Abstract
Primary data for the temperature dependent solubility of HCN in water do not presently exist for low concentrations of HCN at environmentally or physiologically relevant temperatures. Henry’s Law constant (KH, M/atm) for the vapor-solution equilibrium of HCN was determined in 0.1 M sodium phosphate buffer (adjusted to pH 9.00±0.03 at 296.6±0.1 K) from 287–311 K. Stable gas phase concentrations of HCN are generated by established techniques, via air equilibration of aqueous cyanide partitioned by a microporous membrane. The effluent gaseous HCN, in equilibrium with the constant temperature aqueous cyanide, was collected in dilute NaOH and determined by a spectrophotometrically using cobinamide. The KH of HCN may be expressed as ln KH (M/atm) = (8205.7±341.9)/T − (25.323±1.144); r2 = 0.9914) where T is the absolute temperature in K. This corresponds to 9.02 and 3.00 M/atm at 25 and 37.4 °C, respectively, compared to actual measurements of 9.86 and 3.22 at 25.0 and 37.8 °C, respectively. The technique also allows for convenient generation of trace levels of HCN at ppbv-ppmv levels that can be further diluted.
1. INTRODUCTION
Of many different inorganic and organic cyano compounds, hydrogen cyanide (HCN) is the simplest; HCN and its salts (NaCN, KCN, etc.) are well known for their high toxicity (1). Cyanide binds reversibly to the iron containing heme group of cytochrome a3 with resulting inhibition of mitochondrial electron chain transport, decreased energy formation and changes in the cellular redox state producing metabolic acidosis. Like O2, HCN binds to heme Fe(II) in reduced cytochrome a3. However, in the presence of O2 when the iron is oxidized to Fe(III), the binding increases markedly (2). The brain and heart are immediately and readily affected by cyanide (1). Paradoxically, low levels of cyanide are naturally found in biota (HCN at ppbv levels is readily detectable in human breath (3)). Industrial use of HCN and alkali cyanides are important: they are essential components in manufacturing synthetic fibers and plastics, agricultural herbicides, fumigants and insecticides, dyes and pigments, animal feed supplements, chelating agents for water treatment, plating and other specialty chemicals and pharmaceuticals, and in mining and processing gold (1,4). Thousands die annually from fire-associated smoke inhalation, HCN plays a major role in this (5,6). Of the physicochemical properties of HCN that governs its fate, transportation and uptake, its equilibrium aqueous solubility is very important.
Literature values of KH for HCN vary considerably. Dzombak et al. (1) and WHO (7) cite diverse values from multiple sources. In Table 1, we provide a more comprehensive listing but many of these are secondary compilations with no clear indication of the primary data source. Vapor pressures of HCN solutions were listed first in 1928 (8). In the extant literature, the data are often extrapolated to other conditions/temperatures, some of the extrapolations likely are not justifiable (9). Temperatures and/or what concentration range the data actually apply to are often absent (10–11,13–15,18 20).
Table 1.
Single temperature or unspecified temperature values for KH for HCN
| KH, M/atm | t, °C | pHCN, ppmv | Ref | Comments |
|---|---|---|---|---|
| 13.3 | 18 | 138,000 | 8 | Based on the lowest concentration data in this tabulation |
| 9.3 | 25 | 197,000 | 9 | Value extrapolated from above data |
| 8.8–9.6 | ? | ? | 10 | Secondary compilation, no sources are cited |
| 7.5 | ? | ? | 11 | Stated to be effective constant at pH 4, no sources cited |
| 8.7 | 25 | 100 | 12 | Value given in atm/mole fraction, extrapolated to infinite dilution |
| 8.2–8.8 | 25 | 100 | 12,13 | As for previous entry, data from (12), interpreted by authors of (13) |
| 8.8 | ? | 1500 | 13 | As for previous entry, this paper has different values in a table than in text, tabulated values are given here; this result is for a pure cyanide solution |
| 8.4–9.8 | ? | 2190–4150 | 13 | As for previous entry, results for a cyanide bearing synthetic leaching solution |
| 10.5–11.6 | ? | 2020–3030 | 13 | As for previous entry, results for a cyanide based actual zinc leaching solution |
| 13.7 | ? | ? | 1 | Reference 1 cites this as from ref. 14, we were unable to locate this in ref. 14. |
| 12 | 25 | ? | 15 | Secondary source, cites Edwards et al. (1978) that contains no primary data |
| 12.2 | 20 | 2–40 | 16 | Electronically published MS thesis |
| 12.8 | 25 | 318–376 | 17 | |
| 9.4 | ? | ? | 18 | Review, primary source not given |
| 11.9 | 30 | 825 | 19 | As interpreted in (16) |
| 7.6 | 25 | 21 | 20 | Static measurement of headspace by GC. Author suggests that static techniques produce lower KH values |
Table 2 summarizes available data on the temperature dependent KH values of HCN. Dodge and Zabban’s is the only work (21) that contains primary data at three different temperatures, the lowest being 64 °C, much higher than that relevant environmentally/physiologically. Moreover, if these data, obtained by dynamic stripping experiments where incomplete equilibration can occur, are extrapolated to 25 °C, the predicted KH is an order of magnitude greater than most other reported values. Kotlik and Lebedeva (22) measured the HCN vapor pressure over 0.01– 0.5 M solutions of HCN at five different temperatures between 20–95 °C. They provide no primary data but a best fit equation (for conformity, we have converted all original equations to the M/atm units). Some books on HCN/cyanides cite this work (1,23) but by far the work most commonly cited is due to Edwards et al. In their first publication (24), Edwards et al. provided an equation valid over 10–70 °C (their original equation used aqueous concentration in molal units, in the domain of dilute aqueous solutions of our interest, this is not significantly different from molar (M) units). Subsequently they provided an alternate expression valid from 10–140 °C (25). These equations, especially the latter, have been widely cited and used by others in various forms (26,27), one corrected form is due to Yoo et al. (27–29). Ironically, as the source of primary data for the HCN-H2O system, Edwards et al. (24,25) merely cite J. C. Siegle at Du Pont as personal communication. In short, the conditions of these experiments (saturation with pure HCN gas) are far removed from typical environmental conditions and/or the primary data are not accessible to the reader and/or the data are at odds with the bulk of other measurements.
Table 2.
Temperature dependence of KH values for HCN as reported in the literature
| KH, M/atm | t, °C | pHCN, ppmv | Reference | Comments |
|---|---|---|---|---|
| 7.5 | 64 | 19750 | 21 | Most previous treatments assume that ref 21 has also measured results at 18 °C. In fact, these authors merely take that datum from the International critical tables (8). Based on their three experimental temperatures we derive the following linear eqn: ln KH (M/atm) = (6023.6±682.9)/T −15.817±1.922, r2 = 0.9873 |
| 3.0 | 85.5 | 49380 | 21 | r2 drops to 0.8362 if a KH value of 13.3 at 18 C is added to these three points. |
| 1.3 | 100 | 113960 | 21 | |
| 80.3 | 25 | projected | 21 | 25 °C computed from above equation for comparison, it is very far out of line from others. The computed value at 18 °C is 131, order of magnitude greater than the value of 13.3 obtained from (8). |
| See Eqn in Comments | 20–95 | 8400–64600 | 22 | Best fit eqn.: ln KH (M/atm) = (2931.0/T − 7.7897)±0.11; primary data not given, t= 20–95°C range was studied for C = 0.01–0.50 M. To estimate range of gas concn, we conservatively assume 0.50 M at 20°C and 0.01 M at 95°C. |
| 7.7 | 25 | not known, estimated ~10000+ ppm | 22 | 25°C value computed for comparison, based on the above equation. |
| See Eqn in Comments | 10–70 | unknown, likely very high | 24 | ln KH (M/atm)= 212343/T − 7605.09 +1319.22*ln T − 2.08347*T valid over 10–70 °C, No primary source data which is cited as personal communication. |
| 10.1 | 25 | unknown | 24 | 25°C value computed for comparison, based on the above eqn. |
| See Eqn in Comments | 10–140 | unknown, likely very high | 25 | ln KH (M/atm) = 49068.8/T − 1446.005 + 241.82*ln T − 0.315014*T valid over 10–140 °C, No primary source data: cited as personal communication. Used by many in various forms, see, e.g., (26) |
| 11.6 | 25 | unknown | 25 | 25°C value computed for comparison, based on the above eqn. |
| See Eqn in Comments | 0–100 | See Comments | 27 | Says that it uses (25) and (28) as data source, when in fact (25) contains no primary data and (28) describes the dissociation constant of the ammonium ion. Cited as the sole source of KH by some authoritative compilations, e.g., ATSDR (29). Eqn given: ln KH (M/atm) = 6302.0/T −9.5850 − 3.1704*ln T + 0.03147*T |
| 17.8 | 25 | See Comments | 27 | 25°C value computed for comparison based on the above equation. |
| See Eqn in Comments | 14–38 | 0.8–36 | This work | ln KH (M/atm) = (8205.7±341.9)/T− (25.323±1.144), r2 =0.9914, 24 measurement conditions each in triplicate, the value computed for 25 °C is 9.02 |
| 9.86 | 25 | 0.8–36 | This work | This mean experimental value is within 10% of the value calculated from the temperature dependence equation. |
Our interest in HCN stems from its role in smoke inhalation and its potential use by terrorists as a released toxic gas. In this paper, we have determined the KH for HCN over a temperature range (287– 311 K) that spans the ambient to physiological range. We used a dynamic equilibrium system where equilibrium is established at the liquid-gas interface within a length of hydrophobic microporous tubing that is immersed in a cyanide bearing solution of known pH and ionic strength. Since its original introduction (30) this technique has been widely used for the evaluation of KH and for generation of trace standard gas (31–39). A novel highly sensitive photometric assay for the measurement of HCN is also reported.
2. EXPERIMENTAL SECTION
2.1 Reagents
All chemicals used were reagent grade or better and 18.2 MΩ·cm Milli-Q water (www.millipore.com) was used throughout. Pure cobinamide was produced by acid hydrolysis of cobalamin (www.sial.com) following Broderick et al. (40). The stock aqueous KCN solution was calibrated by a standard titrimetric method (41) and stored refrigerated.
2.2 Experimental Arrangements
Measurements were conducted using the arrangement previously reported (31–39). Briefly, ultra high purity grade cylinder N2 was metered through a mass flow controller into a microporous polyvinylidene fluoride (PVDF) membrane tube (Enka, wall thickness 0.3 mm, i.d. 1.0 mm, length 50 cm, type R512) wholly immersed in the HCN generation solution contained in a 500 mL Erlenmeyer flask kept in a constant temperature bath (±0.1 °C). The generation solution consisted of 0.1 M Na2HPO4 solution (adjusted exactly to pH 9.00) to which various concentrations of KCN was added. With sufficient residence time of the flowing air in the porous PVDF tubing, the exit HCN is in chemical and thermal equilibrium with the generation solution. The generated HCN was collected by two serial 30-mL capacity fitted midget bubblers using 10 mL of 50 μM cobinamide in 60 mM NaOH as absorber. An aliquot from the bubbler contents was transferred to a 1 cm path length cuvette and the absorbance values at 580 and 700 nm were read with a photodiode array spectrophotometer (HP 8453, www.agilent.com). In initial experiments, the quantitative collection of cyanide by the upstream bubbler was verified by the lack of any perceptible absorbance change in the contents of the downstream bubbler. Henceforth, the absorbance of the downstream bubbler contents was measured only as a check and not for every sample.
pH was measured with a Φ71 pH meter (Beckman) by an Orion Ross combination electrode calibrated immediately before measurement by bracketing NIST-traceable pH 7 and 10 standard buffers. Buffer pH measurements were made at 23.5±0.1 °C, prior to cyanide addition. Solution manipulation after cyanide addition, including pH measurement, can cause loss of cyanide and thus overestimation of the total cyanide concentration in solution. But up to 0.76 mM KCN was added to the 100 mM Na2HPO4 buffer and this can affect the pH of the buffer, albeit slightly. We therefore measured the pH of the cyanide containing buffer not in the solution actually used for HCN generation but in an identically treated separate aliquot.
The effluent gas was collected for either 10 or 20 min, based on the anticipated pHCN. The system was allowed to equilibrate for 20 h before any actual measurement was made. For any given combination of cyanide solution concentration and temperature, triplicate measurements were made.
CAUTION
Cyanide is extremely toxic and hazardous HCN is easily evolved. Care must be taken to avoid skin contact and inhalation/ingestion. The entire experimental setup was located in a well-ventilated hood. Exit HCN generated in our experiments was trapped in a bubbler containing alkaline hypochlorite (5% bleach solution containing added alkali) before disposal (42). Comparable measures should be taken if similar experiments are performed.
3. RESULTS AND DISCUSSION
3.1. Cobinamide based colorimetric assay for cyanide
For structures of cobalamins and cobinamide, see (43). Hydroxycobalamin binds cyanide with a relatively high affinity (it is used to treat smoke inhalation victims (44)). The colorimetric detection of cyanide by cyanocobalamin to form dicyanocobalamin was recently suggested (45). However, with a detection limit of 0.6 mM in a purely aqueous medium, it has limited utility. Cobinamide is the penultimate precursor in the biosynthesis of cyanocobalamin. It binds cyanide with 1010 times greater affinity than cyanocobalamin and may be a better cyanide poisoning antidote (46,47). Cobinamide also undergoes a greater absorbance change than does any of the cobalamins and can thus be used for sensitive photometric measurement of cyanide, down to low μM levels.
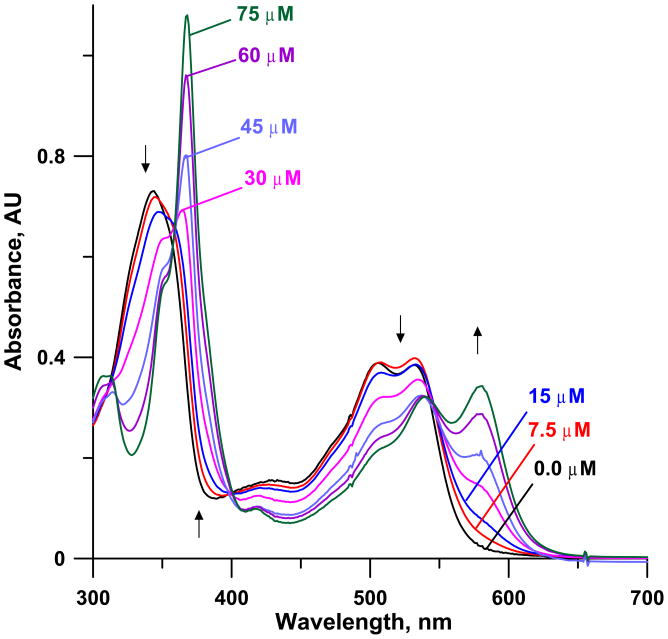
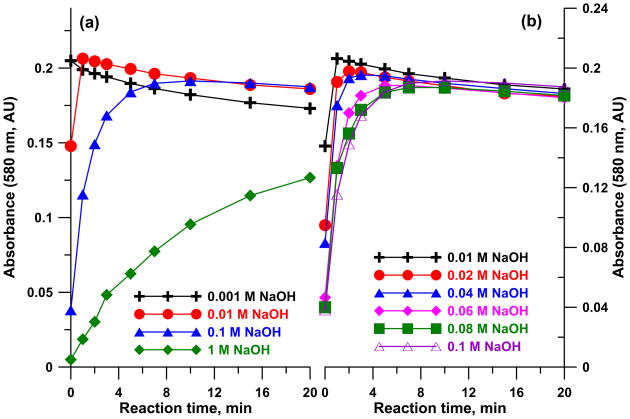
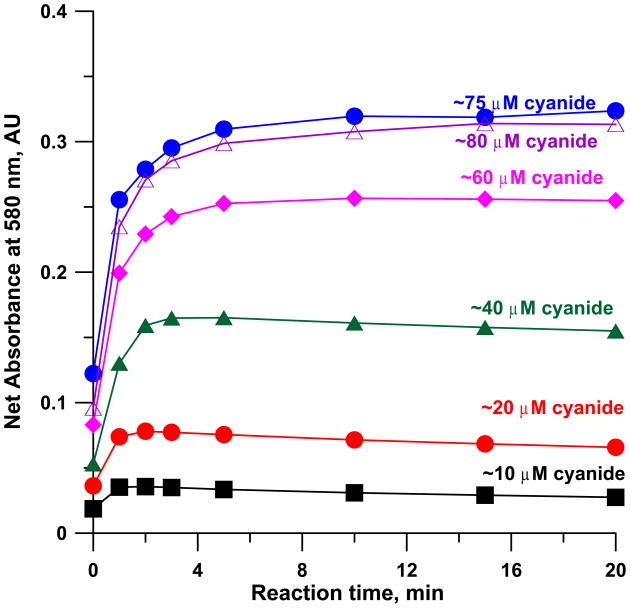
Figure 1 shows the spectra of 50 μM cobinamide in 60 mM NaOH with 0–75 μM cyanide added. It will be observed that the bands at ~365 and 580 nm increase in absorbance upon cyanide addition while the band at ~340 nm and the pair of bands centred at ~520 nm decrease in absorbance with cyanide addition. Similar experiments were first conducted with the same concentration of cobinamide in water, in 0.1 M phosphate and borate buffer solution (each at pH 9.00). While results were similar, the final absorbance changed significantly with time (See Figure S1–S3 in the supporting information (SI) for details). While reproducible timing is not an issue in any automated procedure (e.g., flow-injection or similar procedure) where the collected cyanide is reacted with the reagent on-line, it is problematic in manual assays, especially when cobinamide is already in the absorber. We found that when cobinamide is made in an appropriate NaOH concentration, the product absorbance becomes stable after a short period (Figure 2). With 60 mM NaOH, after >~ 3 min, the response is reasonably stable (Figure 3); calibration slopes after reaction times >5 min are essentially invariant (detailed numerical data can be seen in Table S1 in SI). We will describe cobinamide-based cyanide determination in greater detail elsewhere. Presently, we measured three “unknown” samples, nominally 20.0, 40.0 and 60.0 μM in triplicate by the present method and by the Chloramine-T/pyridine-barbituric acid standard method (48), each calibrated independently. There was no statistical difference between the analytical results at the 95% confidence level by the one tailed t-test and the correlation coefficient between the two sets of analytical results was 0.9992. In addition, with the HCN generation and capture arrangement (50 μM cobinamide in 60 mM NaOH absorber), A580nm increased linearly both with the sampling time (up to 20 min under these conditions, r2 = 0.9967, after this the dicyano complex begins to form and the slope actually increases) and the flow rate (9–30 sccm, r2 = 0.9985); details can be viewed in Figure S4 in Supporting Information. These data suggest that alkaline cobinamide can be reliably used for cyanide measurement. Of course, batch mode measurements cannot take advantage of the ultimate capabilities of such a method; we will describe sub-micromolar limits of detection in a flow system in a separate article in the near future.
Figure 1.
Spectral change in 50 μM cobinamide prepared in 60 mM NaOH upon the addition of 0–75 μM cyanide. Spectra taken 10 min. after cyanide addition.
Figure 2.
Absorbance change at 580 nm at different reaction times (60 μM cyanide reacts with 50 μM cobinamide) (a) prepared in 0.001–1 M NaOH solution, (b) prepared in 0.01–0.1 M NaOH.
Figure 3.
Temporal change in absorbance at 580 nm after 10–75 M cyanide reacts with 50 μM cobinamide in 60 mM NaOH.
3.2 The Henry’s Law Constant of HCN
A high HCN to CN− ratio (as at a low pH) induces volatilization losses soon as the solution is made and this needs to be avoided. To maintain reasonable proximity to physiological pH, we have chosen a pH of 9 as a compromise. Of course, HCN is partially ionized at this pH and this fractional ionization, dependent on the temperature dependent dissociation constant of HCN, KD, must be accurately ascertained. We have chosen to use KD as given in (1):
| (1) |
where pKD is 9.24 at 298.15 K, ΔHr, 298 is the standard enthalpy of reaction at 298.15 K, 135.1 kJ/mol (49). The concentration of undissociated hydrogen cyanide in solution, [HCN] can now be computed from
| (2) |
Here pH and the activity of the cyanide ion, γCN−, are both temperature dependent quantities and require further considerations.
3.2.1. Buffer pH change upon cyanide addition and temperature correction
The pH of a 0.1 M pH 9.012 Na2HPO4 buffer increased in pH 0.006, 0.027, 0.047 and 0.079 pH units upon the incorporation of 0.2, 0.4, 0.6 and 0.8 mM cyanide. These data fit the exponential relationship (within these limits)
| (3) |
With an r2 of 0.9911. The delta pH values anticipated for the specific amounts of cyanide added to the generation solutions were obtained by interpolation with eqn. 3. Calibration buffers of pH 7 and 10 used here also specify pH values at 10–40 °C. We calibrated the pH meter at each generation temperature with these buffers, using the temperature specified pH values. The temperature equilibrated pH o the 0.1 M Na2HPO4 solution was measured at that specific temperature with the meter calibrated at that temperature. The difference in pH from that measured at 23.5 °C was sigmoidal in shape, the pH increased by ~0.2 pH units at the lowest temperature studied and decreased by 0.05 pH units by 35 °C this difference remaining constant up to 38 °C. The actual pH of the cyanide-bearing generation solution at any specific temperature was thus adjusted by this temperature induced change.
3.2.2. Activity coefficient of cyanide
Scatchard and Breckenridge (50) have measured the mean activity coefficient in 0.1 m Na2HPO4 solution to be 0.480 (see also Table 12-3-1 A, Harned and Owen (51)). At this concentration, the difference between molality and molarity is insignificant. From the literature values of Ka values for phosphoric acid (2.15, 7.20 and 12.38, respectively, ref. (52)), in iterative procedures (by initially assuming the ionic strength to be 0.3 M based on the formal concentration and using the Extended Debye-Hückel Equation (EDHE, see ref. (53)), we find that the self consistent ionic strength is 0.299 M; 98+% of the P-species remains the hydrogenphosphate dianion (ion size parameter (see ref. 53) for H2PO4− was assumed to be 4.5 A). For a solution of this ionic strength, the mean ionic activity coefficient in a 0.1 M Na2HPO4 solution by EDHE (we assume ion size parameter of 4 A for Na+ and 4.5 A for H2PO4−, resulting in a mean distance of closest approach to be 4.25 A) will be 0.482, remarkably close to the experimental measurement in (50). We can therefore use EDHE with confidence for the present purpose: we use this in the form:
| (4) |
Where γCN− is the activity coefficient of cyanide, D is the dielectric constant of water, T is the absolute temperature in K, I is the ionic strength and a is the mean distance of closest approach in angstroms. In the present case the last parameter pertains to the approach between Na+ and CN−. Given 3 A as the ion size parameter for CN− and 4 A for Na+ (53), we use a = 3.5 A. The reader interested in calculation details is referred to the Excel® spreadsheet in the supporting information. γCN− showed little dependence on temperature over this limited experimental range, remaining essentially constant at 0.673±0.003. In dilute solutions, the activity coefficient of unionized HCN (aq) may be regarded as unity.
3.2.3. Equilibrium data and comparison
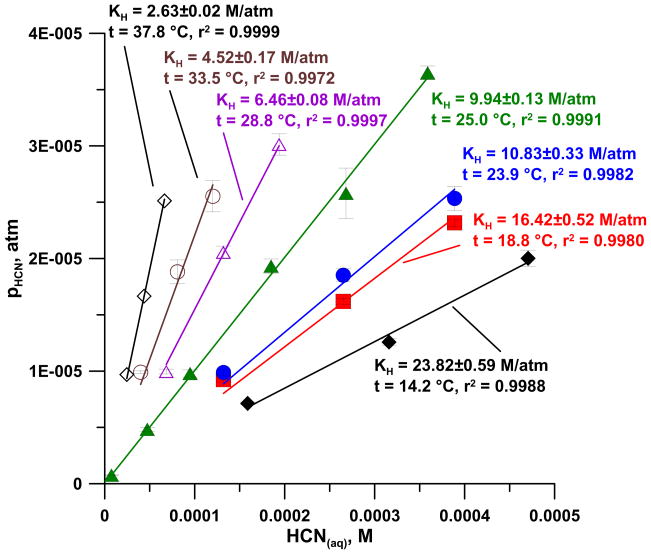
At 6 different temperatures from 14.2 to 37.8 °C, we used three different total cyanide concentrations in the generation solution, 0.18–0.20, 0.38, and 0.57 mM. In addition, three additional concentrations, 0.015, 0.094 and 0.76 mM, were tested at 25 °C, the equilibrium gaseous HCN concentrations ranged from 700 ppbv to 40 ppmv; detailed results are presented in Figure 4.
Figure 4.
Vapor pressures of HCN in equilibrium with 75 μM – 0.36 mM HCN(aq) at 7 different temperatures (n = 3 each).
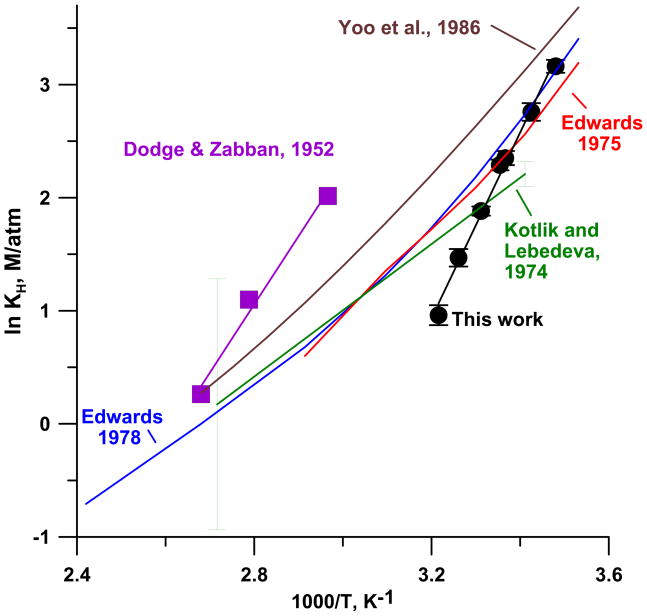
Figure 5 shows the ln KH values obtained in this work plotted against reciprocal absolute temperature and compared with the other data describing temperature dependence in the literature. It will be observed that at temperatures below 25 °C, the results from this work are quite close to the results given by the Edwards et al. expressions (24, 25) while the Kotlik-Lebedeva equation (22) agrees with our value at 28.8 °C and over/under predicts our data at temperatures above/below this temperature. Yoo et al.’s (27) KH expression runs 0.4 ln units higher than Edwards et al. (1978) and is thus also consistently above our data. Dodge and Zabban’s data (21) show much higher KH values than all others but it is remarkable that the slope of their data is remarkably close to ours. At 25 °C, our measured value is 9.86 and that predicted from our temperature dependent equation is 9.02. The values in Table 1 for which the temperature is explicitly 25 °C average to 9.82±2.09, this agreement is thus remarkably good.
Figure 5.
The temperature dependent Henry’s Law constant for HCN from this work compared to data (Dodge and Zabban, 1952) and equations in the literature. The error bars (n = 9–18 for each point in this work, n= 23 for the linear fit of Kotlik and Lebedeva) indicate ±1 standard deviation.
At higher temperatures, especially as we approach physiological temperature at the high end of our experimental range, our data progressively produce lower KH values relative to the other expressions, this deserves comment. One salient difference between our work and the previous temperature dependent data largely lie in the absolute concentration range involved. Dimerization of HCN have been invoked in diverse situations, from interstellar clouds (54) to prebiotic synthesis of purine precursors from aqueous HCN (55,56). If there is any such dimerization in solution, at higher aqueous HCN concentration, the effect will be greater on KH and will shift KH to higher values. If this is the case, it would also suggest that HCN dimerization is promoted at higher temperatures. It is interesting that all the other data in Figure 5 except that of Dodge and Zabban (21) were obtained under static conditions. If the equilibration between dimeric and monomeric aqueous HCN is not rapid on the stripping time scale, it will explain why Dodge and Zabban’s data, also obtained under dynamic conditions, predicts a similar H as our results.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health Grant No. NINDS U01 NS058030-S2 and in part through National Science Foundation Grant No. CHE-0821969.
Footnotes
Supporting Information Available
The Supporting Information includes an Excel file that illustrates aspects of calculations made and a pdf file that illustrates the cobinamide-cyanide reaction in water, and in phosphate and borate buffers, effect of sampling time and flow rate and constancy of calibration at different reaction times. These materials are available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Jian Ma, Department of Chemistry and Biochemistry, University of Texas, 700 Planetarium Place, Arlington, TX 76019-0065. Phone: 817-272-3171, Fax: 817-272-3808.
Purnendu K. Dasgupta, Email: Dasgupta@uta.edu, Department of Chemistry and Biochemistry, University of Texas, 700 Planetarium Place, Arlington, TX 76019-0065. Phone: 817-272-3171, Fax: 817-272-3808
William Blackledge, Department of Medicine, University of California, San Diego, La Jolla, CA 92093-0652.
Gerry R. Boss, Department of Medicine, University of California, San Diego, La Jolla, CA 92093-0652
Literature cited
- 1.Dzombak DA, Ghosh RS, Wong-Chong GM. Cyanide in water and soil: Chemistry, risk and management. CRC press; Boca Raton, FL: 2006. pp. p2pp. p238–240.pp. p41pp. p60–61. [Google Scholar]
- 2.National Research Council. Combined Exposures to Hydrogen Cyanide and Carbon Monoxide in Army Operations: Initial Report. National Academies Press; 2008. p. p8. [PubMed] [Google Scholar]
- 3.Stamyr K, Vaittinen O, Jaakola J, Guss J, Metsala M, Johanson G, Halonen L. Background levels of hydrogen cyanide in human breath measured by infrared cavity ring down spectroscopy. Biomarkers. 2009;14:285–291. doi: 10.1080/13547500902903048. [DOI] [PubMed] [Google Scholar]
- 4.Pesce LD. Kirk-Othmer Encyclopedia of Chemical Technology. Vol. 7. Wiley; New York: 1993. Cyanides; pp. p326–327. [Google Scholar]
- 5.Prien T, Traber DL. Toxic smoke compounds and inhalation injury - a review. Burns. 1988;14:451–460. doi: 10.1016/s0305-4179(88)80005-6. [DOI] [PubMed] [Google Scholar]
- 6.Alarie YC. Toxicity of fire smoke. Crit Rev Toxicol. 2002;32:259–289. doi: 10.1080/20024091064246. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Hydrogen cyanide and cyanides: human health aspects. Geneva: 2004. [Accessed January 09, 2009]. Concise international chemical assessment document 61; p. p6. http://www.who.int/ipcs/publications/cicad/en/cicad61.pdf. [Google Scholar]
- 8.Physics, chemistry and technology. III. McGraw-Hill; New York: 1928. International critical tables of numerical data; p. p365. Vol 3. [Google Scholar]
- 9.Hine J, Weimar RD., Jr Carbon basicity. J Am Chem Soc. 1965;87:3387–3396. [Google Scholar]
- 10.Smith A, Mudder T. The chemistry and treatment of cyanidation wastes. Mining Journal Books, Ltd; London: 1991. [Google Scholar]
- 11.Gaffney JS, Streit GE, Spall WD, Hall JH. Beyond acid rain: do soluble oxidants and organic toxins interact with SO2 and NOx to increase ecosystem effects? Environ Sci Technol. 1987;21:519–524. doi: 10.1021/es00160a001. [DOI] [PubMed] [Google Scholar]
- 12.Dodge BF, Zabban W. Disposal of plating room wastes: V. Batch volatilization of hydrogen cyanide from aqueous solutions of cyanides. Plating. 1952;11:1235–1244. [Google Scholar]
- 13.Avedesian MM, Spira P, Kanduth H. Stripping of HCN in a packed tower. Can J Chem Eng. 1983;61:801–806. [Google Scholar]
- 14.Doudoroff P, Leduc G, Schneider CR. Acute toxicity to fish of solutions containing complex metal cyanides, in relation to concentrations of molecular hydrocyanic acid. Trans Am Fish Soc. 1966;95:6–22. [Google Scholar]
- 15.Li Q, Jacob DJ, Bey I, Yantosca RM, Zhao Y, Kondo Y, Notholt J. Atmospheric hydrogen cyanide (HCN): biomass burning source, ocean sink? Geophys Res Letter. 2000;27:357–360. [Google Scholar]
- 16.Lötter NH. MS Thesis. University of Pretoria; Pretoria, S. Africa: 2006. Cyanide volatilisation from gold leaching operations and tailing storage facilities. [Google Scholar]
- 17.Pattrick G. Technical memorandum, Process chemistry division. MINTEK; Randburg, S. Africa: 2000. Studies of hydrogen cyanide volatility from cyanidation slurry. [Google Scholar]
- 18.Chatwin TD, Trepanowski JJ. Utilisation of soils to mitigate cyanide releases. Proc. 3rd Western Regional Conf. Precious Metals, Coal, Environ; Rapid City, S. Dakota. September 23–26; pp. 201–220. [Google Scholar]
- 19.Heath AR, Rumball JA, Browner REJA. A method for measuring HCN(g) emission from CIP/CIL tanks. Minerals Engineering. 1998;11:749–761. [Google Scholar]
- 20.Lye P, Cricelli R, Corbyn Z, Hannah H, Heter H, May P. Amira Project P420B, Gold proceesing technology, Module 3: Cyanide and the environment. A.J. Parker Research Center fotr Hydrometallurgy, Murdoch University; Australia: 2004. Chemical measurement. [Google Scholar]
- 21.Dodge BF, Zabban W. Disposal of plating room wastes: IV. Batch volatilization of hydrogen cyanide from aqueous solutions of cyanides. Plating. 1952;10:1133–1139. [Google Scholar]
- 22.Kotlik SB, Lebedeva GN. Equilibrium pressures of HCN and NH3 over aqueous solutions. Zh Prikl Khim. 1974;47:444–446. [Google Scholar]
- 23.Bodek I, Lyman W, Reehl WP, Rosenblatt DH, editors. Environmental inorganic chemistry: properties, processes, and estimation methods. Pergamon Press; New York: 1988. pp. p10.13–3. [Google Scholar]
- 24.Edwards TJ, Newman J, Prausnitz JM. Thermodynamics of aqueous solutions containing volatile weak electrolytes. AIChE J. 1975;21:248–259. [Google Scholar]
- 25.Edwards TJ, Maurer G, Newman J, Prausnitz JM. Vapor-liquid equilibria in multicomponent aqueous solutions of volatile weak electrolytes. AIChE J. 1978;24:966–976. [Google Scholar]
- 26.Kümmel R. Liquid/vapour equilibriums of aqueous electrolyte solutions of volatile pollutants. Acta Hydrochim Hydrobiol. 1985;13:109–114. [Google Scholar]
- 27.Yoo KP, Lee SY, Lee WH. Ionization and Henry’s Law constants for volatile, weak electrolyte water pollutants. Kor J Chem Eng. 1986;3:67–72. [Google Scholar]
- 28.Bates RG, Pinching GD. Acidic dissociation constant of ammonium ion at 0-Degrees to 50-Degrees C, and the base strength of ammonia. J Res NatBur Stand. 1949;42:419–430. [Google Scholar]
- 29.The Agency for Toxic Substances and Disease Registry. Toxicological profile for cyanide. Atlanta, GA: US Department of Health and Human Services; [Accessed January 9, 2009]. p. p135. http://www.atsdr.cdc.gov/toxprofiles/tp8-c4.pdf. [PubMed] [Google Scholar]
- 30.Hwang H, Dasgupta PK. Thermodynamics of the hydrogen peroxide-water system. Environ Sci Technol. 1985;19:255–258. doi: 10.1021/es00133a006. [DOI] [PubMed] [Google Scholar]
- 31.Dasgupta PK, Dong S. Solubility of ammonia in liquid water and generation of trace levels of standard gaseous ammonia. Atmos Environ. 1986;20:565–570. doi: 10.1021/es00148a016. [DOI] [PubMed] [Google Scholar]
- 32.Dong S, Dasgupta PK. Solubility of gaseous formaldehyde in liquid water and generation of trace standard gaseous formaldehyde. Environ Sci Technol. 1986;20:637–640. doi: 10.1021/es00148a016. [DOI] [PubMed] [Google Scholar]
- 33.Shepson PB, Mackay E, Muthuramu K. Henry’s law constants and removal processes for several atmospheric β-hydroxy alkyl nitrates. Environ Sci Technol. 1996;30:3618–3623. [Google Scholar]
- 34.Treves K, Shragina L, Rudich Y. Henry’s law constants of some β-, γ- and δ-hydroxy alkyl nitrates of atmospheric interest. Environ Sci Technol. 2000;34:1197–1203. [Google Scholar]
- 35.Gautier C, Calvé SL, Mirabel P. Henry’s law constants measurements of alachlor and dichlorvos between 283 and 298 K. Atmos Environ. 2003;37:2347–2353. [Google Scholar]
- 36.Feigenbrugel V, Calvé SL, Mirabel P, Louis F. Henry’s law constant measurements for phenol, o-, m-, and p-cresol as a function of temperature. Atmos Environ. 2004;38:5577–5588. [Google Scholar]
- 37.Feigenbrugel V, Calvé SL, Mirabel P. Temperature dependence of Henry’s law constants of metolachlor and diazinon. Chemosphere. 2004;57:319–327. doi: 10.1016/j.chemosphere.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Xie Z, Calvé SL, Feigenbrugel V, Preuβ TG, Vinken R, Ebinghaus R, Ruck W. Henry’s law constants measurements of the nonylphenol isomer 4(3′,5′-dimethyl-3′-heptyl)-phenol, tertiary octylphenol and γ-hexachlorocyclohexane between 278 and 298 K. Atmos Environ. 2004;38:4859–4868. [Google Scholar]
- 39.Reyes-Pérez E, Calvé SL, Mirabel P. UV absorption spectrum and Henry’s law constant of EPTC. Atmos Environ. 2008;42:7940–7946. [Google Scholar]
- 40.Broderick KE, Singh V, Zhuang S, Kambo A, Chen JC, Sharma VS, Pilz RB, Boss GR. Nitric oxide scavenging by the cobalamin precursor cobinamide. J Biol Chem. 2005;280:8678–8685. doi: 10.1074/jbc.M410498200. [DOI] [PubMed] [Google Scholar]
- 41.American Public Health Association. Standard Methods for the Examination of Water and Wastewater. 21. Washington, DC: 2005. Method 4500-CN- D: Titrimetric Method. [Google Scholar]
- 42.Kubáň V, Dasgupta PK. Selective determination of gases by two-stage membrane-differentiated flow injection analysis: determination of trace hydrogen cyanide in the presence of large concentrations of hydrogen sulfide. Anal Chem. 1992;64:1106–1112. doi: 10.1021/ac00025a008. [DOI] [PubMed] [Google Scholar]
- 43.Weinberg JB, Chen Y, Jiang N, Beasley BE, Salerno JC, Ghosh DK. Inhibition of nitric oxide synthase by cobalamins and cobinamides. Free Radic Biol Med. 2009;46:1626–1632. doi: 10.1016/j.freeradbiomed.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borron SW, Baud FJ, Barriot P, Imbert M, Bismuth C. Prospective study of hydroxocobalamin for acute cyanide poisoning in smoke inhalation. Ann Emerg Med. 2007;49:794–801. doi: 10.1016/j.annemergmed.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 45.Zelder FH. Specific colorimetric detection of cyanide triggered by a conformational switch in Vitamin B12. Inorg Chem. 2008;47:1264–1266. doi: 10.1021/ic702368b. [DOI] [PubMed] [Google Scholar]
- 46.Broderick KE, Potluri P, Zhuang S, Scheffler IE, Sharma VS, Pilz RB, Boss GR. Cyanide detoxification by the cobalamin precursor cobinamide. Exper Biol Med. 2006;231:641–649. doi: 10.1177/153537020623100519. [DOI] [PubMed] [Google Scholar]
- 47.Broderick KE, Balasubramanian M, Chan A, Potluri P, Feala J, Belke DD, McCulloch A, Sharma VS, Pilz RB, Bigby TD, Boss GR. The cobalamin precursor cobinamide detoxifies nitroprusside-generated cyanide. Exper Biol Med. 2007;232:789–798. [PubMed] [Google Scholar]
- 48.American Public Health Association. Standard Methods for the Examination of Water and Wastewater. 21. APHA; Washington DC: 2005. Method 4500-CN- (E: Colorimetric Method) [Google Scholar]
- 49.Lide DR, Kehiaian HV. CRC handbook of thermophysical and thermochemical data. CRC Press; Boca Raton, FL: 1994. p. p146. [Google Scholar]
- 50.Scatchard G, Breckenridge PC. Isotonic Solutions. II. The chemical potential of water in aqueous solutions of potassium and sodium phosphates and arsenates at 25. J Phys Chem. 1954;58:596–602. [Google Scholar]
- 51.Harned HS, Owen BB. The Physical Chemistry of Electrolytic Solutions. 3. Reinhold; New York: 1958. p. p734. [Google Scholar]
- 52.Dean JA, editor. Langes’s Handbook of Chemistry. McGraw-Hill; New York: 1986. Table 5–7. [Google Scholar]
- 53.Laitinen HA, Harris WE. Chemical Analysis. 2. McGraw-Hill; New York: 1975. pp. 9–16. [Google Scholar]
- 54.Smith IWM, Talbi D, Herbst E. The production of HCN dimer and more complex oligomers in dense interstellar clouds. Astronom Astrophys. 2009;369:611–615. [Google Scholar]
- 55.Kliss RM, Matthews CN. Hydrogen cyanide dimerization and chemical evolution. Proc Nat Acad Sci. 1962;48:1300–1306. doi: 10.1073/pnas.48.8.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kikuchi O, Watanabe T, Satoh Y, Inadomi Y. Ab initio GB study of prebiotic synthesis of purine precursors from aqueous hydrogen cyanide: dimerization reaction of HCN in aqueous solution. J Mol Struct-Theochem. 2000;507:53–62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.