Abstract
This article will focus on the impact of patient age on outcomes following esophageal resection as well as potential strategies to improve perioperative management of geriatric patients undergoing esophagectomy for cancer.
INTRODUCTION
CANCER INCIDENCE AND MORTALITY
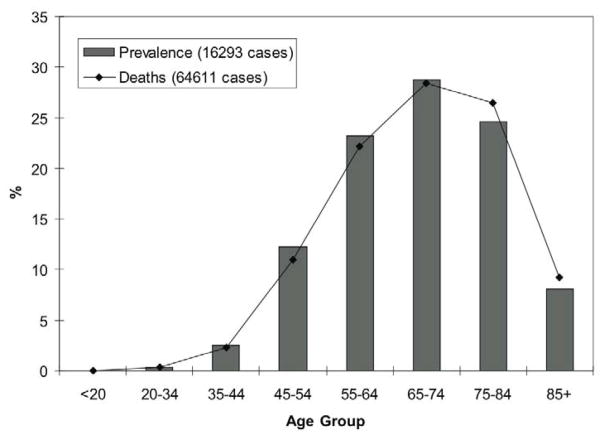
Esophageal cancer remains a highly-lethal malignancy, with an annual death rate, 7.9 men/100,000 and 1.7 women/100,000, nearly matching its annual incidence of 7.9 men/100,000 and 1.8 women/100,000. These rates have been slowly increasing across the entire US population. The majority of cases of esophageal cancer occurs in patients 65 years of age or older, with over one-third of incident cases and deaths affecting patients 75 years of age or older (Figure 1) [1–3]. Of patients older than 65 years of age undergoing esophagectomy captured in the linked Surveillance, Epidemiology and End Results (SEER)-Medicare databases, 34% are 75 years old or greater. Although esophageal resection remains the mainstay of treatment for patients with potentially resectable carcinoma, patient age continues to be a major factor in clinical decision-making.
Figure 1.
Distribution of new cases of (bar) and deaths due to (line) esophageal cancer among cohorts of age, 2001–2005, Surveillance, Epidemiology and End Results. Data from [2].
The Worldwide Esophageal Cancer Collaborative recently evaluated survival among 4,725 patients undergoing esophagectomy alone, without preoperative or adjuvant chemotherapy or radiation at thirteen institutions worldwide[4]. In this study, the average patient age was 62±11 years, and 75% of patients underwent esophagectomy in the 1990s and 2000s. Patient survival differed significantly and distinctively by all staging criteria including tumor, lymph node, metastasis stage, histology, grade and number of involved lymph nodes. Demographically, region of world and patient race were not determinants for patient survival. In this cohort of patients, increasing age was a significant adverse risk factor for overall long-term survival, with patients 70 years of age or greater having worse survival than patients in younger deciles of age. Operative mortality varied from 0% to 7% among the institutions whose data were included in this study. Although this collaborative was assembled with the primary goal of developing a revised esophageal cancer staging system, these international data, accumulated from specialized centers, suggest that patient age has significant impact on early and long-term survival following esophagectomy for cancer, as will be discussed in this review.
Utilizing the SEER-Medicare databases, Paulson and colleagues identified the rate of resection in a study cohort of 2,386 patients with resectable (Stage I, II or III) esophageal cancer diagnosed between 1997 and 2002[5]. In this cohort they found that 813 patients (34%) had received esophagectomy. Among other factors found to be associated with a lower likelihood for undergoing operation, including non-white race, residence in high-poverty area and greater number of co-morbidities, these authors found that increasing age was associated significantly with decreased likelihood of operation. Patients aged 75–79 years were half as likely to undergo esophagectomy as those patients aged 65–74 years. Older patients with stage II or III esophageal cancer, although generally considered resectable, also were less likely to undergo operation compared with the younger ones. Overall 5-year survival was significantly better for patients undergoing operation than non-surgically treated patients, 28% and 10% respectively, even when adjusting for patient and tumor characteristics, including age, comorbidity burden, socioeconomic region, race, and tumor stage (hazard ratio, 0.69; p<0.001, Cox proportional hazards model). While this study focused on the issue of health care disparities based on race and socioeconomic status, the findings also demonstrate that increasing patient age continues to be a determinant in delivery of appropriate treatment, including esophagectomy, for patients with esophageal cancer.
Similar findings can be gleaned from a population-based study of the National Cancer Registry, Ireland, in which the authors found that older patients were more likely to be referred for non-operative management such as chemotherapy and/or radiation therapy, or no treatment at all, rather than resection for curative intent. Among 3,165 patients diagnosed with esophageal cancer from 1994 to 2001, 982 underwent resection, but when compared with patients less than 60 years of age, the likelihood for resection was significantly lower among older cohorts by 33%, 74% and 93% for patients aged 60–69, 70–79 and 80+, respectively. There was limited analysis of the reasons for this practice pattern, although the authors speculated that these differences might in part be attributed to the lack of centralized care and limited availability of specialized cancer services [6].
MORTALITY — POPULATION-BASED DATA
Several population-based studies have demonstrated a consistent adverse effect of increasing age and mortality following esophagectomy. Using data obtained as part of the Department of Veterans Affairs (VA) National Surgical Quality Improvement Program (NSQIP)[7], Bailey and colleagues identified 1,777 patients undergoing esophagectomy, including 1,509 (85%) for cancer, between 1991 and 2000, at 109 Department of Veterans Affairs Medical Centers[8]. Overall mortality was 10% and one or more perioperative complications occurred in nearly 50% of these patients. While increasing age was found to be a significant risk factor in multivariate analysis for both 30-day mortality and morbidity, limited data were published describing the age distribution of this study population. The odds ratio for mortality was 1.05, indicating a 5% increased risk of 30-day mortality for every increasing year of age. A diagnosis of malignancy did not present an increased risk for 30-day mortality or complication in the study population.
Utilizing the Swedish national cancer registry, Rouvelas and colleagues identified 764 patients undergoing resection alone from 1987 to 2000 for esophageal carcinoma, including 302 subjects aged 66–75, and 140 subjects 75 years or greater[9]. Overall 30-day mortality was 7.3%. Although patients with increasing age were at greater risk for mortality, this was not statistically significant, with hazard ratio of 1.28 (95% confidence interval, 0.96–1.72) for patients 75 years or greater.
Cronin-Fenton and colleagues from the National Cancer Registry, Ireland, identified 3,165 patients diagnosed with esophageal cancer or gastroesophageal junction carcinomas from 1994 to 2001, including 1026 (32%) patients, aged 70–79, and 665 (21%) patients, 80 years or greater [6]. In this sample, 982 (31%) patients underwent cancer-directed operation, including 611 patients for esophageal carcinoma and 316 patients for gastroesophageal carcinoma. Data regarding associated comorbidities at the time of diagnosis were not available in this registry. While 30-day mortality was increased by nearly 50% among patients older than 70 years compared with younger patients, this was not statistically significant (hazard ratio, 1.49, 95% confidence interval, 0.95–2.33). Overall long-term survival was significantly worse among older patients (hazard ratio, 1.52, 95% confidence interval, 1.28–1.80).
Population-based administrative databases in the United States have provided a broad perspective of outcomes following esophagectomy allowing evaluation of effects due to a variety of factors including hospital teaching status, geographic region or socioeconomic status. Early studies demonstrated a significant relationship between case volume and outcomes at both the hospital[10,11] and surgeon[12] level which have led to a general inquiry into the structures and processes of care that might be important determinants of the volume-outcome relationship[13,14]. Analyses of these databases also provide more insight into the outcomes of infrequently performed operations, such as esophagectomy, in older patients.
Finlayson and colleagues accessed the Health Care Financing Administration’s MEDPAR and denominator files for 1994 to 1999 and found that early mortality among 4,080 patients aged 65 to 99 undergoing esophagectomy was 13.6%. When this study population was stratified by age cohorts, mortality ranged from 10.7% for the youngest cohort of patients aged 65 to 69, to 18.9% or greater for patients 80 years or older[15]. Using a broader study population of 5,282 patients obtained from the all-payer 1995–1997 Nationwide Inpatient Sample (NIS), representative of an estimated 20% of the United States population, Finlayson and colleagues observed that operative mortality was 8.1% among older patients (65 years of age or greater) undergoing operation at high-volume (>9 esophagectomies annually) hospitals compared with 19.3% at low-volume (<4 esophagectomies annually) hospitals (adjusted odds ratio, 0.38, 95% confidence interval 0.24 – 0.62), whereas among patients less than 65 years of age, hospital volume did not appear to be a significant factor for operative mortality[16].
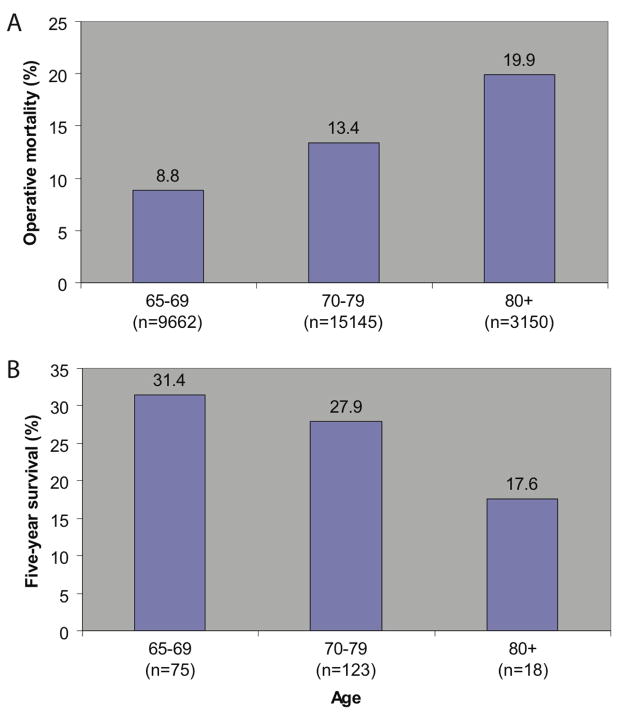
These investigators explored the early and long-term outcomes of esophagectomy in a larger sample of 27,957 patients, 65 years old or greater, from the 1994–2003 Nationwide Inpatient Sample, including 15,145 (54.2%) patients 70–79 years, and 3,150 (11.3%) patients 80 years or older[17]. In this sample the frequency of patients with 2 or more comorbid conditions increased significantly from 58.0% in patients 65 to 69 years of age, to 61.7% and 65.0% in the older age cohorts. Operative mortality (defined as death before hospital discharge) was significantly different and increased with each age cohort, from 8.8% to 13.4% and 19.9% (p<0.0001).
Patient functional status in this cohort was determined by patient discharge status. Compared with 83.5% of patients 65–69 years of age, significantly fewer patients (p<0.0001), 74.6% and 54.2% in the older cohorts, were discharged to home. Instead, 42% of older patients (80 years or greater) were discharged to a skilled nursing facility or other type of long-term care facility, compared with 14.2% of patients 65–69 years of age.
Long-term survival was determined in a second analytic cohort using the 1992–2001 SEER-Medicare linked databases, representing approximately 14% of the US population. Overall 5-year survival was significantly worse among octogenarians (80 years of age and older) undergoing esophagectomy, compared with patients aged 65–69 (Figure 2), although these data were derived from only 216 patients identified in the SEER-Medicare linked databases.
Figure 2.
A, Operative mortality following esophagectomy, 1994–2003 Nationwide Inpatient Sample. B, Overall five-year survival following esophagectomy for cancer, 1992–2001 SEER-Medicare linked database. Data from J Am Coll Surg, vol. 205, Finlayson E, Fan Z and Birkmeyer JD, “Outcomes in octogenarians undergoing high-risk cancer operation: a national study,” pp. 729–734, © 2007, with permission from Elsevier.
Although such studies can carry greater statistical power and capture outcomes across the spectrum of hospital and provider systems on a scale that might not be feasible in most single center reports, administrative databases, even when linked to cancer registries, lack the clinical detail that permit better understanding of the underlying processes and structures of care that are likely determinants of patient outcomes, particularly for esophagectomy[18]. Population-wide studies based on administrative databases should be interpreted with caution and should be utilized for purposes of hypothesis development, rather than policy implementation, particularly if applied to specific patient cohorts such as the elderly.
MORTALITY— CENTER-SPECIFIC REPORTS
Poon and colleagues have demonstrated that increasing age remains a significant risk factor for operative (30-day) mortality, but should not be the only factor for consideration of resection. In a retrospective evaluation of 737 patients, including 167 patients 70 years or greater, undergoing esophagectomy for carcinoma from 1982 to 1996, they observed significantly increased (p<0.02) operative mortality of 7.2% among patients 70 years or greater, compared with 3.0% in younger patients[19]. In the older group, 25% (42 patients) received a transhiatal approach compared with 12% (71 patients) of younger patients. Despite this higher operative mortality, hospital mortality (same hospitalization up to 6 months following operation) did not differ significantly, occurring in 18.0% of the older patients compared with 14.4% in the younger group of patients. Overall long-term survival was significantly worse (p<0.01) among older patients, who had median and 5-year survivals of 16 months and 26%, respectively, compared with 33 months and 35%, respectively, in the younger cohort. When non-cancer related deaths were censored from analysis, long-term survival was equivalent, with median and 5-year survivals of 28 months and 32%, respectively, in patients 70 years or greater, and 37 months and 37%, respectively, in younger patients. In a separate report evaluating 434 patients with esophageal squamous cell carcinoma undergoing esophagectomy from 1990 to 2002, this group has observed that patient age did not appear to differ among patients with or without technical complications, defined as anastomotic leak, recurrent laryngeal nerve palsy, chylothorax, conduit ischemic necrosis, postoperative hemorrhage requiring reoperation, wound dehiscence and delayed gastric emptying[20].
In a series of 773 patients with esophageal cancer undergoing esophagectomy from 1990 to 2003, with over 95% patients receiving thoracotomy, Abunasra and colleagues found that operative (30-day and hospital) mortality occurred in 4.8% (37 patients)[21]. When this study population was divided into quartiles by age, those in the oldest cohort, 73 years and older, were at over 4 times higher risk for operative mortality compared with patients 60 years or less. When analyzed as a continuous variable, each year of increasing age carried an odds ratio of 1.07 (95% confidence interval, 1.02–1.12), indicating nearly doubled risk of early mortality for each decade of increasing age. Other risk factors included obstructive lung disease and cervical or upper third esophageal tumor location.
Moskovitz and colleagues reported a single-center experience evaluating 751 patients, 50 years or older, undergoing esophagectomy[22] between 1996 and 2005. Younger patients, who had fewer co-morbidities and lower perioperative mortality than patients 50 years or older, were excluded from this analysis to avoid any bias due to improved outcomes in this generally lower-risk population. Octogenarians comprised 4% (31 patients) of this experience, and 10% (76 patients) were of age 75–79 years. Overall, 75% of patients underwent thoracotomy, with slightly fewer thoracotomies, but not statistically significant, performed in octogenarians. Postoperative mortality, defined as 60-day or in-hospital mortality, was significantly increased in these older cohorts, with mortality of 19.4% in the octogenarian cohort, compared with mortality of 5.1% (hazard ratio, 3.9; p < 0.01; 95% confidence interval, 1.5 to 10.6) for the entire study cohort. Although intraoperative blood loss and rates of perioperative complication (pulmonary, cardiovascular, infection, anastomotic leak) did not differ significantly between the younger and older cohorts in the study, older patients had longer hospital lengths of stay. Longer-term overall survival also was significantly worse among octogenarians, with median survival of 17 months compared with 47 months among 637 patients aged 50–75. These authors concluded that advanced age remains a significant risk factor for perioperative mortality and diminished long-term survival, independent of patient co-morbidities. Although older patients had similar rates of perioperative complication, their hospital mortality and lengths of stay were increased, leading the authors to suggest that older patients have less physiologic reserve and capacity to survive these complications, consistent with findings from an earlier report from this group. In contrast to the findings of Ferri et al[20], Rizk and colleagues found that patients older than 75 years experienced worse 30-day and one year survival if their operation was associated with perioperative complication, with hazard ratio, 0.61 (95% confidence interval, 0.43–0.81) for patients without perioperative complication [23]. These and earlier finding are listed Table 1.
Table 1.
Impact of patient age on outcomes following esophageal resection for cancer, selected recent studies
| Author | Study Period Data Source |
Age cohorts | Study Population | Operative mortality (%) | Five-year survival | Overall survival Adjusted hazard ratio |
P |
|---|---|---|---|---|---|---|---|
| Bailey et al [8] (2003) | 1991–2000 VA-NSQIP | continuum | 1777 (1509 cancer) | 9.8 | 1.05/year | 0.0001 | |
| Rouvelas et al [9] (2005) | 1987–2000 Cancer Registry, Sweden | <55 | 104 | 27.4 | 1.00 (reference) | ||
| 55–65 | 218 | 26.0 | 1.08 (0.82–1.42) | NS | |||
| 66–75 | 302 | 25.0 | 1.01 (0.84–1.41) | NS | |||
| >75 | 140 | 22.8 | 1.28 (0.96–1.72) | NS | |||
| Cronin-Fenton et al. [6] (2007) | 1994–2001 Cancer Registry, Ireland | <70 | No Surgery: 1705 | 1.00 (reference) | 0.99 | ||
| 70+ | 1.00 (0.89–1.12) | ||||||
| <70 | Resection: 927 | 1.00 (reference) | <0.001 | ||||
| 70+ | 1.52 (1.28–1.80) | ||||||
| Finlayson et al. [17] (2007) | 1994–2003 NIS | 65–69 | 9662 | 6.7 | |||
| 70–79 | 15145 | 9.3 | |||||
| 80+ | 3150 | 15.5 | <0.0001 | ||||
| 1992–2001 SEER-Medicare | 65–69 | 75 | 31.4 | ||||
| 70–79 | 123 | 27.9 | |||||
| 80+ | 18 | 17.6 | 0.02 | ||||
| Poon et al. [19] (1998) | 1982–1996 Clinical database | <70 | 570 | 3.0 | 35.0 | <0.02/<0.01 | |
| 70+ | 167 | 7.2 | 26.0 | ||||
| Alexiou et al.[34] (1998) | 1987–1997 Clinical database | <70 | 337 | 4.7 | 25.1 | NS | |
| 70–79 | 150 | 6.7 | 21.2 | ||||
| 80–86 | 36 | 5.6 | 19.8 | ||||
| Abunasra et al. [21] (2005) | 1990–2003 Clinical database | <59.5 | 193 | 2.1 | 1.0 (reference) | 0.009 | |
| 59.5–67.8 | 193 | 3.6 | 2.06 (0.52–8.22) | ||||
| 67.9–73.2 | 194 | 4.1 | 1.55 (0.36–6.72) | ||||
| >73.2 | 193 | 9.3 | 4.87 (1.35–17.55) | ||||
| Moskovitz et al. [22] (2006) | 1996–2005 Clinical database | <50 | 107 | 1.9 | 1.0 (reference) | ||
| 50–59 | 228 | 4.8 | |||||
| 60–70 | 285 | 5.6 | |||||
| 70–79 | 207 | 7.3 | |||||
| 80+ | 31 | 19.4 | 3.9 (1.5–10.6) | <0.01 | |||
| Median survival (months): | |||||||
| 50–75 | 46.7 | ||||||
| 75–79 | 29.1 | ||||||
| 80+ | 16.8 | 0.01 | |||||
| Five-year survival (%) | |||||||
| Current report (2009) | 1993–2008 Clinical database | continuum | 1251 | 2.6 | 39.7 | 1.022/year | <0.0001 |
| <55 | 241 | 0.8 | 59 (95% CI: 41, 55) | ||||
| 55–64 | 390 | 3.3 | 42 (36, 48) | ||||
| 65–74 | 418 | 2.2 | 38 (33, 43) | ||||
| >=75 | 202 | 4.0 | 28 (21, 35) |
Abbreviations: SEER, Surveillance, Epidemiology, and End Results; NIS, Nationwide Inpatient Sample; VA-NSQIP, Department of Veterans’ Administration National Surgical Quality Improvement Program; NS, no statistically significant difference; N/A, not available
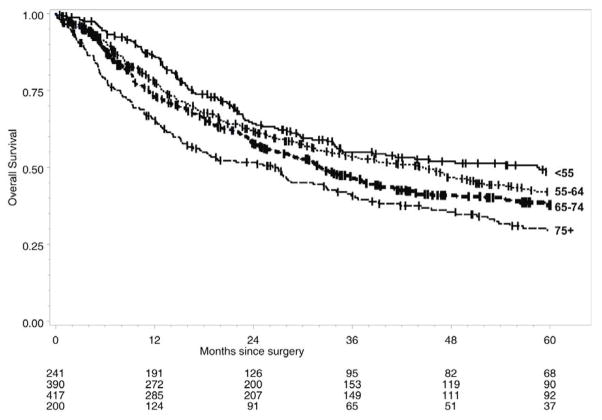
In a previous report of outcomes in patients undergoing esophagectomy at our institution between 1976 and 2006 we found that both hospital mortality and operative blood loss decreased significantly between early and later eras of this overall experience [24]. Over the last fifteen years at our institution, between 1993 and 2008, reflecting our more recent experience, 1251 patients, including 620 patients aged 65 or greater, have undergone esophagectomy for esophageal cancer. Transhiatal esophagectomy without thoracotomy was performed in 1212 (97%) patients. Overall 30-day and hospital mortality was 2.6%, without any differences noted between younger and older patient cohorts. When evaluated by decade of age, overall long-term survival was significantly worse for patients 65 years of age or older, compared with the younger age cohorts (Figure 3). Cox proportional hazards analysis in this population indicated that increasing age at operation was a significant risk factor for worse overall survival with hazard ratio of 1.022 (95% confidence interval, 1.014 – 1.031). Increasing age at operation remained a significant adverse risk factor for disease-specific survival, although to a lesser degree, with hazard ratio of 1.012 (95% confidence interval, 1.001 – 1.022) per year of increasing age, respectively (manuscript in preparation).
Figure 3.
Kaplan-Meier overall survival following esophagectomy for esophageal cancer, stratified by age, University of Michigan, 1993–2008. .
Although considerable debate continues regarding the relative risks and benefits of esophageal resection with or without thoracotomy, whether operation approach influences perioperative outcomes or long-term survival in older patients is not well studied. Single-center reports such as discussed above and elsewhere [25–31] reinforce the opinion that operation approach does not appear to influence outcomes, including long-term survival, following esophagectomy in older patients.
OVERALL COMPLICATIONS
Few studies have sought expressly to evaluate patient age as a risk factor for postoperative complication following esophagectomy. Evaluation of the Swedish esophageal cancer registry identified 275 patients undergoing esophagectomy between 2001 and 2003, including 112 patients 70 years of age or greater. In the entire study cohort, 46% experienced one or more complications, including anastomotic leak (9%), pulmonary complications (18%), cardiac, hepatic or renal complications (17%) or technical complications such as intraoperative bleeding, recurrent laryngeal nerve injury, or need for reoperation (9%). Patients treated with preoperative chemotherapy and/or radiation therapy had a slightly increased complication rate that was not statistically significant. Although patient age was not a significant risk factor for development of postoperative complications, patients who sustained multiple (3 or more) postoperative complications were more likely to be older[32].
Several single center studies have reported differing conclusions regarding the association between age, perioperative complication and mortality following esophagectomy for cancer. Atkins and colleagues identified 379 patients undergoing esophagectomy including 341 patients who underwent resection for cancer between 1996 and 2002 [33]. Operative (30-day) mortality was 5.8%, with at least one complication occurring in 64% of patients. Multivariate analysis indicated that only increasing age and occurrence of pneumonia were risk factors for increased operative mortality, although in univariate analysis dysphagia, anastomotic leak, and increased comorbidities were also found to be associated significantly with operative mortality.
In contrast to a subsequent report from the same institution[21] (discussed earlier in this text), Alexiou and colleagues found that increasing age alone did not have a significant adverse impact on perioperative complications, operative mortality or long-term survival among 523 patients undergoing esophagectomy [34]. Notably, a significantly greater proportion of older patients were found to be unfit for operation. In this study, 686 patients had been assessed for operation between 1987 and 1997, with nearly 20% of older (80–86 years) patients evaluated excluded from operation, compared with only 2% and 5% found to be inoperable among the younger cohorts, aged less than 70 and 70–79 years, respectively. When patients undergoing operation were stratified by these deciles of age, no significant differences were observed in terms of perioperative complication or early mortality. Moreover, since long-term survival was equivalent among the different age cohorts the authors concluded that among highly selected older patients the survival benefit of esophageal resection can be similar to that of younger counterparts, as had been suggested by others[19].
POSTOPERATIVE DELIRIUM
Post-operative delirium occurs in approximately 10%–15% of patients undergoing non-cardiac general surgery[35,36] including esophagectomy[37], and ranges as high as 50% or greater particularly in older patients[38]. Delirium has been associated with other major post-operative complications, particularly myocardial infarction, pneumonia, pulmonary edema and respiratory failure. Moreover, delirium appears to increase both hospital and intermediate-term mortality as well as likelihood for transfer to long-term care[39]. Although there are limited data evaluating the impact of delirium in older patients following esophagectomy, risk factors for development of postoperative delirium following non-cardiac surgery include age 70 years or greater, poor cognitive or functional status, self-reported alcohol abuse, abnormal preoperative serum sodium, potassium or glucose levels[35]. Additionally, factors during hospitalization such as use of physical restraints, malnutrition, use of a bladder catheter, addition of 3 or more medications and adverse outcomes from iatrogenic events can precipitate postoperative delirium particularly among patients with pre-disposing risks for delirium[36,40]. These factors have been utilized in risk profiles in hospitalized general medicine patients and those undergoing abdominal or thoracic operation[39,41] but remain to be validated among patients undergoing esophageal resection.
QUALITY OF LIFE
In evaluating an operation designed to preserve the ability to eat and also to provide satisfactory and durable oncologic outcomes, formal assessment of postoperative quality of life has been largely qualitative and there are few reports evaluating quality of life following esophagectomy for carcinoma in older patients. Using the Medical Outcomes Study 36-Item Short-Form Health Survey (MOS SF-36), Deschamps evaluated postoperative measures of quality of life in eight areas: general health (health perception), daily activities (physical functioning), work (role - physical), emotional problems (role - emotional), social activities (social functioning), nervousness/depression (mental health), pain (bodily pain), and vitality (energy/fatigue). Following esophagectomy for Barrett’s esophagus with high-grade dysplasia, older patients were more likely to report diminished physical function and performance at work, compared with the national standard. In contrast, among patients undergoing resection for carcinoma, although self-perception of physical functioning was significantly diminished in this entire cohort, patient age was not associated with any significant decline in the eight areas assessed by the MOS SF-36 [42].
It behooves the operating surgeon to assure that patients receive thorough preoperative education and informed patient consent regarding the potential risks and benefits of esophageal resection. Following operation and after medical recovery, subsequent discussions regarding prognostic implications of the operative findings might be tempered by patient preference, although this has not been established for patients undergoing esophagectomy. In a recent survey-based analysis of patient preferences for disclosure of prognostic information, patient age did not influence the predominant desire for a detailed discussion of potential outcomes and treatment options. The majority of patients (80%) preferred that their surgeon start the discussion regarding prognosis but patients were significantly older (median age, 69 years vs. 60 years) among those who wished to initiate or to defer this discussion completely [43].
SUMMARY
Although studies differ in their definition of the older patient, when considered as a continuum, increasing age is associated with greater operative mortality. Complication rates also appear to be significantly higher with advancing age, possibly due to limited physiologic reserve. As understanding of risk factors for perioperative morbidity and mortality following esophagectomy has improved, investigators have sought to develop models for risk stratification [44–46] in which patient age is a significant but not the sole determinant of prospective assessment of risk for complication or mortality. Such prognostic indicators, if validated among independent patient cohorts, can serve as useful adjuncts in decision-making with appropriate clinical judgment.
In addition, reported patient survival differs dramatically between rates reported by single centers and rates observed in population-based studies, with operative mortality rates typically lower in single-center reports. While such reports are usually issued from groups with higher operative volume that might be a surrogate for surgical experience, it is also possible that the association between operation volume and improved outcomes reflects optimization of institution-specific infrastructure and/or clinical care pathways[37]. As these processes of care evolve, they should be tailored with attention to differences in the care of older patients with esophageal cancer. Whether widespread application of such processes of care then can lead to less perioperative mortality and fewer complications as well as improved long-term survival remains untested.
Acknowledgments
We thank Dr. Mark B Orringer for his critical comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Birkmeyer JD, Sun Y, Wong SL, et al. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245(5):777–783. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2005. National Cancer Institute; Bethesda, MD: 2008. http://seer.cancer.gov/csr/1975_2005/ based on November 2007 SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.Chang AC, Ji H, Birkmeyer NJ, et al. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg. 2008;85(2):424–429. doi: 10.1016/j.athoracsur.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esoph. 2009;22(1):1–8. doi: 10.1111/j.1442-2050.2008.00901.x. [DOI] [PubMed] [Google Scholar]
- 5.Paulson EC, Ra J, Armstrong K, et al. Underuse of esophagectomy as treatment for resectable esophageal cancer. Arch Surg. 2008;143(12):1198–1203. doi: 10.1001/archsurg.143.12.1198. [DOI] [PubMed] [Google Scholar]
- 6.Cronin-Fenton DP, Sharp L, Carsin AE, et al. Patterns of care and effects on mortality for cancers of the oesophagus and gastric cardia: A population-based study. Eur J Cancer. 2007;43(3):565–575. doi: 10.1016/j.ejca.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Khuri SFMD, Daley JMD, Henderson WP, et al. The Department of Veterans Affairs’ NSQIP: The first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. Ann Surg. 1998;228(4):491–507. doi: 10.1097/00000658-199810000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg. 2003;75(1):217–222. doi: 10.1016/s0003-4975(02)04368-0. [DOI] [PubMed] [Google Scholar]
- 9.Rouvelas I, Zeng W, Lindblad M, et al. Survival after surgery for oesophageal cancer: a population-based study. Lancet Oncol. 2005;6(11):864–870. doi: 10.1016/S1470-2045(05)70347-8. [DOI] [PubMed] [Google Scholar]
- 10.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280(20):1747–1751. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 11.Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 12.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 13.Birkmeyer JD, Sun Y, Goldfaden A, et al. Volume and process of care in high-risk cancer surgery. Cancer. 2006;106(11):2476–2481. doi: 10.1002/cncr.21888. [DOI] [PubMed] [Google Scholar]
- 14.Meguid RA, Weiss ES, Chang DC, et al. The effect of volume on esophageal cancer resections: What constitutes acceptable resection volumes for centers of excellence? J Thorac Cardiovasc Surg. 2009;137(1):23–29. doi: 10.1016/j.jtcvs.2008.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finlayson EV, Birkmeyer JD. Operative mortality with elective surgery in older adults. Eff Clin Pract. 2001;4(4):172–177. [PubMed] [Google Scholar]
- 16.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138(7):721–725. doi: 10.1001/archsurg.138.7.721. [DOI] [PubMed] [Google Scholar]
- 17.Finlayson E, Fan Z, Birkmeyer JD. Outcomes in octogenarians undergoing high-risk cancer operation: a national study. J Am Coll Surg. 2007;205(6):729–734. doi: 10.1016/j.jamcollsurg.2007.06.307. [DOI] [PubMed] [Google Scholar]
- 18.Kozower BD, Stukenborg GJ, Lau CL, et al. Measuring the quality of surgical outcomes in general thoracic surgery: should surgical volume be used to direct patient referrals? Ann Thorac Surg. 2008;86(5):1405–1408. doi: 10.1016/j.athoracsur.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 19.Poon RT, Law SY, Chu KM, et al. Esophagectomy for carcinoma of the esophagus in the elderly: results of current surgical management. Ann Surg. 1998;227(3):357–364. doi: 10.1097/00000658-199803000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferri LE, Law S, Wong KH, et al. The influence of technical complications on postoperative outcome and survival after esophagectomy. Ann Surg Oncol. 2006;13(4):557–564. doi: 10.1245/ASO.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 21.Abunasra H, Lewis S, Beggs L, et al. Predictors of operative death after oesophagectomy for carcinoma. Br J Surg. 2005;92(8):1029–1033. doi: 10.1002/bjs.5049. [DOI] [PubMed] [Google Scholar]
- 22.Moskovitz AH, Rizk NP, Venkatraman E, et al. Mortality increases for octogenarians undergoing esophagogastrectomy for esophageal cancer. Ann Thorac Surg. 2006;82(6):2031–2036. doi: 10.1016/j.athoracsur.2006.06.053. [DOI] [PubMed] [Google Scholar]
- 23.Rizk NP, Bach PB, Schrag D, et al. The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg. 2004;198(1):42–50. doi: 10.1016/j.jamcollsurg.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Orringer MB, Marshall B, Chang AC, et al. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg. 2007;246(3):363–372. doi: 10.1097/SLA.0b013e31814697f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nigro JJ, DeMeester SR, Hagen JA, et al. Node status in transmural esophageal adenocarcinoma and outcome after en bloc esophagectomy. J Thorac Cardiovasc Surg. 1999;117(5):960–968. doi: 10.1016/S0022-5223(99)70377-6. [DOI] [PubMed] [Google Scholar]
- 26.Hulscher JBF, van Sandick JW, Tijssen JGP, et al. The recurrence pattern of esophageal carcinoma after transhiatal resection. J Am Coll Surg. 2000;191(2):143–148. doi: 10.1016/s1072-7515(00)00349-5. [DOI] [PubMed] [Google Scholar]
- 27.Hulscher JBF, van Sandick JW, de Boer AGEM, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347(21):1662–1669. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 28.McCulloch P, Ward J, Tekkis PP. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. Br Med J. 2003;327(7425):1192–1197. doi: 10.1136/bmj.327.7425.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rentz J, Bull D, Harpole D, et al. Transthoracic versus transhiatal esophagectomy: a prospective study of 945 patients. J Thorac Cardiovasc Surg. 2003;125(5):1114–1120. doi: 10.1067/mtc.2003.315. [DOI] [PubMed] [Google Scholar]
- 30.Gockel I, Heckhoff S, Messow C, et al. Transhiatal and transthoracic resection in adenocarcinoma of the esophagus: Does the operative approach have an influence on the long-term prognosis? W J Surg Oncol. 2005;3(1):40. doi: 10.1186/1477-7819-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yekebas EF, Schurr PG, Kaifi JT, et al. Effectiveness of radical en-bloc-esophagectomy compared to transhiatal esophagectomy in squamous cell cancer of the esophagus is influenced by nodal micrometastases. J Surg Oncol. 2006;93(7):541–549. doi: 10.1002/jso.20544. [DOI] [PubMed] [Google Scholar]
- 32.Viklund P, Lindblad M, Lu M, et al. Risk factors for complications after esophageal cancer resection: a prospective population-based study in Sweden. Ann Surg. 2006;243(2):204–211. doi: 10.1097/01.sla.0000197698.17794.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atkins BZ, Shah AS, Hutcheson KA, et al. Reducing hospital morbidity and mortality following esophagectomy. Ann Thorac Surg. 2004;78(4):1170–1176. doi: 10.1016/j.athoracsur.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 34.Alexiou C, Beggs D, Salama FD, et al. Surgery for esophageal cancer in elderly patients: the view from Nottingham. J Thorac Cardiovasc Surg. 1998;116(4):545–553. doi: 10.1016/S0022-5223(98)70159-X. [DOI] [PubMed] [Google Scholar]
- 35.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134–139. [PubMed] [Google Scholar]
- 36.Anderson D. Preventing delirium in older people. Br Med Bull. 2005;73–74(1):25–34. doi: 10.1093/bmb/ldh048. [DOI] [PubMed] [Google Scholar]
- 37.Low D, Kunz S, Schembre D, et al. Esophagectomy—It’s not just about mortality anymore: standardized perioperative clinical pathways improve outcomes in patients with esophageal cancer. J Gastrointest Surg. 2007;11(11):1395–1402. doi: 10.1007/s11605-007-0265-1. [DOI] [PubMed] [Google Scholar]
- 38.Flinn DR, Diehl KM, Seyfried LS, et al. Prevention, diagnosis and management of post-operative delirium in older adults. J Am Coll Surg in review. 2009 doi: 10.1016/j.jamcollsurg.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249(1):173–178. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 40.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852–857. [PubMed] [Google Scholar]
- 41.Ganai S, Lee KF, Merrill A, et al. Adverse outcomes of geriatric patients undergoing abdominal surgery who are at high risk for delirium. Arch Surg. 2007;142(11):1072–1078. doi: 10.1001/archsurg.142.11.1072. [DOI] [PubMed] [Google Scholar]
- 42.Deschamps C, Nichols FC, 3rd, Cassivi SD, et al. Long-term function and quality of life after esophageal resection for cancer and Barrett’s. Surg Clin North Am. 2005;85(3):649–656. xi. doi: 10.1016/j.suc.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 43.Lagarde S, Franssen S, van Werven J, et al. Patient preferences for the disclosure of prognosis after esophagectomy for cancer with curative intent. Ann Surg Oncol. 2008;15(11):3289–3298. doi: 10.1245/s10434-008-0068-y. [DOI] [PubMed] [Google Scholar]
- 44.Aust JB, Henderson W, Khuri S, et al. The impact of operative complexity on patient risk factors. Ann Surg. 2005;241(6):1024–1027. doi: 10.1097/01.sla.0000165196.32207.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shende MR, Waxman J, Luketich JD. Predictive ability of preoperative indices for esophagectomy. Thorac Surg Clin. 2007;17(3):337–341. doi: 10.1016/j.thorsurg.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Lagarde SM, Reitsma JB, Maris A-KD, et al. Preoperative prediction of the occurrence and severity of complications after esophagectomy for cancer with use of a nomogram. Ann Thorac Surg. 2008;85(6):1938–1945. doi: 10.1016/j.athoracsur.2008.03.014. [DOI] [PubMed] [Google Scholar]