Abstract
Opioid treatment program patients and staff often have concerns that smoking cessation may jeopardize abstinence from illicit drug use. In this study, we evaluated whether smoking abstinence produced with a two-week contingency-management (CM) intervention was associated with relapse to illicit drug use among patients enrolled in opioid maintenance. Opioid-maintenance patients who were stable in treatment and abstinent from illicit drugs were enrolled in a 14-day smoking-cessation study. Participants were dichotomized into Abstainers (> 90% smoking-negative samples, n=12) and Smokers (< 10% smoking-negative samples, n=16). Illicit drug assays included opioids, oxycodone, propoxyphene, cannabis, amphetamines, cocaine and benzodiazepines. There were no differences between the Abstainers and Smokers, with 99% and 96% of samples testing negative for all illicit drugs in each group, respectively. Data from this study provide no evidence that smoking cessation among stable opioid-maintained patients undermines drug abstinence and lend support for programs that encourage smoking cessation during drug abuse treatment.
Introduction
Methadone maintenance (MM) represents one of the most widely used and effective treatments for opioid dependence, with over 200,000 patients in the United States receiving this opioid agonist medication in a clinic-based setting annually (DASIS, 2006). A second opioid-agonist medication, buprenorphine, was more recently approved for the treatment of opioid dependence in 2002. Available from trained physicians in an office-based setting, buprenorphine also has been demonstrated effective in treating opioid dependence (see Johnson et al., 2003 for a comprehensive review). While both forms of agonist treatment have been proven to reduce illicit opioid use and maintain patients in treatment, rates of cigarette smoking remain substantially higher among opioid-maintained individuals than in the general population. For example, compared to 25% in the general U.S. adult population (CDC, 2005; SAMHSA, 2007), prevalence of current smoking among MM patients is 84 – 94% (Nahvi, Richter, Li, Modali & Arnsten, 2006; Clemmey, Brooner, Chutuape, Kidorf & Stitzer, 1997; Richter, Gibson, Ahulwalia & Schmelzle, 2001). While specific data is not yet available on the prevalence of smoking among buprenorphine-maintained patients, it is reasonable to assume a similar prevalence among these patients as well (e.g., Mello, Lukas & Mendelson, 1985).
As is the case in the general population, smoking in opioid-treatment patients is associated with increased morbidity and mortality (Engstrom, Adamsson, Alleback & Rydberg, 1991; Hser, McCarthy, & Anglin, 1994). The ten-year mortality rate of opioid-dependent smokers is estimated to be four-fold greater than that of opioid-dependent nonsmokers (Hser et al., 1994), and individuals who abuse alcohol and other drugs are more likely to die of tobacco-related disorders than problems related to their drug use (Hurt et al., 1996). An effective smoking cessation intervention among patients enrolled in opioid treatment could significantly reduce the economic and health-related costs associated with their smoking. Methadone and buprenorphine programs may offer an excellent setting for implementing smoking-cessation interventions as many patients achieve prolonged periods of abstinence from illicit drug use and remain engaged in treatment for extended periods of time. This set of conditions could support the frequent and, if necessary, prolonged clinical contact to help facilitate success with smoking cessation. Opioid maintenance programs adhere to a uniform set of state and federal regulations (Federal Register 42 CFR, Section 8.12, 2001), which could greatly facilitate the dissemination of an effective smoking-cessation intervention in this population across the country.
Many opioid treatment patients express serious interest in quitting smoking (Clark, Stein, McGarry & Gogineni, 2001; Clemmey et al, 1997; Frosch, Shoptaw, Jarvik, Rawson & Ling, 1998; Kozlowski, Skinner, Kent & Pope, 1989; Richter, Gibson, Ahluwalia & Schmelzle, 2001; Sees & Clark, 1993). For example, as many as 80% of MM smokers report a desire to quit, approximately half report making at least one serious attempt to quit in the past year, and approximately three quarters report being willing to participate in a program if it were made available by their clinic (Nahvi et al., 2006; Richter et al., 2001). Despite such interest in smoking cessation, few clinics seem to offer programs to patients (Fuller et al, 2007; Knapp, Rosheim, Meister & Kottke, 1993; Olsen, Alford, Horton & Saitz, 2005; Richter, Choi & Alford, 2005). One commonly-cited barrier is the belief that smoking cessation may undermine patients’ abstinence from other drugs (Bobo, Slade & Hoffman, 1995). A recent survey of MM clinics, for example, reported that 26% of clinic directors had encouraged a patient to delay quitting smoking because they considered alcohol and illicit drug use the top priority and believed that patients should not attempt to modify too many behaviors at once (Richter, 2006). Another survey of MM patients noted that 32% had been encouraged to delay quitting smoking during treatment (Richter, McCool, Okuyemi, Mayo & Ahluwalia, 2002).
An ongoing program of research by our group provides a unique opportunity to investigate whether quitting smoking during opioid-maintenance treatment is associated with relapse to illicit drug use. Specifically, data were examined from one completed pilot study (Dunn, Sigmon, Thomas, Heil & Higgins, 2008) and one ongoing clinical trial that used a contingency-management (CM) intervention to promote smoking cessation among methadone- and buprenorphine-maintained patients. CM has been widely shown to reduce drug use by providing non-drug reinforcers contingent upon biochemical confirmation of abstinence (Higgins, Alessi & Dantona, 2002; Higgins, Silverman & Heil, 2008). We have been examining the potential efficacy of using voucher-based CM to promote smoking cessation among opioid-maintained patients. Thus far, participants assigned to abstinence-contingent voucher delivery have achieved significantly more smoking abstinence and longer durations of continuous abstinence than those assigned to a control condition involving noncontingent voucher delivery. Besides its therapeutic utility, CM also can serve as an effective tool for investigating a variety of scientific questions related to changes in drug use (Sigmon, Lamb & Dallery, 2008). In our studies on smoking cessation in methadone and buprenorphine maintenance patients, the ability of CM to experimentally produce smoking abstinence (largely in the Contingent group) and no abstinence (in the Noncontingent group) in a prospectively randomized sample of smokers provided an opportunity to directly compare rates of illicit drug use between these two experimental conditions.
Methods
Participants
Twenty-eight participants were recruited from local methadone treatment programs (n=16) and office-based buprenorphine providers (n=12). Eleven subjects participated in an initial pilot study and 17 in an ongoing clinical trial (described below). For both studies, eligible participants had to report smoking ≥ 10 cigarettes per day for at least 1 year and be on a stable methadone or buprenorphine maintenance dose for the past 30 days. Also, because illicit opioid and cocaine use can directly increase smoking rates (e.g., Chait & Griffiths, 1994; Roll, Higgins & Tidey, 1997) and cannabis use could confound carbon monoxide testing, participants needed to be free from regular illicit drug use (> 70% negative urine specimens during past 30 days). Participants provided consent in advance to allow staff to collect from treatment providers the clinical information necessary to determine eligibility.
Participants were on average 39% male, 31 years old, and had completed 13 years of education (Table 1). They reported smoking 20 cigarettes per day and 89% reported a prior quit attempt with a median quit duration of 60 days. Mean (standard deviation) methadone and buprenorphine maintenance doses were 106 (51) and 18.5 (8) mgs, respectively. Participants had been maintained at their current dose for an average 193 (419) days.
Table 1.
Demographic and Smoking Characteristics
| Overall (n=28) | Abstainers (n=12) | Smokers (n=16) | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Male (%) | 39 | 50 | 31 | 0.44 |
| Age (yrs) | 31.3 (8.8) | 36.7 (10.6) | 30.2 (7.5) | 0.47 |
| Education (yrs) | 12.5 (1.3) | 12.3 (1.6) | 12.5 (1.1) | 0.80 |
| Opioid Treatment Characteristics | ||||
| Methadone dose (mg) | 106.9 (51.4) | 127.9 (52.9) | 90.7 (46.7) | 0.16 |
| Buprenorphine dose (mg) | 18.5 (8.8) | 16.4 (9.2) | 20.0 (8.9) | 0.51 |
| Length of time at current dose (days) | 193.2 (519.2) | 136.8 (154.8) | 238.3 (550.1) | 0.54 |
| Negative urines in prior 30 days (%) | 90 | 88 | 91 | 0.72 |
| Smoking Characteristics | ||||
| Cigarettes smoked per day | 20.0 (7.1) | 20.0 (5.3) | 20.0 (8.4) | 0.99 |
| Age first cigarette smoked (yrs) | 12.4 (3.0) | 12.5 (3.7) | 12.3 (2.5) | 0.87 |
| Living with a smoker (%) | 61 | 50 | 69 | 0.44 |
| Nicotine yield of typical cigarette (mg) | 1.0 (0.2) | 0.9 (0.2) | 1.0 (0.2) | 0.18 |
| Tried to quit smoking (%) | 89 | 100 | 81 | 0.24 |
| Longest successful quit duration (days) | 60 (30, 270)a | 60 (30, 456)a | 90 (45, 270)a | 0.93 |
| Baseline cotinine (ng/ml) | 1493.9 (710.0) | 1173.7 (554.3) | 1734 (734.3) | 0.04 |
| Baseline CO (ppm) | 15.0 (7.1) | 11.1 (7.3) | 18.0 (5.3) | 0.01 |
| FTND | 5.9 (1.5) | 5.3 (1.8) | 6.1 (1.2) | 0.76 |
Values represent average (SD) unless otherwise indicated.
Represents interquartile range
Study Details
Full details of this smoking intervention have been reported elsewhere (Dunn et al., 2008. Briefly, participants completed an intake assessment consisting of a demographic and smoking questionnaire, the Fagerstrom Test of Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker & Fagerstrom, 1991) and the Addiction Severity Index (ASI; McLellan et al., 1985). They were also asked whether a counselor or healthcare professional had ever discussed with them the importance of quitting smoking, if they had ever been advised to delay quitting or to never quit smoking, and how much they thought cigarette smoking harms their health. Follow-up assessments were completed at 14 and 30 days after quit date and included the above questionnaires and collection of urine and breath samples. Participants received $35 per follow-up, independent of smoking status.
Eligible participants were randomly assigned to the Contingent (n=11) or Noncontingent (n=17) experimental group and visited the clinic daily for 14 consecutive days. While the duration of this intervention was relatively brief, the aim was simply to demonstrate the efficacy of a behavioral intervention in promoting initial abstinence in opioid-maintained smokers. Considering that a positive relationship has been well-documented between smoking abstinence during the initial two weeks of the quit attempt and longer-term outcomes (Gourlay, Forbes, Marriner, Pethica & McNeil, 1994; Higgins, Heil, Dumeer, Thomas, Solomon, & Bernstein, 2006; Kenford, Liore, Jorenby, Smith, Wetter & Baker, 1994; Yudkin, Jones, Lancaster & Fowler, 1996), promising levels of initial smoking abstinence with this two-week intervention would bode well for longer-term outcomes. Indeed, evidence that initial continuous abstinence can be established with an intensive intervention early in the quit attempt will support future work by our group to develop an intervention that sustains smoking abstinence for the longer term.
At each visit, participants provided breath and urine samples and reported the number of cigarettes smoked since the last visit. Breath and urine specimens were analyzed immediately for biochemical verification of smoking status. Breath carbon monoxide (CO) levels were assessed using hand-held meters (Bedfont EC50 Smokerlyzer, Bedfont Scientific Ltd., Kent, England). Urinary levels of cotinine, a metabolite of nicotine, were measured using an on-site enzyme multiplied immunoassay test (EMIT) (Syva Co., San Jose, CA). The abstinence criterion for earning vouchers was defined as a breath CO ≤ 6 ppm on Days 1–5 of the study and as a urine cotinine ≤ 80 ng/ml on Days 6–14. Because of the relatively short half-life of CO (4 hrs), smokers can meet the 6 ppm abstinence criterion within 12–24 hours of stopping smoking (SRNT Subcommittee on Biochemical Verification, 2002). With the relatively long half-life of cotinine, several continuous days of abstinence are needed to meet the abstinence criterion (SRNT Subcommittee on Biochemical Verification, 2002). Therefore, CO was used early in the intervention to allow us to reinforce initial smoking abstinence, and the cotinine measure was used later to provide a more sensitive test likely to detect even low levels of ongoing smoking. We have found this method of transitioning from CO to cotinine for monitoring of smoking status to be effective for promoting smoking abstinence with CM in prior research by our group (Higgins, et. al., 2004).
Contingent participants earned voucher-based incentives for samples that met the abstinence criteria. The first negative sample earned $9.00 and values escalated by $1.50 with each subsequent negative sample. Additional bonuses were available to further promote early and complete smoking abstinence, and a reset contingency was included to discourage relapse (Roll & Higgins, 2000). Contingent participants could earn a maximum of $362.50 in vouchers for continuous abstinence during the 14-day study. Noncontingent participants earned vouchers independent of smoking status and yoked to the Contingent group. In the first study, Noncontingent participants were yoked to an individual Contingent participant such that the amount and schedule of their voucher payments were yoked to that of a Contingent group partner (but independent from their own smoking status). In the second study, Noncontingent participants were yoked to the average earnings of the Contingent group from the pilot study, meaning that the amount and schedule of their voucher payments were yoked to the average payment amount obtained by the Contingent group in the pilot trial (but independent from their own smoking status). The purpose of this yoking procedure was to generally equate the amounts of vouchers, clinic contact, monitoring, and material support were equal received by each experimental group.
There was a low occurrence of pharmacotherapy use by participants in these two studies. Due to our use of urinary cotinine as a measure of smoking status, participants were not permitted to use nicotine replacement, as this would confound the cotinine assay. During the initial pilot study, no pharmacotherapy was provided as part of the study and participants reported at each daily visit any medications taken since last visit. Overall, 14% of participants in this report used pharmacotherapy, with 3 Contingent and 1 Noncontingent participants using bupropion and 1 Contingent participant receiving varenicline from an outside physician.
Analyses of Illicit Drug Use
All urine samples collected during the study and at 30-day follow-up were analyzed for illicit drugs using an EMIT assay on an on-site Microgenics MCG240 analyzer. Samples were tested for amphetamine (at a 1000 ng/ml cutoff), benzodiazepines (200 ng/ml), cannabis (50 ng/ml), cocaine (300 ng/ml), opioids (300 ng/ml), oxycodone (100 ng/ml) and propoxyphene (300 ng/ml). Methadone and buprenorphine assays were also used but these data are not included in this report as they simply reflected the maintenance pharmacotherapy being received by each participant.
Data Analysis
Participants were dichotomized post-hoc into Abstainer and Smoker groups, based on the amount of smoking abstinence achieved during the study. There was a low occurrence of missing samples during the study (3%). All missing samples were considered smoking-positive for implementation of the CM intervention and for analyses of smoking outcomes; missing samples were not included in analyses on illicit drug use. Examination of the data revealed a bimodal distribution, whereby participants provided either a majority of smoking-negative or smoking-positive samples. Based on this distribution, all participants who provided > 90% smoking-negative samples were categorized as Abstainers (n=12) and those who provided < 10% smoking-negative samples as Smokers (n=16). As might be expected most Abstainers (92%) had been randomly assigned to the Contingent experimental group during the parent trials and all Smokers (100%) were members of the Noncontingent group.
Abstainers and Smokers were compared on demographic and drug use characteristics using chi square tests for categorical variables and t-tests or Wilcoxon Rank Sum tests for continuous variables. Chi square tests also were used to compare the two groups on the percentage of samples that tested positive for each illicit drug during the study and at 30-day follow-up. Analyses were performed using SAS statistical software (SAS Institute, Cary, NC). Statistical significance was based on α=.05.
Results
With respect to smoking-related outcomes, Abstainers provided significantly more smoking-negative samples during the 14-day intervention than Smokers (97% vs. 1%, p<.01). This difference persisted at the 30-day follow-up, with 25% of urine samples provided by Abstainers testing negative for recent smoking compared to 0% of samples among Smokers (p< .05, data not shown).
With regards to demographic and smoking characteristics, there were no significant differences between Abstainers and Smokers on opioid treatment variables. The two groups were also largely similar in smoking characteristics, with the exception of Abstainers having lower cotinine and CO levels than Smokers at baseline, respectively (Table 1). There were no significant group differences on ASI composite scores at intake or 30-day follow-up (not shown). Overall, 89% of participants reported having discussed the importance of quitting smoking with their doctor. Forty-three percent reported that a counselor had advised them to delay quitting smoking because it may interfere with their opioid treatment, and 4% reported that a counselor had instructed them to never quit smoking. Despite this, 100% of participants reported that they believed their cigarette smoking presented a serious health risk. There were no significant differences between Abstainers and Smokers in the percentage who had discussed with a doctor the importance of quitting smoking (92% vs. 88%), had been advised to delay quitting (50% vs. 38%) or had been advised to never quit smoking (0% vs. 6%), respectively.
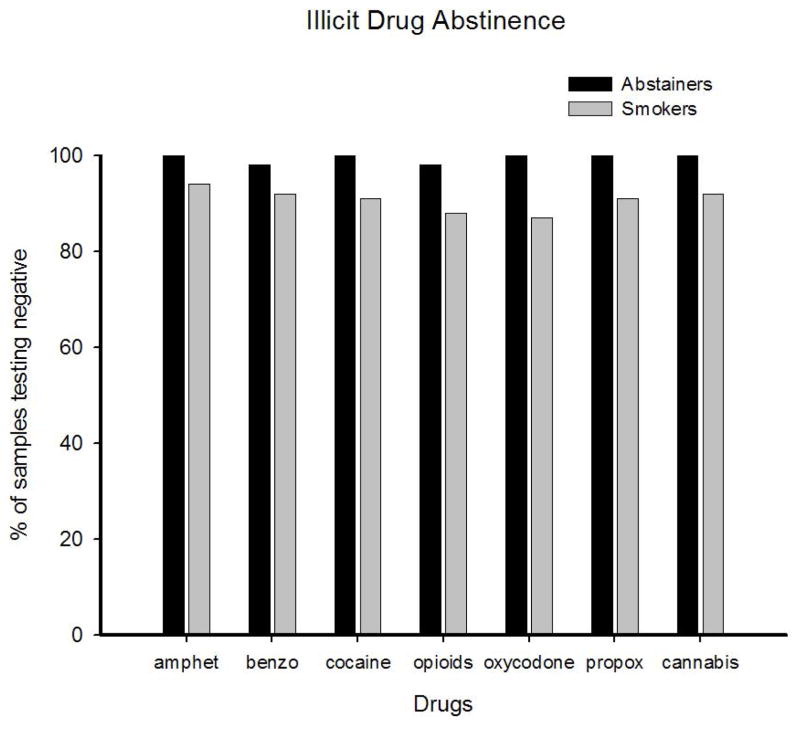
There were no differences between Abstainers and Smokers in illicit drug use during the smoking intervention, with 99% and 96% of samples testing negative for all illicit drugs in each group, respectively. Data on individual illicit drug assays are presented in Figure 1. Though there appeared to be a trend toward greater illicit drug abstinence among Abstainers, there were no statistically-significant differences between the two groups for any drug. At 30-day follow-up, 96% of participants in the Smoker and Abstainer groups provided negative urine results for any illicit drug (data not shown); there were no significant group differences.
Figure 1.
Represents abstinence from smoking and illicit-drug use. Percent of negative samples provided by Abstainers (black bars) and Smokers (gray bars) for each illicit drug collapsed across the 14-day intervention. Individual illicit drugs are (from left): amphetamines (amphet), benzodiazepines (benzo), cocaine, opioids, oxycodone, propoxyphene (propox) and cannabis.
Discussion
Results from this pilot study provide no evidence that quitting smoking during methadone or buprenorphine treatment increases use of illicit drugs in this sample of stable maintenance patients. Patients in the present study who provided >90% smoking-negative samples maintained near-perfect levels of illicit drug abstinence and there was no difference in drug use between those who were essentially smoking-abstinent and those who continued to smoke cigarettes throughout the study. These findings are consistent with data from several prior studies. For example, two recent studies examining smoking cessation among MM patients found that quitting smoking was associated with either no change in illicit drug use (Reid et al., in press) or an increase in opioid- and cocaine-negative urines (Shoptaw et al., 2002). Several additional reports have noted that smoking cessation in more general substance abuse treatment settings does not appear to increase alcohol or drug use (Miller, Hendrick & Taylor, 1983; Hurt et al., 1994) and also may be associated with a decreased urge to use alcohol or drugs (Bobo, Gilchrist, Schilling, Noach & Schinke, 1987; Campbell, Wander, Stark & Holbert, 1995; Prochaska, Delucchi & Hall, 2004). Overall, data from this study replicate those results using a more experimentally rigorous methodology than has generally been used previously. They also may extend the findings to buprenorphine-maintained smokers, though additional research with a larger sample of buprenorphine-maintained patients will be necessary to address this question more definitively.
These data would argue against relapse risk as a rationale for not encouraging smoking cessation, at least among stable maintenance patients. This finding may offer important clinical implications. In this study, 100% of patients believed cigarette smoking is harmful to their health. While the majority of participants in our study had discussed the importance of quitting smoking with a healthcare professional, 43% of those participants had received advice not to attempt quitting smoking at some point and 4% reported being instructed against any attempt to ever quit smoking. The high prevalence of smoking and increased risk of smoking-related morbidity and mortality would seem to underscore the need to explore smoking cessation for at least patients who are relatively stable in their opioid treatment. There is evidence that community methadone and other substance abuse treatment clinics are beginning to emphasize the importance of, and provide support for, quitting smoking. In terms of policy, the American Society of Addiction Medicine has issued a policy statement in support of treating nicotine dependence among substance abusers (American Society of Addiction Medicine, 2001), and the state of New Jersey now requires substance abuse facilities to address cigarette smoking among their patients (Hoffman et al., 1997). Reports also show that an increasing number of MM programs are providing patients with smoking cessation aids (McCool, Richter & Choi, 2005) and establishing bans on indoor cigarette smoking (Knapp et al., 1993; Richter, Choi & Alford, 2005). Interestingly, one study examining staff attitudes towards smoking cessation found that significantly more staff supported a smoking intervention after one was instituted, suggesting that experience with providing an intervention may improve staff attitudes toward smoking cessation during drug treatment (Hurt, Croghan, Offord, Eberman & Morse, 1995).
Several potential limitations of these findings should be noted. First, because participants in this smoking cessation trial had to be relatively stable with regard to their opioid and other drug use, our findings cannot address effects of smoking cessation on drug use among unstable patients. That said, large portions of opioid maintenance patients are often remarkably stable and abstinent from illicit drugs for long periods. As such, it seems reasonable that they may be appropriate candidates for making a quit attempt during treatment. Second, it is possible that the findings seen with this brief voucher-based CM intervention may not generalize to other types of smoking-cessation treatments. Future research efforts should assess illicit drug outcomes during other types of smoking-cessation interventions as well as with the commonly-used pharmacotherapies for quitting smoking. A final limitation is the limited sample size used in the present report. Future studies will be important to replicate and extend these findings with a larger number of participants. Overall, our findings provide no evidence that smoking cessation increases drug use among stable opioid-maintained patients. This information provides additional support for programs that aim to encourage smoking cessation during drug abuse treatment.
Acknowledgments
We would like to thank Colleen Thomas and Gary Badger for their statistical assistance, and Lukas Lewis for his assistance in performing urine toxicology testing. This research was supported in part by research (R01 DA019550) and training (T32 DA007242) grants from the National Institute on Drug Abuse.
Biographies
Kelly E. Dunn M.S., is a NIDA predoctoral fellow in the Human Behavioral Pharmacology Laboratory at the University of Vermont. Her area of research is behavioral interventions to reduce smoking among opioid-maintained patients, and brief buprenorphine detoxification for the treatment of prescription opioid abuse.
Contact Information: UHC-SATC Room 1415, 1 South Prospect Street, Burlington VT 05401. Phone: 802-656-8701. Fax: 802-656-5793. kdunn@uvm.edu
Stacey Sigmon, Ph.D., Associate Professor, Psychiatry, University of Vermont. Research interests: Behavioral and pharmacological treatments for opioid dependence; Behavioral interventions for smoking cessation; Individual differences in response to stimulants and other drugs. Dr. Sigmon also is the Director of The Chittenden Center methadone program, which currently treats 225+ opioid-dependent patients.
Contact Information (Corresponding Author): SATC-UHC, Room 1415, 1 South Prospect Street Burlington, VT 05401. Phone: 802-656-9987 Fax: 802-656-5793 stacey.sigmon@uvm.edu
Edward Reimann B.A., is a Research Assistant in the Human Behavioral Pharmacology Laboratory at the University of Vermont. His area of research is behavioral interventions to reduce smoking behavior among opioid-maintained patients.
Contact Information: UHC-SATC Room 1415, 1 South Prospect Street, Burlington VT 05401. Phone: 802-656-5411. Fax: 802-656-5793. ereimann@uvm.edu
Sarah H. Heil, Ph.D., is a Research Assistant Professor of Psychiatry and Psychology at the University of Vermont. Her primary areas of research include behavioral and pharmacological approaches to the treatment of substance use disorders in pregnant and recently postpartum women.
Contact Information: Substance Abuse Treatment Center, Rm. 1415 UHC, 1 So. Prospect St., Burlington, VT 05401. Phone: 802-656-8712 Fax: 802-656-5793 sarah.heil@uvm.edu
Stephen Higgins, Ph.D., is Professor of Psychiatry and Psychology at the University of Vermont. His research blends human laboratory and treatment outcome studies elucidating behavioral and pharmacological factors involved is substance use disorders.
Contact Information: Substance Abuse Treatment Center, Rm. 1415 UHC, 1 So. Prospect St., Burlington, VT 05401. Phone: 802-656-8714 Fax: 802-656-579 shiggins@uvm.edu
References
- American Society of Addiction Medicine . Board of Director’s public policy statement on nicotine dependence and tobacco. Journal of Addictive Diseases. 1997;16(3):99–104. [Google Scholar]
- Bobo JK, Gilchrist LD, Schilling RF, Noach B, Schinke SP. Cigarette smoking cessation attempts by recovering alcoholics. Addictive Behaviors. 1987;12(3):209–215. doi: 10.1016/0306-4603(87)90030-x. [DOI] [PubMed] [Google Scholar]
- Bobo JK, Slade J, Hoffman AL. Nicotine addiction counseling for chemically dependant patients. Psychiatric Services. 1995;46(9):945–947. doi: 10.1176/ps.46.9.945. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Wander N, Stark MJ, Holbert T. Treating cigarette smoking in drug-abusing clients. Journal of Substance Abuse Treatment. 1995;12(2):89–94. doi: 10.1016/0740-5472(95)00002-m. [DOI] [PubMed] [Google Scholar]
- CDC. Annual smoking-attributable mortality, years of potential life lost, and economic costs --- United States, 1997–2001. Morbidity and Mortality Weekly Report. 2005;55:625–628. [PubMed] [Google Scholar]
- Chait LD, Griffiths RR. Effects of methadone on human cigarette smoking and subjective ratings. The Journal of Pharmacology and Experimental Therapeutics. 1984;229(3):636–640. [PubMed] [Google Scholar]
- Clark JG, Stein MD, McGarry KA, Gogineni A. Interest in smoking cessation among injection drug users. The American Journal on Addictions. 2001;10(2):159–166. doi: 10.1080/105504901750227804. [DOI] [PubMed] [Google Scholar]
- Clemmey P, Brooner R, Chutuape MA, Kidorf M, Stitzer M. Smoking habits and attitudes in a methadone maintenance treatment population. Drug and Alcohol Dependence. 1997;44(2–3):123–132. doi: 10.1016/s0376-8716(96)01331-2. [DOI] [PubMed] [Google Scholar]
- Drug Abuse and Alcohol Treatment Information System (DASIS) Office of Applied Studies. SAMHSA; 2006. Facilities operating opioid treatment programs: 2005. [Google Scholar]
- Dunn KE, Sigmon SC, Thomas CS, Heil SH, Higgins ST. Voucher-based contingent reinforcement of smoking abstinence among methadone-maintained patients: A pilot study. Journal of Applied Behavior Analysis. 2008;41(4):527–538. doi: 10.1901/jaba.2008.41-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom A, Adamsson C, Allebeck P, Rydberg U. Mortality in patients with substance abuse: a follow-up in Stockholm County, 1973–84. International Journal of Addictions. 1991;26(1):91–106. doi: 10.3109/10826089109056241. [DOI] [PubMed] [Google Scholar]
- Federal Register 42 CRF Part 8. Final Rule. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration; 2001. Opioid drugs in maintenance and detoxification treatment of opiate addiction. [PubMed] [Google Scholar]
- Frosch DL, Shoptaw S, Jarvik ME, Rawson RA, Ling W. Interest in smoking cessation among methadone maintained outpatients. Journal of Addictive Disorders. 1998;17(2):9–19. doi: 10.1300/J069v17n02_02. [DOI] [PubMed] [Google Scholar]
- Fuller BE, Guydish J, Tsoh J, Reid MS, Resnick M, Zammarelli L, Ziedonis DM, Sears C, McCarty D. Attitudes towards the integration of smoking cessation treatment into drug abuse clinics. Journal of Substance Abuse Treatment. 2007;32(1):53–60. doi: 10.1016/j.jsat.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Prospective study of factors predicting outcome of transdermal nicotine treatment in smoking cessation. British Medical Journal. 1994;309(6958):842–846. doi: 10.1136/bmj.309.6958.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Alessi SM, Dantona RL. Voucher-based incentives: a substance abuse treatment innovation. Addictive Behaviors. 2002;27(6):887–910. doi: 10.1016/s0306-4603(02)00297-6. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dumeer AT, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking-cessation outcomes in pregnant women. Drug and Alcohol Dependence. 2006;85:138–141. doi: 10.1016/j.drugalcdep.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Solomon LJ, Lussier JP, Abel RL, Lynch ME, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine and Tobacco Research. 2004;6(6):1015–1020. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Silverman K, Heil SH, editors. Contingency Management in the Treatment of Substance Use Disorders: A Science-based Treatment Innovation. New York: The Guilford Press; 2008. [Google Scholar]
- Hoffman AL, Kantor B, Leech T, Lindberg D, Order-Connors B, Schreiber J, Slade J. Drug-free is nicotine-free: Addressing tobacco in the treatment and prevention of other addictions. New Brunswick, NJ: St. Peter’s Medical Center; 1997. [Google Scholar]
- Hser YI, McCarthy WJ, Anglin MD. Tobacco use as a distal predictor of mortality among long-term narcotic addicts. Preventative Medicine. 1994;23(1):61–69. doi: 10.1006/pmed.1994.1009. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Croghan IT, Offord KP, Eberman KM, Morse RM. Attitudes towards nicotine dependence among chemical dependency unit staff: before and after a smoking cessation trial. Journal of Substance Abuse Treatment. 1995;12(4):247–252. doi: 10.1016/0740-5472(95)00024-y. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Eberman KM, Croghan IT, Offord KP, Davis LJ, Morse RM, Palmen MA, Bruce BK. Nicotine dependence treatment during inpatient treatment for other addictions: a prospective intervention trial. Alcoholism: Clinical and Experimental Research. 1994;18(4):867–872. doi: 10.1111/j.1530-0277.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ. Mortality following inpatient addictions treatment: role of tobacco use in a community-based cohort. Journal of the American Medical Association. 1996;275(14):1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Strain EC, Amass L. Buprenorphine: How to use it right. Drug and Alcohol Dependence. 2003;70:S59–S77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation: who will quit with and without the nicotine patch. Journal of the American Medical Association. 1994;271(8):589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Knapp JM, Rosheim CL, Meister EA, Kottke TE. Managing tobacco dependence in chemical dependency treatment facilities: A survey of current attitudes and policies. Journal of Addictive Diseases. 1993;12(4):89–104. doi: 10.1300/J069v12n04_07. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Skinner W, Kent C, Pope MA. Prospects for smoking treatment in individuals seeking treatment for alcohol and other drug problems. Addictive Behavior. 1989;14(3):273–278. doi: 10.1016/0306-4603(89)90058-0. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr H, O’Brien CP. New data from the Addiction Severity Index: Reliability and validity in three centers. Journal of Nervous and Mental Disorders. 1985;173(7):412–422. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- McCool RM, Richter K, Choi WS. Benefits of and barriers to providing smoking treatment in methadone clinics: findings from a National Study. The American Journal of Addictions. 2005;14(4):358–366. doi: 10.1080/10550490591003693. [DOI] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Mendelson JH. Buprenorphine effects on cigarette smoking. Psychopharmacology. 1985;86(4):417–425. doi: 10.1007/BF00427902. [DOI] [PubMed] [Google Scholar]
- Miller WR, Hedrick KE, Taylor CA. Addictive behaviors and life problems before and after behavioral treatment of problem drinkers. Addictive Behaviors. 1983;8(4):403–412. doi: 10.1016/0306-4603(83)90041-2. [DOI] [PubMed] [Google Scholar]
- Nahvi S, Richter K, Li Xuan Modali L, Arnsten J. Cigarette smoking and interest in quitting in methadone maintenance patients. Addictive Behaviors. 2006;31(11):2127–2134. doi: 10.1016/j.addbeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Olsen Y, Alford DP, Horton NJ, Saitz R. Addressing smoking cessation in methadone programs. Journal of Addictive Diseases. 2005;24(2):33–48. doi: 10.1300/J069v24n02_04. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. Journal of Consulting and Clinical Psychology. 2004;72(6):1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- Reid MS, Fallon B, Sonne S, Flammino F, Nunes EV, Jiang H, Kourniotis E, Lima J, Brady R, Burgess C, Arfken C, Pihlgren E, Giordano L, Starosta A, Robinson J, Rotrosen J. Smoking cessation treatment in community-based substance-abuse rehabilitation programs. Journal of Substance Abuse Treatment. doi: 10.1016/j.jsat.2007.08.010. in press. [DOI] [PubMed] [Google Scholar]
- Richter KP. Good and bad times for treating cigarette smoking in drug treatment. Journal of Psychoactive Drugs. 2006;38(3):311–315. doi: 10.1080/02791072.2006.10399857. [DOI] [PubMed] [Google Scholar]
- Richter KP, Choi WS, Alford DP. Smoking policies in U.S. outpatient drug treatment facilities. Nicotine & Tobacco Research. 2005;7(3):475–480. doi: 10.1080/14622200500144956. [DOI] [PubMed] [Google Scholar]
- Richter KP, Gibson CA, Ahluwalia JS, Schmelzle KH. Tobacco use and quit attempts among methadone maintenance clients. American Journal of Public Health. 2001;91(2):296–299. doi: 10.2105/ajph.91.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, McCool RB, Okuyemi KS, Mayo MS, Ahluwalia JS. Patients’ views on smoking cessation and tobacco harm reduction during drug treatment. Nicotine & Tobacco Research. 2002;4(Suppl 2):S175–S182. doi: 10.1080/1462220021000032735. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug and Alcohol Dependence. 2000;58(1–2):103–109. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Tidey JW. Cocaine use can increase cigarette smoking: evidence from laboratory and naturalistic settings. Experimental and Clinical Psychopharmacology. 1997;5(3):263–268. doi: 10.1037//1064-1297.5.3.263. [DOI] [PubMed] [Google Scholar]
- Sees KL, Clark HW. When to begin smoking cessation in substance abusers. Journal of Substance Abuse Treatment. 1993;10(2):189–195. doi: 10.1016/0740-5472(93)90044-3. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Lamb RJ, Dallery J. Contingency management for reducing tobacco use. In: Higgins ST, Silverman K, Heil SH, editors. Contingency Management in the Treatment of Substance Use Disorders: A Science-based Treatment Innovation. New York: The Guilford Press; 2008. pp. 99–119. [Google Scholar]
- Shoptaw S, Rotheram-Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, Rawson RA, Ling W. Smoking cessation in methadone maintenance. Addiction. 2002;97(10):1317–1328. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- Society for Research on Nicotine and Tobacco (SRNT) Subcommittee on Biochemical Verification. . Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2006 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2007. Office of Applied Studies, NSDUH Series H-32, DHHS Publication No. SMA 07-4293. [Google Scholar]
- Yudkin PL, Jones L, Lancaster T, Fowler GH. Which smokers are helped to give up smoking using transdermal nicotine patches? Results from a randomized, double-blind, placebo-controlled trial. British Journal of General Practice. 1996;46(404):145–148. [PMC free article] [PubMed] [Google Scholar]