | 8 | 47 | 8 |
| PL/J | 6 | 30 | 39 | 2 | 37 | 3 | 16 | 2 | 53 | 3 |
| RBA/Dn | 2 | 24 | 35 | 0 | 25 | 0 | 13 | 4 | 43 | 4 |
| RBF/DnJ | 6 | 27 | 33 | 3 | 23 | 4 | 13 | 3 | 40 | 4 |
| RF/J | 5 | 8 | 36 | 2 | 24 | 2 | 15 | 0 | 38 | 3 |
| RHJ/Le hrrh–J/+ | 5 | 8 | 39 | 4 | 31 | 4 | 22 | 9 | 51 | 13 |
| RIIIS/J | 5 | 8 | 35 | 4 | 26 | 2 | 16 | 2 | 36 | 2 |
| SEC/1ReJ | 5 | 8 | 36 | 2 | 26 | 2 | 19 | 2 | 42 | 3 |
| *SENCARA/PtJ | 6 | 8 | 40 | 0 | 33 | 3 | 23 | 5 | 53 | 5 |
| *SENCARB/PtJ | 6 | 7 | 36 | 2 | 28 | 6 | 22 | 24 | 45 | 3 |
| SENCARC/PtJ | 6 | 7 | 35 | 0 | 23 | 3 | 12 | 3 | 41 | 4 |
| SENCARC/PtJ | 5 | 43 | 43 | 3 | 41 | 11 | 32 | 20 | 59 | 10 |
| SF/CamEi | 4 | 32 | 39 | 3 | 30 | 4 | 14 | 3 | 43 | 3 |
| SHR/GnEi | 5 | 22 | 38 | 3 | 26 | 4 | 16 | 4 | 45 | 4 |
| SJL/J | 6 | 7 | 34 | 2 | 26 | 4 | 13 | 3 | 38 | 5 |
| SJL/J | 6 | 49 | 38 | 4 | 32 | 6 | 17 | 4 | 46 | 10 |
| SM/J | 5 | 9 | 36 | 2 | 27 | 4 | 17 | 3 | 41 | 4 |
| SM/J | 6 | 58 | 37 | 3 | 28 | 4 | 20 | 10 | 40 | 8 |
| SPRET/Ei | 5 | 19 | 33 | 3 | 18 | 3 | 10 | 0 | 33 | 3 |
| ST/bJ | 5 | 9 | 35 | 0 | 25 | 5 | 12 | 3 | 38 | 3 |
| ST/bJ | 6 | 30 | 35 | 0 | 21 | 2 | 13 | 3 | 37 | 3 |
| SWR/J | 6 | 16 | 38 | 4 | 29 | 4 | 12 | 3 | 43 | 7 |
| SWR/J | 5 | 27 | 45 | 8 | 38 | 3 | 19 | 4 | 42 | 8 |
| *YBR/Ei | 8 | 14 | 40 | 5 | 34 | 8 | 19 | 4 | 43 | 3 |
| *YBR/Ei | 6 | 52 | 43 | 9 | 39 | 6 | 21 | 13 | 46 | 11 |
| Total number of mice tested | 430 | |||||||||
| Grand means/S.D.s for all mice | 38 | 3 | 29 | 3 | 18 | 4 | 44 | 3 | ||
Strains that exhibit hearing loss when tested at older ages (see Table 3b).
In Table 2, we report results for 430 normal hearing mice from 60 different inbred strains, thus establishing a reliable reference for normal ABR thresholds. Despite the extensive genetic variability among inbred strains, as well as the variability in ages (7–39 weeks) of the mice tested, the grand means and standard deviations (in dB SPL) of the normal hearing mice were remarkably uniform for each tested stimulus (Table 2, bottom line): click (38 ± 2.7) and tone bursts of 8 kHz (29 ± 3.4), 16 kHz (18 ± 4.2), and 32 kHz (44 ± 3.2). These grand means were very similar to those obtained from the control CBA/CaJ strain (Table 1) and may serve as references for comparing other strains of mice judged to exhibit a significant elevation of ABR thresholds.
To be certain that the grand means did not obscure some age-related differences, the normal hearing mice were divided into two groups according to their age when tested: 196 were tested at 7–14 weeks of age, and 223 were tested at 16–39 weeks of age (Table 2). No significant differences in ABR thresholds were observed between these two different age groups (P > 0.05). For the mice tested at 7–14 weeks, the mean ABR thresholds were 38 (click), 28 (8 kHz), 17 (16 kHz) and 43 (32 kHz) dB SPL; for the mice tested at 16–39 weeks, the average values were 39, 30, 18, and 44, respectively. Likewise, no significant gender differences in thresholds were observed between female and male mice (P > 0.05). For female mice, the mean ABR thresholds were 38 (click), 29 (8 kHz), 18 (16 kHz), and 44 (32 kHz) dB SPL; for male mice, the average values were 40, 30, 19, and 44, respectively.
3.2. Inbred strains of mice with elevated ABR thresholds
All strains with mean ABR threshold values above 55 (click), 40 (8 kHz), 35 (16 kHz), or 60 (32 kHz) dB SPL were considered hearing impaired and are presented in Table 3. For reference, the grand means for ABR thresholds of normal hearing mouse strains are listed at the top of Table 3. Nineteen strains or substrains of mice exhibited elevated ABR thresholds before 13 weeks of age, for either one, two or all of the four stimuli (Table 3a). Sixteen strains with normal hearing when tested before 13 weeks of age exhibited hearing loss when tested at older ages. These 16 strains with late-onset hearing loss are denoted by asterisks in Table 2 and results for older, hearing-impaired mice from these strains are presented in Table 3b.
Table 3.
Inbred strains with elevated ABR thresholds (dB SPL). A total of 326 mice from 19 strains with early onset hearing loss and 82 mice from 16 strains with late onset hearing loss were tested
| Strain | Number mice | Age (weeks) | Clicks |
8 kHz |
16 kHz |
32 kHz |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| mean | S.D. | mean | S.D. | mean | S.D. | mean | S.D. | |||
| Normal grand means | 430 | 38 | 3 | 29 | 3 | 18 | 4 | 44 | 3 | |
| a. Strains with early onset hearing loss (before 13 weeks of age) | ||||||||||
| 129/J | 5 | 9 | 52 | 8 | 47 | 8 | 33 | 12 | 69 | 7 |
| 129/J | 5 | 13 | 59 | 11 | 49 | 7 | 42 | 10 | 72 | 9 |
| 129/J | 12 | 28 | 87 | 15 | 65 | 15 | 76 | 13 | 87 | 11 |
| 129/ReJ | 12 | 9 | 52 | 9 | 45 | 8 | 33 | 10 | 62 | 10 |
| 129/ReJ | 5 | 12 | 62 | 6 | 54 | 11 | 37 | 8 | 73 | 9 |
| 129/ReJ | 5 | 23 | 81 | 7 | 70 | 5 | 65 | 6 | 94 | 7 |
| 129/SvJ | 4 | 9 | 53 | 9 | 55 | 12 | 41 | 5 | 68 | 12 |
| 129/SvJ | 5 | 13 | 46 | 4 | 36 | 4 | 21 | 4 | 55 | 5 |
| 129/SvJ | 6 | 45 | 100 | 13 | 81 | 13 | 77 | 13 | 102 | 11 |
| A/J | 5 | 8 | 46 | 4 | 33 | 6 | 38 | 8 | 53 | 6 |
| A/J | 5 | 10 | 48 | 8 | 30 | 5 | 59 | 7 | 49 | 11 |
| A/J | 5 | 13 | 54 | 7 | 38 | 8 | 70 | 8 | 56 | 4 |
| A/J | 6 | 30 | 73 | 10 | 64 | 14 | 76 | 10 | 89 | 14 |
| A/J | 7 | 40 | 106 | 10 | 90 | 13 | 93 | 9 | 110 | 9 |
| ALR/LtJ | 4 | 26 | 117 | 5 | 104 | 0 | 99 | 0 | 119 | 0 |
| ALS/LtJ | 1 | 9 | 60 | 55 | 55 | 70 | ||||
| BUB/BnJ | 6 | 3 | 93 | 5 | 69 | 9 | 60 | 8 | 74 | 19 |
| BUB/BnJ | 8 | 8 | 102 | 14 | 83 | 17 | 79 | 17 | 101 | 14 |
| BUB/BnJ | 5 | 13 | 101 | 4 | 86 | 7 | 78 | 8 | 103 | 9 |
| BUB/BnJ | 5 | 20 | 117 | 4 | 102 | 4 | 97 | 4 | 117 | 4 |
| C57BLKS/J | 7 | 11 | 46 | 9 | 41 | 14 | 36 | 15 | 61 | 16 |
| C57BLKS/J | 4 | 15 | 41 | 5 | 33 | 6 | 18 | 3 | 55 | 4 |
| C57BLKS/J | 6 | 45 | 67 | 7 | 52 | 14 | 78 | 6 | 79 | 17 |
| C57BR/cdJ | 6 | 7 | 48 | 7 | 48 | 8 | 23 | 7 | 61 | 9 |
| C57BR/cdJ | 8 | 20 | 62 | 19 | 61 | 18 | 43 | 24 | 82 | 18 |
| C57BR/cdJ | 10 | 34 | 75 | 15 | 74 | 15 | 49 | 16 | 89 | 15 |
| C57BR/cdJ | 8 | 68 | 88 | 12 | 76 | 15 | 61 | 15 | 97 | 9 |
| C57L/J | 5 | 9 | 69 | 4 | 73 | 4 | 47 | 6 | 83 | 8 |
| C57L/J | 6 | 32 | 88 | 8 | 78 | 8 | 58 | 8 | 101 | 12 |
| C57L/J | 14 | 42 | 85 | 17 | 77 | 13 | 53 | 18 | 93 | 14 |
| C57L/J | 6 | 50 | 73 | 8 | 69 | 10 | 40 | 8 | 93 | 5 |
| C57L/J | 5 | 70 | 91 | 15 | 78 | 18 | 60 | 20 | 104 | 13 |
| DBA/2J | 6 | 3 | 43 | 4 | 28 | 4 | 28 | 18 | 53 | 12 |
| DBA/2J | 5 | 5 | 61 | 14 | 44 | 11 | 51 | 18 | 67 | 13 |
| DBA/2J | 5 | 8 | 55 | 8 | 46 | 9 | 62 | 10 | 67 | 12 |
| DBA/2J | 5 | 11 | 65 | 4 | 48 | 10 | 69 | 4 | 72 | 9 |
| DBA/2J | 5 | 14 | 68 | 15 | 52 | 17 | 60 | 7 | 72 | 16 |
| DBA/2J | 6 | 28 | 106 | 16 | 96 | 9 | 90 | 11 | 110 | 11 |
| DBA/2J | 2 | 44 | 107 | 17 | 87 | 24 | 95 | 6 | 119 | 0 |
| I/LnJ | 7 | 12 | 49 | 15 | 48 | 17 | 27 | 16 | 59 | 19 |
| MA/MyJ | 5 | 6 | 36 | 2 | 27 | 6 | 32 | 19 | 52 | 22 |
| MA/MyJ | 7 | 13 | 45 | 6 | 31 | 3 | 52 | 11 | 59 | 4 |
| MA/MyJ | 5 | 42 | 95 | 32 | 80 | 27 | 79 | 14 | 103 | 22 |
| NOD.B10-H2b | 13 | 10 | 96 | 20 | 91 | 12 | 90 | 11 | 111 | 14 |
| NOD.NON-H2nb1 | 5 | 11 | 64 | 14 | 66 | 25 | 79 | 13 | 88 | 26 |
| NOD.NON-Thy1a | 5 | 8 | 71 | 9 | 93 | 4 | 91 | 2 | 108 | 4 |
| NOD/LtJ | 5 | 3 | 59 | 4 | 46 | 5 | 64 | 4 | 78 | 11 |
| NOD/LtJ | 8 | 9 | 83 | 9 | 86 | 14 | 88 | 14 | 109 | 14 |
| NOD/LtJ | 6 | 13 | 107 | 16 | 103 | 4 | 98 | 4 | 118 | 4 |
| NOR/LtJ | 5 | 9 | 85 | 12 | 74 | 17 | 81 | 9 | 100 | 14 |
| NOR/LtJ | 5 | 15 | 103 | 22 | 95 | 10 | 93 | 13 | 115 | 8 |
| NOR/LtJ | 3 | 34 | 119 | 0 | 104 | 0 | 99 | 0 | 119 | 0 |
| SKH2/J-hr | 5 | 10 | 89 | 25 | 67 | 22 | 69 | 22 | 90 | 26 |
| SKH2/J-hr | 3 | 46 | 116 | 2 | 104 | 0 | 99 | 0 | 119 | 0 |
| SKH2/J-hr | 4 | 63 | 117 | 2 | 99 | 6 | 99 | 0 | 117 | 5 |
| Number of mice tested | 326 | |||||||||
| b. Strains with late onset hearing loss (beyond 13 weeks of age) | ||||||||||
| 129/SvEMS−+Ter?/J | 8 | 35 | 53 | 18 | 54 | 12 | 26 | 15 | 67 | 13 |
| A/WySnJ | 4 | 34 | 46 | 8 | 33 | 6 | 38 | 12 | 48 | 6 |
| C3H/HeSnJ | 6 | 45 | 47 | 8 | 45 | 13 | 28 | 12 | 61 | 9 |
| C57BL/6J | 7 | 100 | 106 | 11 | 84 | 10 | 78 | 16 | 105 | 9 |
| C58/J | 3 | 30 | 62 | 3 | 50 | 5 | 43 | 3 | 72 | 8 |
| CE/J | 6 | 50 | 62 | 10 | 44 | 7 | 37 | 9 | 63 | 8 |
| DBA/1J | 5 | 48 | 57 | 4 | 40 | 6 | 42 | 17 | 59 | 7 |
| DBA/1LacJ | 5 | 33 | 74 | 11 | 67 | 21 | 81 | 16 | 82 | 14 |
| KK/H1J | 5 | 50 | 56 | 28 | 49 | 29 | 33 | 25 | 60 | 31 |
| LG/J | 4 | 48 | 61 | 17 | 51 | 8 | 33 | 12 | 66 | 14 |
| LP/J | 4 | 31 | 96 | 23 | 76 | 22 | 79 | 19 | 98 | 19 |
| LP/J | 4 | 49 | 118 | 2 | 96 | 6 | 92 | 5 | 117 | 2 |
| MRL/MpJ | 6 | 30 | 40 | 3 | 51 | 11 | 26 | 19 | 67 | 14 |
| P/J | 5 | 19 | 54 | 22 | 46 | 20 | 23 | 21 | 60 | 22 |
| SENCARA/PtJ | 3 | 44 | 53 | 15 | 40 | 23 | 55 | 10 | 73 | 28 |
| SENCARB/PtJ | 5 | 39 | 55 | 23 | 42 | 25 | 36 | 20 | 64 | 23 |
| YBR/Ei | 2 | 60 | 58 | 25 | 58 | 25 | 38 | 25 | 68 | 25 |
| Number of mice tested | 82 | |||||||||
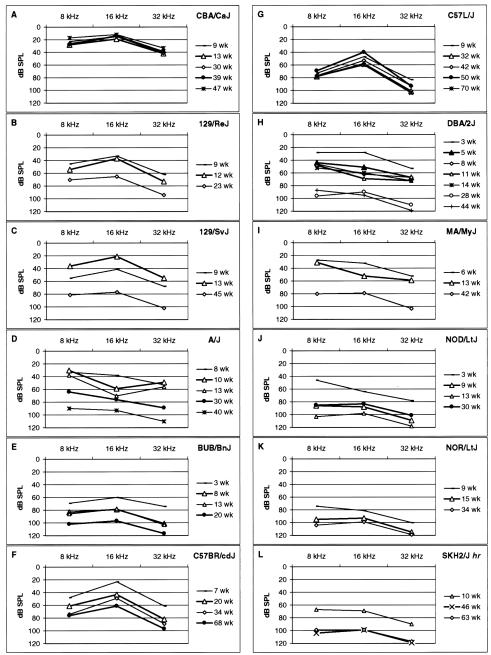
Severity and time of onset of hearing impairment varied among strains (Fig. 2). To classify strains according to the severity of their hearing loss, we define here three levels of impairment as measured by ABR thresholds: 20–40 dB SPL above normal means = mild impairment, 41–60 dB above normal means = intermediate impairment, and greater than 60 dB above normal means = severe impairment. With these criteria, strains 129/J, 129/ReJ, 129/SvJ, C57BR/cdJ, C57/J, DBA/2HaSmn, I/LnJ, and MA/MyJ have mild hearing impairment; strains A/J, DBA/2J, and SKH2/J intermediate impairment; and strains BUB/BnJ, NOR/LtJ, and NOD/LtJ severe impairment before 13 weeks of age.
Fig. 2.
ABR audiometric profiles for inbred strains with significant hearing impairment. For each strain and age of mice tested, ABR threshold values (in dB SPL) are plotted against the auditory stimulus frequency (in kHz). The normal hearing CBA/CaJ control strain is shown for comparison (A).
ABR thresholds increased with age for all of the hearing impaired inbred strains (Table 3; Fig. 2). For example, the A/J strain (Fig. 2D) exhibits a mild impairment at 8–10 weeks, an intermediate impairment at 13–30 weeks, and a severe impairment at 40 weeks of age. Although the NOD/LtJ (Fig. 2J) strain already exhibits intermediate hearing impairment at 3 weeks, it further progresses to severe hearing loss by 9 weeks of age.
For some strains, ABR thresholds varied significantly depending on the stimulus frequency. Different strains exhibited different frequency sensitivities. For example, the A/J strain (Fig. 2D) is most vulnerable to hearing loss at 16 kHz, whereas the C57BR/cdJ (Fig. 2F) and C57L/J (Fig. 2G) strains are least vulnerable to loss at 16 kHz.
Among 326 hearing impaired mice (Table 3a), as was the case with normal hearing mice, there were no statistically significant gender differences in ABR thresholds (P > 0.05). For female mice, the average ABR thresholds were 71 (click), 65 (8 kHz), 61 (16 kHz), and 82 (32 kHz) dB SPL; for male mice, the average values were 74, 68, 64, and 86, respectively.
3.3. Allelism tests
Different inbred strains identified with elevated ABR thresholds may share some of the same underlying genes for hearing loss because of common ancestry. For recessive genes, allelic complementation can be tested by examining F1 hybrids for hearing loss. If hearing loss is due to the same gene loci in both strains tested, then the F1 hybrids inheriting these non-complementing recessive alleles will exhibit hearing loss. If the hearing loss genes are different between the strains, they will complement each other and the F1 hybrids will hear normally.
All F1 hybrids between the good hearing strain CAST/Ei and strains identified with hearing impairment (including 129/ReJ, A/J, ALR/J, BUB/BnJ, C57BR/cdJ, C57L/J, DBA/2J, and NOD/LtJ) exhibited normal hearing, even at advanced ages, indicating that the genes contributing to hearing loss are recessive (data not shown). To see if the inbred strains 129/ReJ, A/J, and NOD.NON-H2nb1 share any hearing loss genes with DBA/2J, we generated F1 hybrids and measured their ABR thresholds. At 3 months of age, both the 129/ReJ and A/J strains appear to complement the DBA/2J strain (the F1 hybrids have normal ABR thresholds; Table 4), suggesting that these two strains do not share all of their hearing loss genes with DBA/ 2J. At 29 weeks of age, however, (DBA/2JUA/J) F1 hybrids do exhibit significant hearing loss (Table 4), suggesting that these two strains share at least one gene contributing to hearing loss. The elevated ABR thresholds of (NOD.NON-H2nb1×DBA/2J) and (NOD.NON-H2nb1×ALR/LtJ) F1 hybrids, even by 13 weeks of age (Table 4), indicate that these three strains probably share all of the same hearing loss genes.
Table 4.
Allelism tests for recessive hearing loss genes
| Strain | Results | Number of mice |
Age (weeks) |
Clicks |
8 kHz |
16 kHz |
32 kHz |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parents F×M | mean | S.D. | mean | S.D. | mean | S.D. | mean | S.D. | |||
| 129/ReJ×DBA/2J | − | 5 | 31 | 45 | 15 | 31 | 11 | 44 | 25 | 51 | 11 |
| C57BR/cdJ×NOD.NON-H2nb1 | − | 5 | 13 | 41 | 4 | 36 | 8 | 17 | 7 | 53 | 8 |
| C57L×DBA/2J | − | 6 | 13 | 37 | 3 | 28 | 3 | 13 | 3 | 50 | 3 |
| C57L×NOD.NON-H2nb1 | − | 10 | 13 | 40 | 2 | 33 | 5 | 16 | 4 | 48 | 5 |
| DBA/2J×129/ReJ | − | 5 | 13 | 38 | 7 | 28 | 3 | 17 | 6 | 43 | 3 |
| DBA/2J×129/ReJ | − | 5 | 31 | 38 | 3 | 27 | 6 | 25 | 15 | 44 | 4 |
| DBA/2J×A/J | − | 5 | 14 | 38 | 4 | 32 | 4 | 26 | 4 | 49 | 4 |
| DBA/2J×A/J | + | 5 | 29 | 53 | 8 | 47 | 14 | 82 | 12 | 73 | 11 |
| NOD.NON-H2nb1×ALR/LtJ | + | 19 | 13 | 72 | 15 | 75 | 15 | 90 | 9 | 98 | 15 |
| NOD.NON-H2nb1×ALR/LtJ | + | 10 | 31 | 114 | 7 | 99 | 7 | 97 | 8 | 115 | 9 |
| NOD.NON-H2nb1×DBA/2J | + | 5 | 13 | 69 | 12 | 66 | 12 | 84 | 13 | 91 | 13 |
| NOD.NON-H2nb1×DBA/2J | + | 10 | 31 | 114 | 7 | 99 | 7 | 97 | 8 | 115 | 9 |
: F1 hybrids with significantly elevated ABR thresholds constituted a positive allelism result.
4. Discussion
4.1. Appropriateness of ABR for assessing hearing sensitivity
Hearing is defined as a perception and, as such, behavioral measures would be most appropriate. However, behavioral thresholds can be confounded by a great many processes, any of which could be aberrant (Henry, 1982). The ABR is an evoked potential measure of auditory activity in the brainstem that is commonly used for prediction of hearing levels in animals and young children. ABRs include several waves; the first wave represents cochlear and auditory nerve activity and the late waves reflect function within the central auditory pathways (Markand, 1994; MÖller, 1994). We notice that peak I of the mouse ABR is robust, whereas peak V is most robust in humans and peak V is frequently undetected in the mouse. The ABR is essentially unaffected by the subject’s state of arousal or to the effects of the majority of sedative-hypnotic drugs and the commonly used anesthetics, even with doses that make patients comatose and render a ‘flat’ EEG (Stockard et al., 1977).
In mice, ABR thresholds have been used successfully as audiometric thresholds in hearing and genetic research (Erway et al., 1993, 1996; Henry et al., 1992; Johnson et al., 1997; Noben-Trauth et al., 1997; Willott et al., 1995). The reliability, sensitivity, non-invasive nature, and relative ease of application makes the ABR a method of choice for a first screen of hearing impairment in mice. Other options for evaluating hearing ability of mice have their own specific limitations and advantages for particular applications. The Preyer reflex, a suprathreshold response, does not measure thresholds and, therefore, can only be used to search for deaf or severely hearing impaired mice. Its simplicity, however, allows for the testing of very large numbers of mice. The whole nerve action potential recorded at the round window is a well-established method for assessing cochlear activity, and gives response thresholds close to those of single units (Johnstone et al., 1979). Round window recordings are generally done in terminal experiments in fully anesthetized mice, because extensive surgery is required. Because of its difficulty and terminal nature, recording from the round window is best done as a secondary screen. Distortion product otoacoustic emissions (DPOAE) can be used either as an alternative screening approach or for detailed study (Parham, 1997). DPOAE is used extensively for testing human infants but only recently has been adapted for use in small mammals (Kim et al., 1996; Sun et al., 1996). As a secondary screen of mice with known hearing impairment, DPOAE may provide additional information about outer hair cell function.
4.2. Reference values for ABR thresholds of inbred mice
The large database generated by this study establishes a reliable reference for hearing ability of inbred mice at ages less than 6 months. The entire database includes hearing properties of individual mice from a variety of strains at different ages. We present a summary of these data in Table 2. ABR measurements for individual mice and updates of tests for additional mice and strains will soon be made accessible on the World Wide Web, with entry from The Jackson Laboratory address: http://abr.jax.org/miceabr/.
Mice can hear over a range of frequencies between 0.5 and 120 kHz; however, normal mice are most sensitive to frequencies of 12–24 kHz (Ehret, 1983). Due to time and equipment limitations for this project, we tested ABR thresholds for stimulus frequencies around this optimal range, at 8, 16, and 32 kHz. ABR wave patterns sometimes varied slightly from strain to strain and even from mouse to mouse. A series of ABR waveforms were obtained (and digitally stored on computer disks) at the threshold (T) for the click stimulus, and also at T+10, T+20 and T+30 dB. These ABR data are not presented here but are available for future analyses of intensity-latency and intensity-amplitude functions among the ABR peaks of all of the strains of mice tested. In this report, we focus on ABR thresholds as indicators of hearing impairment in inbred mouse strains.
The CBA/J and CBA/CaJ inbred substrains have been used extensively as normal controls for hearing research. CBA/CaJ may be preferred because CBA/J mice are very susceptible to otitis media and have retinal degeneration (Henry and McGinn, 1992). The consistent, low ABR thresholds we obtained in this study for CBA/CaJ mice confirm the good hearing ability of this strain, even at relatively old age (39 weeks, Table 1). The inbred strains CASA/Rk, CAST/Ei, CE/J, CZECH II/Ei, MOLD/Rk, MOLF/Ei, MOLG/Dn, PERC/Ei, SF/CamEi, and SHR/GnEi are recently derived from widely distributed wild populations of Mus musculus subspecies, and SPRET/Ei from a separate species, Mus spretus. These wild-derived inbred strains (Table 2), like CBA/CaJ (Table 1), consistently exhibited low ABR thresholds.
4.3. Characteristics of hearing loss in impaired strains
Hearing loss identified by elevated ABR thresholds (Table 3) is progressive and varies among inbred strains in its frequency specificity, time of onset, and severity. All of the hearing impaired strains had better hearing at younger ages (Table 3, Fig. 2). None of the strains with elevated ABR thresholds had aberrant ABR wave patterns, suggesting that the hearing loss is caused by defects in the peripheral rather than the central part of the hearing system.
Different strains exhibit different sensitivities to particular stimulus frequencies, as shown by ABR audiograms (Fig. 2). The A/J strain (Fig. 2D) is most vulnerable to hearing loss at 16 kHz, whereas the C57BR/cdJ (Fig. 2F) and C57L/J (Fig. 2G) strains are least vulnerable to loss at 16 kHz. Henry (1982) observed the same frequency sensitivities in A/J and C57BR/cdJ mice, although he did not test A/J mice at 32 kHz. The strain specificity indicates that there is a genetic basis for the frequency-dependent sensitivities. Audiometric profiles of human patients with hearing loss also show frequency-dependent sensitivities; however, genetic contributions to this phenomenon are difficult to determine (Martini et al., 1997).
Some of the inbred strains that have normal hearing ABR thresholds when tested before 13 weeks of age (Table 2) may exhibit late onset or age-related hearing loss. For example, the C57BL/6J strain exhibits AHL and has been used extensively to study presbycusis (Church and Shucard, 1986; Erway et al., 1996; Hunter and Willott, 1987; Johnson et al., 1997; McFadden and Willott, 1994; Parham, 1997; Shone et al., 1991; Walton et al., 1995; Willott et al., 1995). The C57BL/6J mice we tested for this study still had normal ABR thresholds at 33 weeks; however, by 100 weeks the same mice had ABR thresholds 60 dB above normal means (Table 3). In contrast to strains with late onset AHL, some inbred strains such as BUB/BnJ, and NOR/LtJ exhibit a severe hearing loss before 8 weeks of age (Fig. 2E,K).
4.4. Hearing impairment in 129-related strains (but not FVB/NJ)
129-related inbred strains are the source of most embryonic stem (ES) cells used to create targeted mutations in mice by homologous recombination. Mutant mice produced from these ES cells consequently have a 129-derived genetic background. 129 substrains have a complex history and extensive genetic variability (Simpson et al., 1997). All four of the 129-related substrains we examined, 129/J, 129/ReJ, 129/SvEms, and 129/SvJ, exhibited slightly elevated ABR thresholds by 9 weeks of age (Table 3). By 28 weeks, two of them, 129/J and 129/ReJ, exhibited an intermediate hearing impairment. The one substrain tested at 45 weeks, 129/SvJ, exhibited a severe hearing impairment (Fig. 2C). Such elevated ABR thresholds within the 129 substrains is of much concern regarding hearing studies conducted on mice derived from 129 ES cells. Genetic contributions from this strain can confound interpretations of hearing loss attributable to the targeted mutation, especially if tested at older ages.
FVB/NJ is another inbred strain commonly used in genetic engineering because of its good reproductive performance and large pronuclei for transgenic microinjections (Taketo et al., 1991). This albino strain originated from Swiss mice established at the National Institutes of Health in 1935. In the early 1970s a subgroup of these mice were found to be sensitive to the B strain of Friend leukemia virus; homozygous mice were then inbred as strain FVB. FVB/NJ mice appear to have good hearing as shown by their low ABR thresholds at 28 weeks of age (Table 2), and thus this strain background will not interfere with measurements of hearing loss attributable to the experimental transgenesis.
4.5. Hearing impairment in NOD-related strains
The NOD (non-obese diabetic)-related strains are used extensively to study insulin-dependent diabetes mellitus in mice. These strains, including NOD/LtJ, NON/LtJ, NOR/LtJ, ALR/LtJ, and ALS/LtJ, are genetically related, all having been derived from Swiss mice (Kikutani and Makino, 1992; Leiter, 1998; Prochazka et al., 1992; Sekiguchi et al., 1990). Four of the NOD-related strains that we examined, NOD/LtJ, NOR/LtJ, ALR/LtJ, and ALS/LtJ, and several NOD/LtJ congenic derivatives, exhibit severe hearing impairment by 12 weeks of age (Table 3), whereas the NON/LtJ strain has normal hearing (Table 2). Although related through common ancestry, the NOD and NON strains have accumulated extensive genetic differences (Leiter, 1998), which could account for their difference in hearing abilities.
Hearing impairment is not associated with diabetes in these NOD-related strains. The diabetes-resistant NOR/LtJ inbred strain (Prochazka et al., 1992) and the resistant NOD.B10-H2b and NOD.NON-H2nb1 congenic strains (Wicker et al., 1993) exhibit hearing impairment as severe as the diabetic NOD/LtJ strain (Table 3). To alleviate husbandry difficulties encountered with diabetic mice, the resistant NOD.NON-H2nb1 congenic strain, rather than its NOD/LtJ progenitor, is preferred for genetic studies of the hearing impairment associated with these strains, as described below.
4.6. Hearing impairment in other strains
Besides some of the NOD-related strains, BUB/BnJ and SKH2/J exhibited the earliest and most severe hearing impairment of the inbred strains we have surveyed (Table 3). The BUB/BnJ inbred strain originated from albino mice of unknown ancestry at Brown University. According to phylogenetic analysis (Atchley and Fitch, 1993), the BUB strain appears to have a complex origin and shows close genetic similarity to SWR, a Swiss albino strain. Because NOD also was derived from Swiss mice, it is possible that NOD and BUB share some of the same underlying genes for hearing impairment. SKH2/J is an inbred strain of hairless mice developed for immune response studies (Smith et al., 1982). Although it is homozygous for the hairless mutation (hr), hearing loss in SKH2/J is not caused by this mutation because two other strains carrying the same hr mutation, HRS/J and RHJ/Le (Table 2), do not exhibit hearing loss. Other SKH2/J strain-specific genes, therefore, must be responsible for this hearing loss.
The hearing loss we discovered in the DBA/2HaSmn and DBA/Lac1 strains probably results from some of the same genes as those for DBA/2J, whose hearing loss was known before this study began (Erway et al., 1993). The C57BLKS/J strain was derived from an admixture of C57BL and DBA and its hearing loss, therefore, is probably also caused by genes from these strains. According to phylogenetic analysis, the I strain shows greatest genetic similarity to DBA/2 (Atchley and Fitch, 1993); this similarity suggests that the hearing impairment we observed in the I/LnJ strain may have some of the same underlying genes as those responsible for the hearing loss in DBA/2J.
LP/J mice are reported to have late onset conductive and sensorineural hearing loss (Henry, 1982; Steel et al., 1987). Our ABR data for this strain confirm this hearing loss; however, the age of onset we observed was later than previously reported by others. LP/J mice exhibited normal ABR thresholds at 11 weeks (Table 2), but significantly elevated thresholds at 31 weeks (Table 3). Although the RBF/DnJ strain has been reported to have hearing loss at 8 weeks of age (Pieke-Dahl et al., 1997), the mice we tested from this strain exhibited normal ABR thresholds, even at 27 weeks of age.
4.7. Further genetic studies
With strain history and genetic relatedness considerations in mind, we have chosen the following eight inbred strains that exhibit hearing impairment for subsequent genetic analysis: 129/ReJ, A/J, BUB/BnJ, C57BR/cdJ, C57L/J, DBA/2J, NOD.NON-H2nb1, and SKH2/J. Matings have been set up between mice from each of these strains and mice from the wild-derived inbred strain CAST/Ei to produce F1 hybrids and backcross mice for inheritance and linkage analysis. CAST/ Ei retains good hearing beyond 12 months of age (Table 2), is genetically distinct from other inbred strains, and is well characterized; therefore, we are using it as our standard linkage testing stock. Results from these genetic studies will be reported in future publications.
4.8. Conclusion
We have tested hearing in 80 inbred strains of mice by ABR threshold analysis. The data for 61 strains of mice with normal hearing establish a valuable reference that represents the ABR thresholds for mice produced and reared under fairly homogeneous conditions. We identified 19 strains or substrains with significant hearing impairment before 13 weeks of age and 16 strains with later onset impairment. The hearing loss in all of these strains is progressive. Information about the hearing ability of inbred strains will be valuable for certain behavioral, pathological and physiological comparisons. For example, the NOD-related strains, important in diabetes and autoimmunity research, exhibit significant hearing loss that may affect interpretations of behavioral responses to experimental treatments. In research involving the assessment of hearing in mutant mice, it is essential to be aware of potential confounding effects of genetic background. Our results for the 129 and FVB strains, extensively used in genetic engineering to produce ‘knockout’ and transgenic mutant mice, are particularly important in this regard. Some of the inbred mouse strains we have identified with elevated ABR thresholds may prove especially important as potential models for human hearing impairment and may help to identify some of the underlying genes.
Acknowledgments
We thank Dr. Ed Leiter, The Jackson Laboratory (TJL), for the ALR, ALS, and NOD-related mice and information about them. We also thank Drs. P. Nishina and V. Letts, TJL, for their careful review of the manuscript. This work was supported by the National Institutes of Health Contract N01 DC62108 from NIDCD and Core Grant CA34196 from NCI. Preliminary findings were presented at the 20th and 21st meetings of the Association for Research in Otolaryngology, St. Petersburg Beach, FL, 1997 and 1998.
References
- Atchley WR, Fitch W. Genetic affinities of inbred mouse strains of uncertain origin. Mol. Biol. Evol. 1993;10:1150–1169. doi: 10.1093/oxfordjournals.molbev.a040070. [DOI] [PubMed] [Google Scholar]
- Brown SD, Steel KP. Genetic deafness – progress with mouse models. Hum. Mol. Genet. 1994;3(Spec. No):1453–1546. doi: 10.1093/hmg/3.suppl_1.1453. [DOI] [PubMed] [Google Scholar]
- Church MW, Shucard DW. Age-related hearing loss in BDF1 mice as evidenced by the brainstem auditory evoked potential. Audiology. 1986;25:363–372. doi: 10.3109/00206098609078400. [DOI] [PubMed] [Google Scholar]
- Deol MS. A gene for uncomplicated deafness in the mouse. J. Embryol. Exp. Morphol. 1956;4:190–195. [Google Scholar]
- Ehret G. Psychoacoustics. In: Willot JF, editor. Auditory Psychobiology of the Mouse. Charles C. Thomas; Springfield, IL: 1983. pp. 13–53. [Google Scholar]
- Erkman L, McEvilly RJ, Luo L, Ryan AK, Hooshmand F, O’Connell SM, Keithley EM, Rapaport DH, Ryan AF, Rosenfeld MG. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996;381:603–606. doi: 10.1038/381603a0. [DOI] [PubMed] [Google Scholar]
- Erway LC, Willott JF, Archer JR, Harrison DE. Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hear. Res. 1993;65:125–132. doi: 10.1016/0378-5955(93)90207-h. [DOI] [PubMed] [Google Scholar]
- Erway LC, Shiau YW, Davis RR, Krieg EF. Genetics of age-related hearing loss in mice. III. Susceptibility of inbred and F1 hybrid strains to noise-induced hearing loss. Hear. Res. 1996;93:181–187. doi: 10.1016/0378-5955(95)00226-x. [DOI] [PubMed] [Google Scholar]
- Gibson F, Walsh J, Mburu P, Varela A, Brown KA, Antonio M, Beisel KW, Steel KP, Brown SD. A type VII myosin encoded by the mouse deafness gene shaker-1. Nature. 1995;374:62–64. doi: 10.1038/374062a0. [DOI] [PubMed] [Google Scholar]
- Hall JW, 3rd, Rupp KA. Auditory brainstem response: recent developments in recording and analysis. Adv. Otorhinolaryngol. 1997;53:21–45. doi: 10.1159/000059038. [DOI] [PubMed] [Google Scholar]
- Henry KR. Age-related auditory loss and genetics: an electrocochleographic comparison of six inbred strains of mice. J. Gerontol. 1982;37:275–282. doi: 10.1093/geronj/37.3.275. [DOI] [PubMed] [Google Scholar]
- Henry KR, McGinn MD. The mouse as a model for human audition. A review of the literature. Audiology. 1992;31:181–189. doi: 10.3109/00206099209081653. [DOI] [PubMed] [Google Scholar]
- Henry KR, McGinn MD, Carter LA, Savoska EA. Auditory brainstem function of the F1 offspring of the cross of CBA/CaJ and AU/SsJ inbred mice. Audiology. 1992;31:190–195. doi: 10.3109/00206099209081654. [DOI] [PubMed] [Google Scholar]
- Hunter KP, Willott JF. Aging and the auditory brainstem response in mice with severe of minimal presbycusis. Hear. Res. 1987;30:207–218. doi: 10.1016/0378-5955(87)90137-7. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear. Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Johnstone JR, Alder VA, Johnstone BM, Robertson D, Yates GK. Cochlear action potential threshold and single unit thresholds. J. Acoust. Soc. Am. 1979;65:254–257. doi: 10.1121/1.382244. [DOI] [PubMed] [Google Scholar]
- Keats BJ, Nouri N, Huang JM, Money M, Webster DB, Berlin CI. The deafness locus (dn) maps to mouse chromosome 19. Mamm. Genome. 1995;6:8–10. doi: 10.1007/BF00350886. [DOI] [PubMed] [Google Scholar]
- Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv. Immunol. 1992;51:285–322. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- Kim DO, Paparello J, Jung MD, Smurzynski J, Sun X. Distortion product otoacoustic emission test of sensorineural hearing loss: performance regarding sensitivity, specificity and receiver operating characteristics. Acta Otolaryngol. (Stockh.) 1996;116:3–11. doi: 10.3109/00016489609137705. [DOI] [PubMed] [Google Scholar]
- Leiter EH. NOD mice and related strains: origin, husbandry, and biology. In: Leiter EH, Atkinson MA, editors. NOD Mice and Related Strains: Research Applications in Diabetes, AIDS, Cancer, and Other Disease. Landes; Austin, TX: 1998. pp. 1–35. [Google Scholar]
- Liu XZ, Walsh J, Mburu P, Kendrick-Jones J, Cope MJ, Steel KP, Brown SD. Mutations in the myosin VIIA gene cause non-syndromic recessive deafness. Nature Genet. 1997a;16:188–190. doi: 10.1038/ng0697-188. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Walsh J, Tamagawa Y, Kitamura K, Nishizawa M, Steel KP, Brown SD. Autosomal dominant non-syndromic deafness caused by a mutation in the myosin VIIA gene (letter) Nature Genet. 1997b;17:268–269. doi: 10.1038/ng1197-268. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Rastan S, Brown SDM. Genetic Variants and Strains of the Laboratory Mouse. Oxford University Press; Oxford: 1996. [Google Scholar]
- Markand ON. Brainstem auditory evoked potentials. J. Clin. Neurophysiol. 1994;11:319–342. doi: 10.1097/00004691-199405000-00004. [DOI] [PubMed] [Google Scholar]
- Martini A, Milani M, Rosignoli M, Mazzoli M, Prosser S. Audiometric patterns of genetic non-syndromal sensorineural hearing loss. Audiology. 1997;36:228–236. doi: 10.3109/00206099709071975. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Willott JF. Responses of inferior colliculus neurons in C57BL/6J mice with and without sensorineural hearing loss: effects of changing the azimuthal location of an unmasked pure-tone stimulus. Hear. Res. 1994;78:115–131. doi: 10.1016/0378-5955(94)90018-3. [DOI] [PubMed] [Google Scholar]
- MÖller AR. Auditory neurophysiology. J. Clin. Neurophysiol. 1994;11:284–308. doi: 10.1097/00004691-199405000-00002. [DOI] [PubMed] [Google Scholar]
- Morton NE. Genetic epidemiology of hearing impairment. Ann. NY Acad. Sci. 1991;630:16–31. doi: 10.1111/j.1749-6632.1991.tb19572.x. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR, Nishina PM. mdfw: a deafness susceptibility locus that interacts with deaf waddler (dfw) Genomics. 1997;44:266–272. doi: 10.1006/geno.1997.4869. [DOI] [PubMed] [Google Scholar]
- Parham K. Distortion product otoacoustic emissions in the C57BL/6J mouse model of age-related hearing loss. Hear. Res. 1997;112:216–234. doi: 10.1016/s0378-5955(97)00124-x. [DOI] [PubMed] [Google Scholar]
- Petit C. Genes responsible for human hereditary deafness: symphony of a thousand. Nature Genet. 1996;14:385–391. doi: 10.1038/ng1296-385. [DOI] [PubMed] [Google Scholar]
- Pieke-Dahl S, Ohlemiller KK, McGee J, Walsh EJ, Kimberling WJ. Hearing loss in the RBF/DnJ mouse, a proposed animal model of Usher syndrome type IIa. Hear. Res. 1997;112:1–12. doi: 10.1016/s0378-5955(97)00087-7. [DOI] [PubMed] [Google Scholar]
- Probst FJ, Fridell RA, Raphael Y, Saunders TL, Wang A, Liang Y, Morell RJ, Touchman JW, Lyons RH, Noben-Trauth K, Friedman TB, Camper SA. Correction of deafness in shaker-2 mice by an unconventional myosin in a BAC transgene (see comments) Science. 1998;280:1444–1447. doi: 10.1126/science.280.5368.1444. [DOI] [PubMed] [Google Scholar]
- Prochazka M, Serreze DV, Frankel WN, Leiter EH. NOR/Lt mice: MHC-matched diabetes-resistant control strain for NOD mice. Diabetes. 1992;41:98–106. doi: 10.2337/diab.41.1.98. [DOI] [PubMed] [Google Scholar]
- Sekiguchi F, Ishibashi K, Katoh H, Kawamoto Y, Ino T. Genetic profile of alloxan-induced diabetes-susceptible mice (ALS) and -resistant mice (ALR) Jikken Dobutsu. 1990;39:269–272. doi: 10.1538/expanim1978.39.2_269. [DOI] [PubMed] [Google Scholar]
- Shone G, Altschuler RA, Miller JM, Nutall AL. The effect of noise exposure on the aging ear. Hear. Res. 1991;56:173–178. doi: 10.1016/0378-5955(91)90167-8. [DOI] [PubMed] [Google Scholar]
- Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nature Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- Smith SM, Forbes PD, Linna TJ. Immune responses in nonhaired mice. Int. Arch. Allergy Appl. Immunol. 1982;67:254–261. doi: 10.1159/000233027. [DOI] [PubMed] [Google Scholar]
- Steel KP. Inherited hearing defects in mice. Annu. Rev. Genet. 1995;29:675–701. doi: 10.1146/annurev.ge.29.120195.003331. [DOI] [PubMed] [Google Scholar]
- Steel KP, Bock GR. Hereditary inner-ear abnormalities in animals. Relationships with human abnormalities. Arch. Otolaryngol. 1983;109:22–29. doi: 10.1001/archotol.1983.00800150026005. [DOI] [PubMed] [Google Scholar]
- Steel KP, Brown SD. Genetics of deafness. Curr. Opin. Neurobiol. 1996;6:520–525. doi: 10.1016/s0959-4388(96)80059-6. [DOI] [PubMed] [Google Scholar]
- Steel KP, Moorjani P, Bock GR. Mixed conductive and sensorineural hearing loss in LP/J mice. Hear. Res. 1987;28:227–236. doi: 10.1016/0378-5955(87)90051-7. [DOI] [PubMed] [Google Scholar]
- Stockard JJ, Rossiter VS, Jones TA, Sharbrough FW. The effects of centrally acting drug on brainstem auditory responses. Electroencephalogr. Clin. Neurophysiol. 1977;43:550–551. [Google Scholar]
- Sun XM, Jung MD, Kim DO, Randolph KJ. Distortion product otoacoustic emission test of sensorineural hearing loss in humans: comparison of unequal- and equal-level stimuli. Ann. Otol. Rhinol. Laryngol. 1996;105:982–990. doi: 10.1177/000348949610501209. [DOI] [PubMed] [Google Scholar]
- Taketo M, Schroeder AC, Mobraaten LE, Gunning KB, Hanten G, Fox RR, Roderick TH, Stewart CL, Lilly F, Hansen CT, et al. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc. Natl. Acad. Sci. USA. 1991;88:2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahava O, Morell R, Lynch ED, Weiss S, Kagan ME, Ahituv N, Morrow JE, Lee MK, Skvorak AB, Morton CC, Blumenfeld A, Frydman M, Friedman TB, King MC, Avraham KB. Mutation in transcription factor POU4F3 associated with inherited progressive hearing loss in humans. Science. 1998;279:1950–1954. doi: 10.1126/science.279.5358.1950. [DOI] [PubMed] [Google Scholar]
- Van Camp G, Smith RJ. Hereditary Hearing Loss Home-page. 1998 World Wide Web URL: http://dnalab-www.uia.ac.be/dnalab/hhh/
- Walton JP, Frisina RD, Meierhans LR. Sensorineural hearing loss alters recovery from short-term adaptation in the C57BL/6 mouse. Hear. Res. 1995;88:19–26. doi: 10.1016/0378-5955(95)00093-j. [DOI] [PubMed] [Google Scholar]
- Wang A, Liang Y, Fridell RA, Probst FJ, Wilcox ER, Touchman JW, Morton CC, Morell RJ, Noben-Trauth K, Camper SA, Friedman TB. Association of unconventional myosin MYO15 mutations with human nonsyndromic deafness DFNB3 (see comments) Science. 1998;280:1447–1451. doi: 10.1126/science.280.5368.1447. [DOI] [PubMed] [Google Scholar]
- Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD, et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374:60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- Wicker LS, DeLarto NH, Pressey A, Peterson LB. Genetic control of diabetes and insulitis in nonobese diabetic mouse: analysis of the NOD.H-2b and B10.H-2nod strains. In: Act FW, Vogel HJ, editors. Molecular Mechanisms of Immunological Self-Recognition. Academic Press; San Diego, CA: 1993. pp. 173–181. [Google Scholar]
- Willott JF. Auditory Psychobiology of the Mouse. Charles C. Thomas; Springfield, IL: 1983. [Google Scholar]
- Willott JF, Erway LC, Archer JR, Harrison DE. Genetics of age-related hearing loss in mice. II. Strain differences and effects of caloric restriction on cochlear pathology and evoked response thresholds. Hear. Res. 1995;88:143–155. doi: 10.1016/0378-5955(95)00107-f. [DOI] [PubMed] [Google Scholar]