Abstract
Curcumin, a yellow pigment and the active component of turmeric, has been shown to protect against carcinogenesis and prevent tumor development in several types of cancer. However, its low bioavailability and potency prevent it from being effective in most chemotherapeutic applications. One potential means of circumventing this problem has been the creation of synthetic curcumin analogues. We tested the efficacy of two such analogues, known as FLLL11 and FLLL12, in human pancreatic cancer cell lines. We compared the impact of curcumin with FLLL11 and FLLL12 on cell viability in five different pancreatic cancer cell lines. Although all three compounds were capable of lowering viability in all cell lines tested, FLLL11 and FLLL12 (IC50 values between 0.28–3.2 and 0.91–3.43λμmol/l, respectively) were substantially more potent than curcumin (IC50 values between 8.67 and 20.35λμmol/l). In addition, FLLL11 and FLLL12 inhibited phosphorylation of signal transducer and activator of transcription 3 and AKT, two cell signaling pathways frequently found persistently active in many forms of cancer. Furthermore, FLLL11 and FLLL12 were found to be more effective than curcumin in inducing apoptosis as evidenced by increased cleavage of PARP and caspase-3 in pancreatic cancer cell lines. These results indicate that the curcumin analogues, FLLL11 and FLLL12, are more effective than curcumin in inhibiting cell viability and inducing apoptosis, and may have translational potential as chemopreventive or therapeutic agents for pancreatic cancer.
Keywords: AKT, curcumin, pancreatic cancer, signal transducer and activator of transcription 3
Introduction
Pancreatic cancer develops when cancerous cells form in the tissues of the pancreas, a large organ that lies horizontally behind the lower part of the stomach. Each year approximately 32λ000 individuals in the United States are diagnosed with pancreatic cancer. According to the American Cancer Society, there were an estimated 37λ170 new cases and 33λ370 deaths from pancreatic cancer in the United States in 2007. Cancer of the exocrine pancreas has a low cure rate and has an overall survival rate of less than 4% [1]. The highest cure rate occurs when the tumor is truly localized to the pancreas; however, this stage of the disease accounts for less than 20% of cases. For those patients with localized disease and small cancers (<2λcm) and with no lymph node metastases or extension beyond the capsule of the pancreas, complete surgical resection can yield actuarial 5-year survival rates of 18–24% [2]. For patients with advanced cancers, the overall survival rate of all stages is less than 1% at 5 years with most patients dying within 1 year [3,4]. Hence, there is an immediate need for better treatment and prevention of pancreatic cancer. The cellular mechanisms contributing to pancreatic cancer are still not well understood, but involve a multistep process of mutations of tumor suppressor genes such as p53, retinoblastoma-1 genes [5] as well as signaling protein dysregulation.
Curcumin is a bioactive component found in the rhizome of the perennial herb Curcuma longa. A polyphenolic compound with intense yellow coloring, curcumin, has been part of therapeutic preparations for centuries because of its wide spectrum of beneficial activities and its safety in relatively large doses [6]. Extensive research has indicated that curcumin can influence multiple cell signaling pathways and has anti-inflammatory, antioxidant, chemopreventive, and chemotherapeutic properties in addition to many others [7]. The anticarcinogenic properties of curcumin continue to be a subject of great interest, and evidence that it can inhibit the initiation, progression, and continued survival of cancerous cells likewise continue to accumulate [7]. Curcumin has antitumor effects against pancreatic cancer in mice tumor models and inhibitory effects in pancreatic cancer cells [8–12]. Despite these promising findings, curcumin is yet to be approved as a chemotherapeutic agent. Testing in animal models and human clinical trials has revealed that the bioavailability of curcumin is low, owing to its poor absorption across the gut, limited tissue distribution, rapid metabolism, and its subsequent elimination from the body [13]. In light of these findings, numerous strategies have been devised to address this limitation of curcumin, including the design and synthesis of novel structural analogues [14]. Two such compounds, termed FLLL11 and FLLL12, were synthesized in our laboratories. In this study, we compared the effects of FLLL11, FLLL12, and curcumin in pancreatic cancer cells. We demonstrated that FLLL11 and FLLL12 are more potent than curcumin in the inhibition of signal transducer and activator of transcription 3 (STAT3) and AKT AQ2phosphorylation, suppression of cell viability, and induction of apoptosis of human pancreatic cancer cell lines.
Materials and methods
Cell culture
Human pancreatic cancer cell lines (PANC-1, BXPC-3, MIA-PACA-2, ASPC-1, and HPAC), and normal human bladder cells were obtained from the American Type Culture CollectionAQ3. These pancreatic cancer cells were maintained in 10% fetal bovine serum (Invitrogen, Carlsbad, California, USA), Dulbecco's modification of Eagle's medium, 1× with 4.5λg/l, l-glutamine, and sodium pyruvate (Mediatech) with 1% penicillin/streptomycin. Immortalized but not malignant human pancreatic duct epithelial cells were provided by Dr Ming-Sound Tsao at the University of Toronto and were maintained in CnT-07CF epidermal keratinocyte medium (CELLnTEC Advanced Cell Systems, Bern, Switzerland) supplemented with 100λU/ml penicillin and 100λmg/ml streptomycin (Invitrogen Life Technologies) and 0.07λmmol/l CaCl2 in addition to the supplements provided by manufacturer. All cell lines were cultured in cell culture incubators, which were set at 37°C and aired with 5% CO2.
Curcumin and curcumin analogues
Curcumin was obtained from Sigma-Aldrich Inc., (St Louis, Missouri, USA). FLLL11 and FLLL12 were prepared by acid-catalyzed condensation of acetone with vanillin and 3,5-dimethoxy-4-hydroxybenzaldehyde, respectively, according to known procedures [15,16]. FLLL11 was initially isolated as a natural product from Curcuma domestica Val. (Zingiberaceae) [17] and later Curcuma longa Linn [18]. FLLL12 is a synthetic analogue of FLLL11 with an additional methoxy substituent at the 5-position of both aromatic rings [19]. The spectral data of both of the synthesized compounds were identical to those reported earlier. Both compounds were synthesized in Dr Pui-Kai's Laboratory and dissolved in dimethylsulfoxide to a final stock concentration of 20λmmol/l. GO-Y030 was provided by Dr Hiroyuki Shibata (Tohoku University, Sendai, Japan).
MTT cell viability assay
PANC-1, BXPC-3, MIA-PACA-2, ASPC-1, HPAC pancreatic cancer cells, HPDE, and normal human bladder cells were seeded in 96-well plates (4000λcells/well) in triplicate in Dulbecco's Modification of Eagle's Medium and 10% fetal bovine serum. The next day, pancreatic cancer cells were treated with 1.0–20λμmol/l of FLLL11 and FLLL12 as well as 2.5–20λμmol/l of curcumin (Sigma-Aldrich) for 72λh. At the end of each time point, 25λμl of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide: #M5655, Sigma-Aldrich] was added to each well of the plate and incubated for 3.5λh. After this, 100λμl of N,N-dimethylformamide (#D4551, Sigma-Aldrich) solubilization solution was added to each well. Plates were left at room temperature overnight to allow complete lysis of cells and read at 450λnm the next day. Microsoft excel was used to analyze the cell viability data. The untreated cells were set at 100% and the cell viability of curcumin-treated, FLLL11-treated, and FLLL12-treated cells was determined relative to untreated cells. Results were presented as bar charts with error bars. IC50 values were calculated using SigmaPlot (Systat Software, IncAQ4.)
Western blot analysis
PANC-1, BXPC-3, MIA-PACA-2, HPAC pancreatic cancer cells, and HPDE cells were treated with 5 and 10λμmol/l of FLLL11 and FLLL12, respectively, or 10 and 20λμmol/l of curcumin (Sigma-Aldrich) for 24–48λh. For western blots, 50λμg of total protein from pancreatic cancer cell lysates were subjected to SDS polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane. Membranes were blotted with phospho-specific STAT3 antibody [Tyrosine 705; #9131 Cell Signaling Tech., (Beverly, Massachusetts, USA)], phospho-independent STAT3 antibody (#9132 Cell Signaling Tech.), phospho-specific AKT antibody (Serine 473; #9271S Cell Signaling Tech.), cleaved PARP antibody (#9546 Cell Signaling Tech.), cleaved caspase-3 (#9661S Cell Signaling Tech.), and GAPDH antibody (#MAB374 Chemicon International Inc., Temecula, California, USA). Membranes were analyzed with enhanced chemiluminescence Plus reagents and scanned with the Storm scanner (Amersham Pharmacia Biotech Inc., Piscataway, New Jersey, USA).
Results
Curcumin analogues, FLLL11 and FLLL12, are more potent than curcumin in inhibiting cell viability in human pancreatic cancer cells.
In this study, we examined the growth suppressive activities of the structural analogues of curcumin, FLLL11, and FLLL12 (Fig. 1), in five independent human pancreatic cancer cell lines, PANC-1, BXPC-3, MIA-PACA-2, ASPC-1, and HPAC. After 72λh of treatments, FLLL11, FLLL12, and curcumin all showed some inhibition of cell viability (Table 1). FLLL11 and FLLL12 exhibited greater inhibition than curcumin in these five pancreatic cancer cell lines. IC50 values of FLLL11 and FLLL12 in these five different human pancreatic cancer cell lines were between 0.28–3.20 and 0.91–3.43λμmol/l, respectively (Table 1), which are substantially more potent than curcumin (IC50 values between 8.67 and 20.35λμmol/l). FLLL11 and FLLL12 showed certain inhibition of cell proliferation in immortalized HPDE cells, but they have less inhibitory effects in normal human bladder cells with IC50 values as high as 206.41 and 886.76λμmol/l, respectively (Table 1).
Fig. 1.
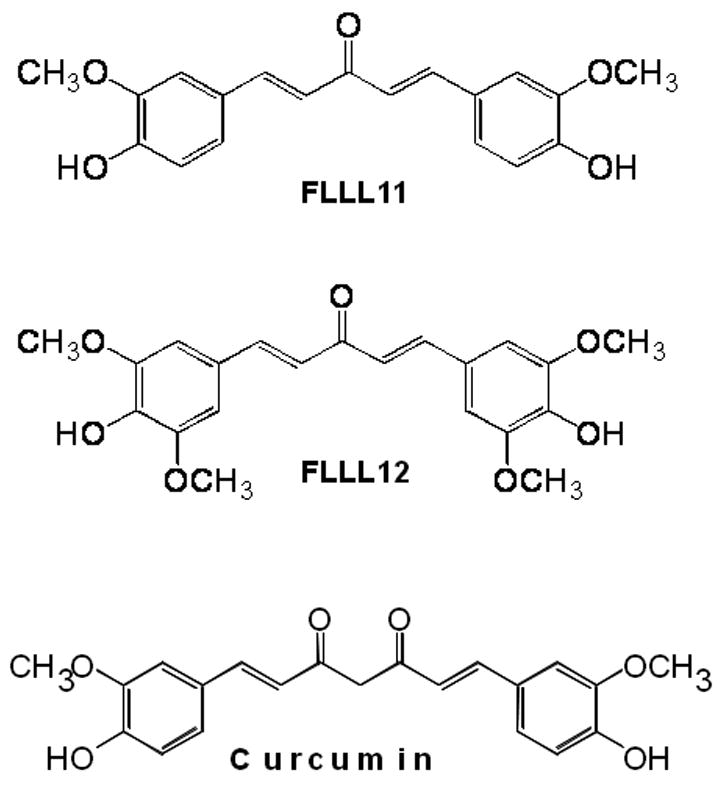
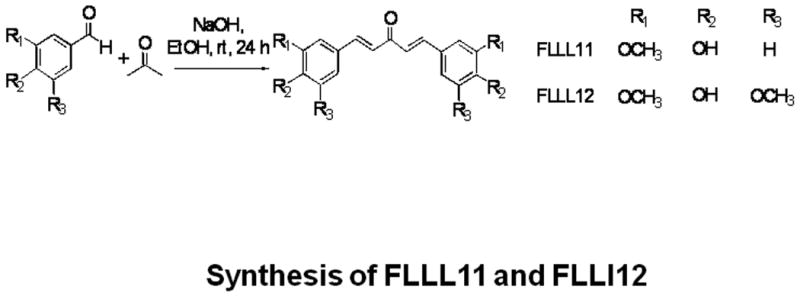
(a) The chemical structures of curcumin, FLLL11, and FLLL12. (b) Synthetic scheme of FLLL11 and FLLL12.
Table 1.
IC50 (μmol/l) of FLLL11, FLLL12, and curcumin in human pancreatic cancer cells and normal human cells
| FLLL11 | FLLL12 | Curcumin | |
|---|---|---|---|
| λPANC-1 | 3.20 | 3.09 | 20.35 |
| BXPC-3 | 0.28 | 1.31 | 8.67 |
| MIA-PACA-2 | 2.52 | 3.43 | 11.16 |
| ASPC-1 | 0.95 | 2.64 | 12.40 |
| HPAC | 0.50 | 0.91 | 15.99 |
| HPDE | 0.44 | 0.26 | 5.60 |
| Bladder | 206.41 | 886.76 | >1000 |
Pancreatic cancer cells or normal human cells were seeded in 96-well plates (4000λcells/well) in triplicate. The next day, pancreatic caner cells were treated with FLLL11, FLLL12, and curcumin for 72λh. The cell viability was analyzed by MTT assays. IC50 values were calculated using Sigma Plot from two to three independent experiments with each dose of drug in triplicate well.
FLLL11 and FLLL12 are more potent than curcumin in inducing STAT3 phosphorylation and apoptosis in human pancreatic cancer cells.
We further examined whether FLLL11 and FLLL12 could inhibit STAT3 phosphorylation in PANC-1, BXPC-3, and HPAC pancreatic cancer cell lines. These cell lines have been shown to have elevated levels of STAT3 phosphorylation. Both FLLL11 and FLLL12 were able to inhibit STAT3 phosphorylation (Tyr705) in these cell lines (Fig. 2a, b and c). In addition, the inhibition of STAT3 phosphorylation seems to correlate with the induction of apoptosis evidenced by increased levels of cleaved caspase-3 (Fig. 2a, b and c) by FLLL11 and FLLL12 in these three independent human pancreatic cancer cell lines. FLLL12 at 5 and 10λμmol/l inhibited STAT3 phosphorylation and induced increased levels of cleaved caspase-3 in PANC-1 pancreatic cancer cells, whereas 5 and 10λμmol/l of FLLL11 were less effective in inhibiting STAT3 phosphorylation and inducing increased levels of cleaved caspase-3 (Fig. 2a). In BXPC-3 pancreatic cancer cells, 5 and 10λμmol/l of FLLL11 and FLLL12, respectively, inhibited STAT3 phosphorylation and induced significant levels of cleaved PARP, whereas 20λμmol/l of curcumin was less effective in inhibiting STAT3 phosphorylation and increasing cleaved caspase-3 (Fig. 2b). FLLL11 and FLLL12 were both able to inhibit AKT phosphorylation at a concentration of 10λμmol/l, whereas a concentration of 20λμmol/l of curcumin was required to inhibited AKT phosphorylation (Fig. 2b). Lastly, 10λμmol/l of both FLLL11 and FLLL12 inhibited STAT3 phosphorylation and increased levels of cleaved caspase-3 in HPAC pancreatic cancer cells, whereas 10λμmol/l of curcumin was less potent (Fig. 2c). However, FLLL11 and FLLL12 do not induce apoptosis in human pancreatic duct epithelial cells as evidence by cleaved caspase-3 (Fig. 2d).
Fig. 2.
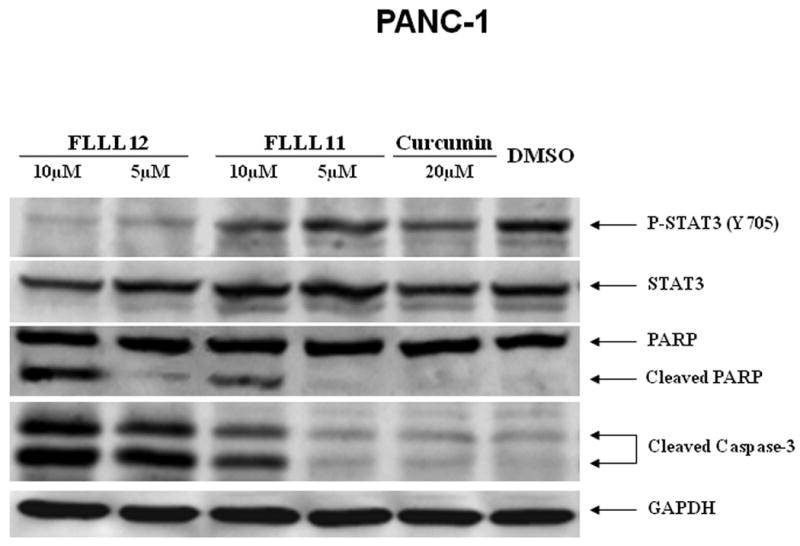
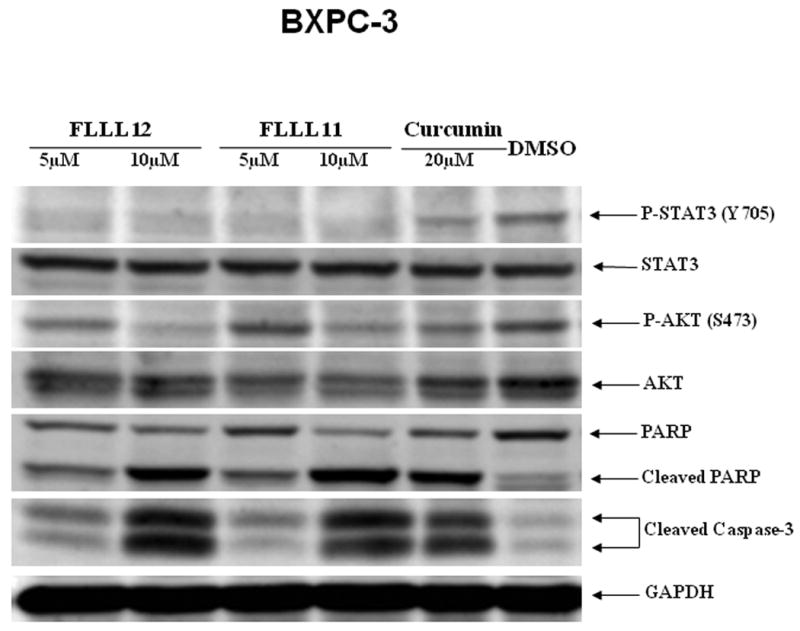
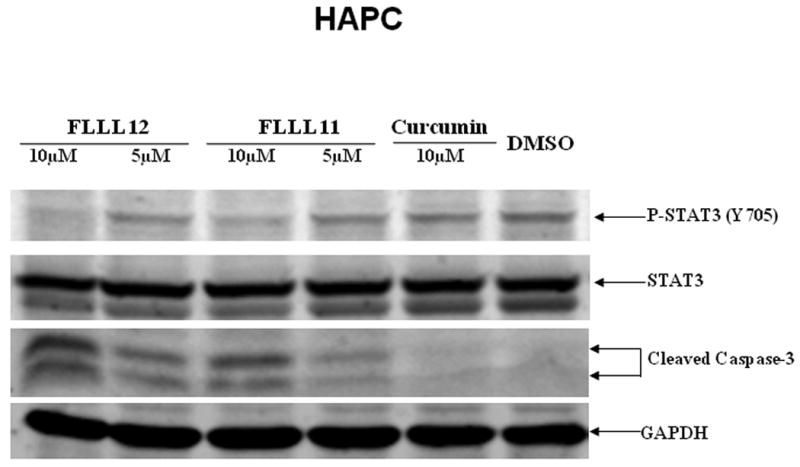
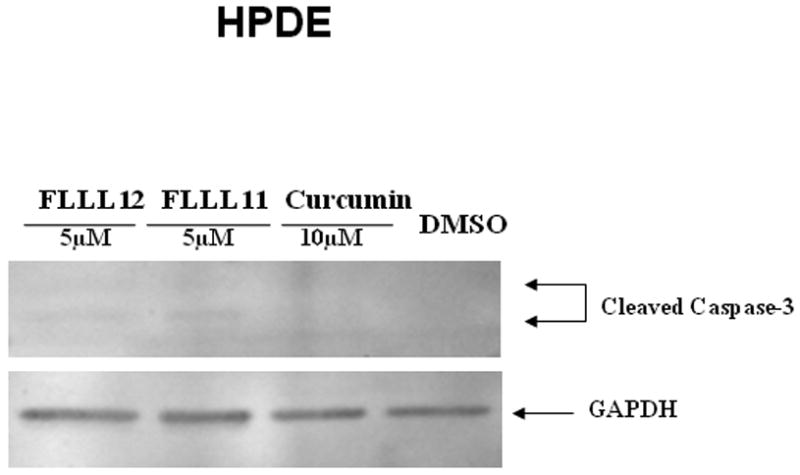
FLLL11 and FLLL12 inhibit STAT3 and/or AKT phosphorylation and induce apoptosis in (a) PANC-1, (b) BXPC-3, and (c) HPAC pancreatic cancer cell lines. However, FLLL11, FLLL12 do not increase cleaved caspase-3 in (d) human pancreatic duct epithelial cells. Cells were treated with 5–10λμmol/l of FLLL11 and FLLL12 as well as 10 or 20λμmol/l of curcumin for 24–48λh. Fifty micrograms of total protein from cell lysates were subjected to SDS polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membrane. Membranes were blotted with phospho-specific STAT3 (Tyrosine 705), phospho-independent STAT3, phospho-specific AKT antibody (Serine 473), cleaved PARP antibody, cleaved caspase-3 antibody, and GAPDH antibody. DMSO, dimethylsulfoxide.
We also compared the effects of FLLL12 and FLLL11 with one of the most potent curcumin analogues, GO-Y030 [19]. The results indicated that FLLL12 has a similar efficacy as GO-Y030 in the inhibition of STAT3 phosphorylation and caspase-3 cleavage in PANC-1 pancreatic cancer cells (Fig. 3a). In HPAC pancreatic cancer cells, GO-Y030 may be slightly more potent (Fig. 3b). Both FLLL12 and GO-Y030 induced increased levels of cleaved PARP and caspase-3 at similar levels in MIA-PACA2, whereas FLLL11 was less potent (Fig. 3c).
Fig. 3.
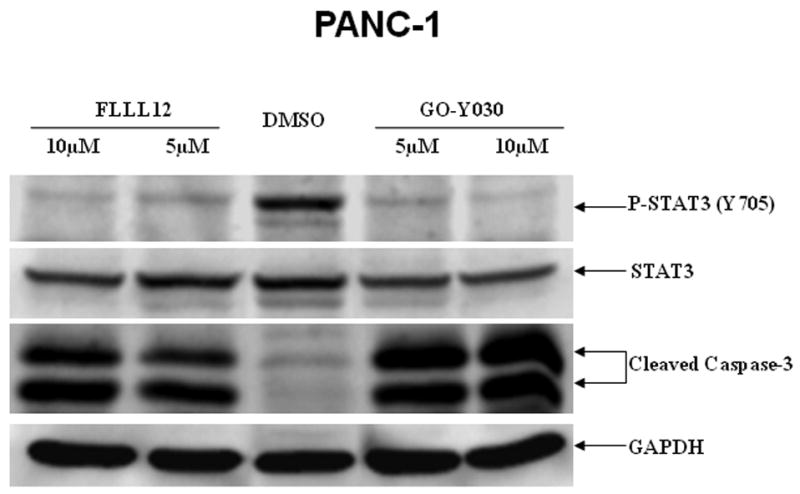
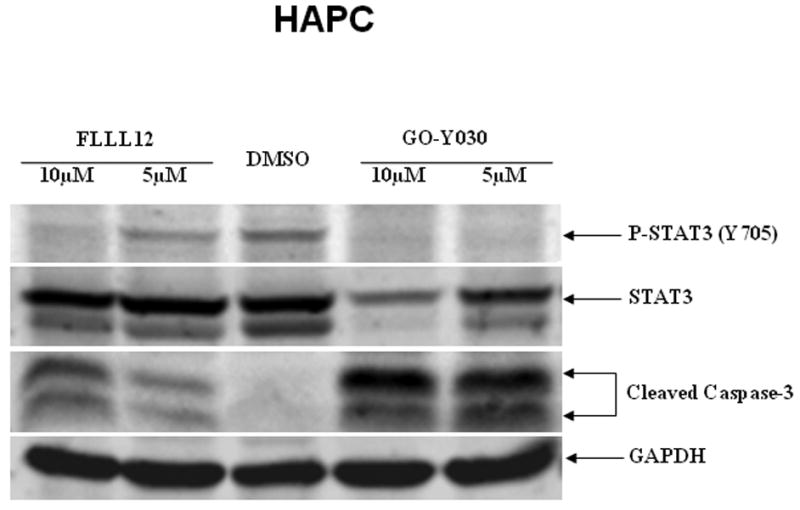
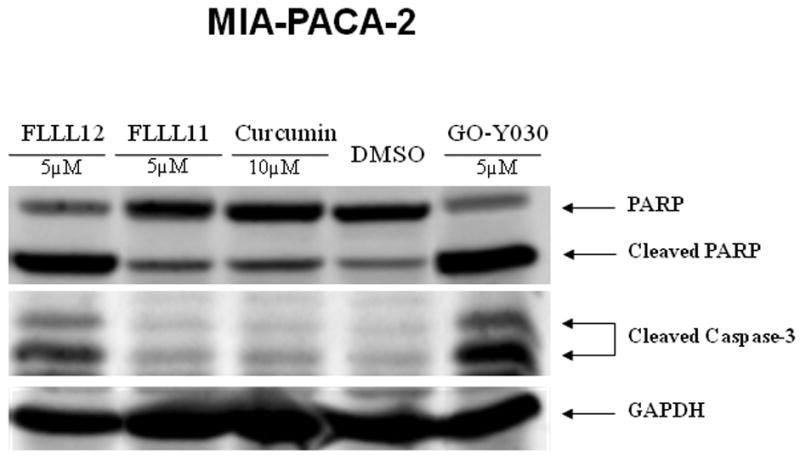
FLLL11, FLLL12, and GO-Y030 inhibit STAT3 phosphorylation and/or induce apoptosis in (a) PANC-1, (b) HPAC, and (c) MIA-PACA-2 pancreatic cancer cell lines. DMSO, dimethylsulfoxide.
Discussion
Some anticancer therapies currently in use are inadequate, not only in terms of their therapeutic efficacy, but also because they have undesirable side effects. In contrast, certain bioactive compounds known as phytochemicals have been shown to exhibit growth suppressive activity and chemopreventive properties against various types of cancers [20]. Curcumin is one of the most widely characterized of the phytochemicals and is the active ingredient in the rhizome of the plant turmeric (Curcuma longa Linn), and has both antioxidant and anti-inflammatory properties [20–22]. Curcumin has been shown to protect against carcinogenesis and prevent tumor formation and development in several types of cancer and also to suppress angiogenesis and metastasis in a variety of animal tumor models [20,21,23–30]. In particular, curcumin has shown inhibitory effects in pancreatic cancer cells in vitro as well as in an orthotopic pancreatic tumor model in vivo [8–12]. The growth suppressive activity of curcumin, as well as its safety, makes it an attractive choice for a chemotherapeutic agent; however, because of its low bioavailablity it may not be sufficient as an effective therapeutic agent in pancreatic cancer. Therefore, more potent analogues of curcumin need to be developed to find therapeutic approaches for pancreatic cancer.
There are several curcumin analogues such as dimethoxycurcumin, EF-24, and others that have been reported earlier [31–34]. We tested two additional analogues of curcumin, FLLL11 and FLLL12. Although the monoketone scaffold present in FLLL11 and FLLL12 is frequently used in the synthesis of curcumin analogues, the origin of the differences in activity between curcumin and these monoketone analogues remains unclear. FLLL11 possesses the same ring substitution pattern as curcumin, 4-hydroxy-3-methoxy substituted aromatic rings, and lacks only one of the carbonyls and the central methylene carbon found in curcumin. Certainly, the shorter tether length between the aromatic rings (5 carbons vs. 7 carbons) and the lack of acidic 1,3-diketone protons may play a role, but little evidence yet exists to substantiate these claims or clearly indicate a difference in affected biological targets. We demonstrated that FLLL11 and FLLL12 are more potent than curcumin in inhibiting STAT3 and AKT phosphorylation. The constitutive activation of STAT3 is frequently detected in pancreatic cancer and may contribute to angiogenesis, metastasis, increased survival, and growth of pancreatic cancer cells and pancreatic tumors in mice models in vivo [35–38]. The constitutively activated AKT expression (phosphorylated AKT) is also a significant prognostic indicator for pancreatic ductal adenocarcinoma [39]. Activated AKT seems to play an important role in proliferation, chemoresistance, and invasion of human pancreatic cancer cells [40–44]. These results support that constitutive STAT3 and AKT signaling seem to be two of the key oncogenic pathways in pancreatic cancer and can serve as attractive therapeutic targets for pancreatic carcinoma. Therefore, the inhibition of cell viability is most likely linked to the inhibition of STAT3 and AKT pathways in these pancreatic cancer cells. Although FLLL11 and FLLL12 are potent in inhibiting pancreatic cancer cell viability, they have less inhibitory effects in normal human bladder cells. We observed that FLLL11 and FLLL12 have certain inhibition of cell proliferation in immortalized HPDE cells. However, we did not observe FLLL11 and FLLL12 induce cleaved caspase-3 in human pancreatic duct epithelial cells (Fig. 3d). These results might be explained by immortalized cells that may become sensitive to the growth suppression by curcumin or its analogues such as FLLL11 and FLLL12. However, HPDE cells are not malignant or oncogenic yet compare to other five pancreatic cancer cell lines. Therefore, fewer survival or antiapoptotic pathway(s) are activated in HPDE cells comparing to cancer cells. Hence, the treatments of FLLL11 and FLLL12 in HPDE cells may not result in the induction of apoptosis by the inhibition of survival or antiapoptotic pathway(s).
In summary, our results showed for the first time that the curcumin analogues, FLLL11 and FLLL12, are potent agents in inhibiting STAT3 and AKT phosphorylation in human pancreatic cancer cells and are more active than curcumin in this inhibition. Our results also showed that FLLL11 and FLLL12 are potent agents to inhibit cell viability and induce apoptosis in pancreatic cancer cells. Therefore, FLLL11 and FLLL12 may have translational potential as novel cancer therapeutics or preventive agents for human pancreatic carcinoma.
Acknowledgments
This work was supported in part by a National Foundation for Cancer Research grant and NIHR21 grant (R21CA133652-01) to Jiayuh Lin. GO-Y030 was kindly provided by Dr Hiroyuki Shibata (Tohoku University, Sendai, Japan).
References
- 1.Greenlee R, Murray T, Bolden S, Wingo P. Cancer statistics. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Yeo C, Abrams R, Grochow L, Sohn T, Ord S, Hruban R, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg. 1997;225:621–633. doi: 10.1097/00000658-199705000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conlon K, Klimstra D, Brennan M. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273–279. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lillemoe K. Current management of pancreatic carcinoma. Ann Surg. 1995;221:133–148. doi: 10.1097/00000658-199502000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruggeri B, Zhang S, Caamano J, DiRado M, Flynn S, Klein-Szanto A. Human pancreatic carcinomas and cell lines reveal frequent and multiple alterations in the p53 and Rb-1 tumor-suppressor genes. Oncogene. 1992;7:1503–1511. [PubMed] [Google Scholar]
- 6.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as Curecumin: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;M:M–M. doi: 10.1007/s00018-008-7452-4. AQ5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:2351–2362. doi: 10.1002/cncr.20605. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106:2503–2513. doi: 10.1002/cncr.21904. [DOI] [PubMed] [Google Scholar]
- 11.Lev-Ari S, Starr A, Vexler A, Karaush V, Loew V, Greif J, et al. Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, down-regulation of COX-2 and EGFR and inhibition of Erk1/2 activity. Anticancer Res. 2006;26:4423–4430. [PubMed] [Google Scholar]
- 12.Lev-Ari S, Zinger H, Kazanov D, Yona D, Ben-Yosef R, Starr A, et al. Curcumin synergistically potentiates the growth inhibitory and pro-apoptotic effects of celecoxib in pancreatic adenocarcinoma cells. Biomed Pharmacother. 2005;59:S276–280. doi: 10.1016/s0753-3322(05)80045-9. [DOI] [PubMed] [Google Scholar]
- 13.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 14.Mosley CA, Liotta DC, Snyder JP. Highly active anticancer curcumin analogues. Adv Exp Med Biol. 2007;595:77–103. doi: 10.1007/978-0-387-46401-5_2. [DOI] [PubMed] [Google Scholar]
- 15.Du ZY, Bao YD, Liu Z, Qiao W, Ma L, Huang ZS, et al. Curcumin analogs as potent aldose reductase inhibitors. Arch Pharm (Weinheim) 2006;339:123–128. doi: 10.1002/ardp.200500205. [DOI] [PubMed] [Google Scholar]
- 16.Du ZY, Liu RR, Shao WY, Mao XP, Ma L, Gu LQ, et al. Alpah-Glucosidase inhibition of natural curcuminoids and curcumin analogs. Eur J Med Chem. 2006;41:213–218. doi: 10.1016/j.ejmech.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Masuda T, Jitoe A, Isobe J, Nakatani N, Yonemori S. Anti-oxidative and anti-inflammatory curcumin-related phenolics from rhizomes of Curcuma domestica. Phytochemistry. 1993;32:1557–1560. [Google Scholar]
- 18.Park SY, Kim DSHL. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: a drug discovery effort against Alzheimer's disease. J Nat Prod. 2002;65:1227–1231. doi: 10.1021/np010039x. [DOI] [PubMed] [Google Scholar]
- 19.Ohori H, Yamakoshi H, Tomizawa M, Shibuya M, Kakudo Y, Takahashi A, et al. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol Cancer Ther. 2006;5:2563–2571. doi: 10.1158/1535-7163.MCT-06-0174. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal B, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Hanif R, Qiao L, Shiff SJ, Rigas B. Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J Lab Clin Med. 1997;130:576–584. doi: 10.1016/s0022-2143(97)90107-4. [DOI] [PubMed] [Google Scholar]
- 22.Shi M, Cai Q, Yao L, Mao Y, Ming Y, Ouyang G. Antiproliferation and apoptosis induced by curcumin in human ovarian cancer cells. Cell Biol Int. 2006;30:221–226. doi: 10.1016/j.cellbi.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 23.Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, et al. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597–601. [PubMed] [Google Scholar]
- 24.Aggarwal BB, Shishodia S, Takada Y, Banerjee S, Newman RA, Bueso-Ramos CE, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11:7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 25.Bachmeier B, Nerlich AG, Iancu CM, Cilli M, Schleicher E, Vene R, et al. The chemopreventive polyphenol Curcumin prevents hematogenous breast cancer metastases in immunodeficient mice. Cell Physiol Biochem. 2007;19:137–152. doi: 10.1159/000099202. [DOI] [PubMed] [Google Scholar]
- 26.Azuine MA, Bhide SV. Chemopreventive effect of turmeric against stomach and skin tumors induced by chemical carcinogens in Swiss mice. Nutr Cancer. 1992;17:77–83. doi: 10.1080/01635589209514174. [DOI] [PubMed] [Google Scholar]
- 27.Azuine MA, Bhide SV. Protective single/combined treatment with betel leaf and turmeric against methyl (acetoxymethyl) nitrosamine-induced hamster oral carcinogenesis. Int J Cancer. 1992;51:412–415. doi: 10.1002/ijc.2910510313. [DOI] [PubMed] [Google Scholar]
- 28.Ikezaki S, Nishikawa A, Furukawa F, Kudo K, Nakamura H, Tamura K, et al. Chemopreventive effects of curcumin on glandular stomach carcinogenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine and sodium chloride in rats. Anticancer Res. 2001;21:3407–3411. [PubMed] [Google Scholar]
- 29.Frank N, Knauft J, Amelung F, Nair J, Wesch H, Bartsch H. No prevention of liver and kidney tumors in Long-Evans Cinnamon rats by dietary curcumin, but inhibition at other sites and of metastases. Mutat Res. 2003;523–524:127–135. doi: 10.1016/s0027-5107(02)00328-7. [DOI] [PubMed] [Google Scholar]
- 30.Gururaj AE, Belakavadi M, Venkatesh DA, Marme D, Salimath BP. Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem Biophys Res Commun. 2002;297:934–942. doi: 10.1016/s0006-291x(02)02306-9. [DOI] [PubMed] [Google Scholar]
- 31.Tamvakopoulos C, Dimas K, Sofianos Z, Hatziantoniou S, Han Z, Liu Z, et al. Metabolism and anticancer activity of the curcumin analogue, dimethoxycurcumin. Clin Cancer Res. 2007;13:1269–1277. doi: 10.1158/1078-0432.CCR-06-1839. [DOI] [PubMed] [Google Scholar]
- 32.Ohtsu H, Xiao Z, Ishida J, Nagai M, Wang H, Itokawa H, et al. Antitumor agents. 217. Curcumin analogues as novel androgen receptor antagonists with potential as anti-prostate cancer agents. J Med Chem. 2002;45:5037–5042. doi: 10.1021/jm020200g. [DOI] [PubMed] [Google Scholar]
- 33.Youssef D, Nichols C, Cameron T, Balzarini J, De Clercq E, Jha A. Design, synthesis, and cytostatic activity of novel cyclic curcumin analogues. Bioorg Med Chem Lett. 2007;17:5624–5629. doi: 10.1016/j.bmcl.2007.07.079. [DOI] [PubMed] [Google Scholar]
- 34.Youssef KM, El-Sherbeny MA, El-Shafie FS, Farag HA, Al-Deeb OA, Awadalla SA. Synthesis of curcumin analogues as potential antioxidant, cancer chemopreventive agents. Arch Pharm (Weinheim) 2004;337:42–54. doi: 10.1002/ardp.200300763. [DOI] [PubMed] [Google Scholar]
- 35.Scholz A, Heinze S, Detjen KM, Peters M, Welzel M, Hauff P, et al. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology. 2003;125:891–905. doi: 10.1016/s0016-5085(03)01064-3. [DOI] [PubMed] [Google Scholar]
- 36.Greten FR, Weber CK, Greten TF, Schneider G, Wagner M, Adler G, et al. Stat3 and NF-kappaB activation prevents apoptosis in pancreatic carcinogenesis. Gastroenterology. 2002;123:2052–2063. doi: 10.1053/gast.2002.37075. [DOI] [PubMed] [Google Scholar]
- 37.Huang C, Cao J, Huang KJ, Zhang F, Jiang T, Zhu L, et al. Inhibition of STAT3 activity with AG490 decreases the invasion of human pancreatic cancer cells in vitro. Cancer Sci. 2006;97:1417–1423. doi: 10.1111/j.1349-7006.2006.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto S, Tomita Y, Hoshida Y, Morooka T, Nagano H, Dono K, et al. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:2846–2850. doi: 10.1158/1078-0432.ccr-02-1441. [DOI] [PubMed] [Google Scholar]
- 40.Arlt A, Gehrz A, Muerkoster S, Vorndamm J, Kruse ML, Folsch UR, et al. Role of NF-kappaB and Akt/PI3λK in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243–3251. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 41.Fahy BN, Schlieman M, Virudachalam S, Bold RJ. AKT inhibition is associated with chemosensitisation in the pancreatic cancer cell line MIA-PaCa-2. Br J Cancer. 2003;89:391–397. doi: 10.1038/sj.bjc.6601037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanno S, Tanno S, Mitsuuchi Y, Altomare D, Xiao G, Testa J. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 2001;61:589–593. [PubMed] [Google Scholar]
- 43.Takeda A, Osaki M, Adachi K, Honjo S, Ito H. Role of the phosphatidylinositol 3′-kinase-Akt signal pathway in the proliferation of human pancreatic ductal carcinoma cell lines. Pancreas. 2004;28:353–358. doi: 10.1097/00006676-200404000-00026. [DOI] [PubMed] [Google Scholar]
- 44.Veit C, Genze F, Menke A, Hoeffert S, Gress T, Gierschik P, et al. Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line-derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells. Cancer Res. 2004;64:5291–5300. doi: 10.1158/0008-5472.CAN-04-1112. [DOI] [PubMed] [Google Scholar]


