Abstract
Arachidonic acid metabolites, eicosanoids, are key contributors to vascular function and improper eicosanoid regulation contributes to the progression of cardiovascular diseases. Epoxyeicosatrienoic acids (EETs) are synthesized from arachidonic acid by epoxygenase enzymes to four regioisomers, 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET. These EETs have interesting beneficial effects like vasodilation, anti-inflammation, and anti-platelet aggregation that could combat cardiovascular diseases. There is mounting evidence that each regioisomeric EET may have unique vascular effects and that the contribution of individual EETs to vascular function differs from organ to organ. Over the past decade EET analogs and antagonists have been synthesized to determine EET structure function relationships and define the contribution of each regioisomeric EET. A number of studies have demonstrated that EET analogs induce vasodilation, lower blood pressure and decrease inflammation. EET antagonists have also been used to demonstrate that endogenous EETs contribute importantly to cardiovascular function. This review will discuss EET synthesis, regulation and physiological roles in the cardiovascular system. Next we will focus on the development of EET analogs and what has been learned about their contribution to vascular function. Finally, the development of EET antagonists and how these have been utilized to determine the cardiovascular actions of endogenous epoxides will be discussed. Overall, this review will highlight the important knowledge garnered by the development of EET analogs and their possible value in the treatment of cardiovascular diseases.
Keywords: epoxyeicosatrienoic acids, endothelium derived hyperpolarizing factor, cardiovascular, inflammation, analogs, agonist and antagonist
INTRODUCTION - EET METABOLISM & REGULATION
Arachidonic acid is a polyunsaturated fatty acid that is present in membrane phospholipids at stereospecific numbering (sn)-2 position. Hormonal and paracrine stimuli can activate phospholipase A2 that catalyzes the release of arachidonic acid from membrane phospholipids. This intracellular arachidonic acid is catabolized by three enzymatic pathways that includes, cyclooxygenase (COX) conversion to prostaglandins, lipoxygenase conversion to leukotrienes and cytochrome P450 (CYP) conversion to epoxyeicosatrienoic acids (EETs) and hydroxyeicosatetraenoic acids. Mammalian CYP epoxygenase enzymes catalyze olefin bond epoxidation of arachidonic acid to generate EETs. These EETs possess a wide array of potent biological actions. This review provides an overview of EETs and the importance for developing EET analogs and antagonists and their possible value in the treatment of cardiovascular diseases.
CYP enzymes are membrane-bound, heme-containing oxidases that oxidize numerous endogenous substances including fatty acids. Epoxygenase enzymes metabolize arachidonic acid to produce EETs (Figure 1). Though several CYP isoforms can generate EETs in humans, the predominant CYP isoforms, CYP2C8, CYP2C9 and CYP2J2, are expressed in the endothelium [1,2]. The predominant rodent CYP isoforms include Cyp2c44 and Cyp2j5 in mice and Cyp2c11, Cyp2c23 and Cyp2j2 in rats [2]. In endothelial cells, CYP isoforms produce four regioisomeric EETs: 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET. Each regioisomer consists of a mixture of the S/R and R/S enantiomers. Production by CYP epoxygenases and vascular activity of these enantiomers can vary between vascular beds and species. CYP2C8 produces 86% 14(R),15(S)-EET and 81% 11(R),12(S)-EET entantiomers whereas CYP2C9 produces these enantiomers as 63% 14(R),15(S)-EET and 31% 11(R),12(S)-EET. CYP2J2 is similar to CYP2C9 and produces 63% 14(R),15(S)-EET and 44% of 11(R),12(S)-EET enatiomers [3,4]. Each enantiomer has different potencies to dilate organ vasculatures. 11(R),12(S)-EET produces vasodilation while 11(S),12(R)-EET was not active in rat renal arteries [5]. 14(S),15(R)-EET induced vascular relaxation in bovine coronary arteries whereas 14(R),15(S)-EET was less active [6,7]. These findings provide support to the notion that different regioisomeric and stereoselective EETs can have differential effects on vascular function.
Figure 1.
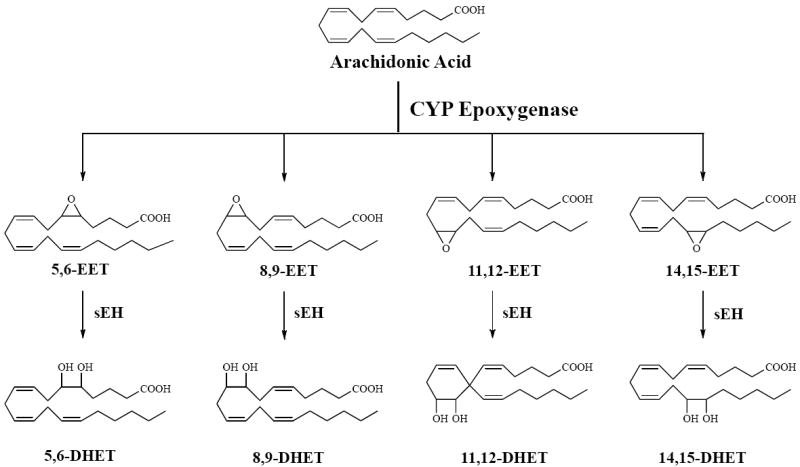
Epoxides are generated from arachidonic acid. Arachidonic acid is converted to epoxyeicosatrienoic acid (EET) by cytochrome P450 (CYP) epoxygenase. EETs primary metabolic fate is conversion to dihydroxyeicosatrienoic acids (DHETs) by the soluble epoxide hydrolase (sEH) enzyme.
Once EETs are formed, they can be further recycled or excreted by several pathways, including esterification into membrane phospholipids, binding to proteins, metabolism to shorter chain molecules by β-oxidation or metabolism by soluble epoxide hydrolase (sEH). EETs can also be esterified into the sn-2 position of phospholipids in kidney and liver tissue and this esterification can potentially modulate cell membrane channel activity [8,9]. Chen et al. [9] reported that esterified EETs inhibit cardiac Ca2+ channel activity in a lipid bilayer. These findings indicate that esterified EETs in phospholipids can contribute to the regulation of cell membrane ion transport. Binding to fatty acid binding proteins (FABP) is another potential mechanism by which EETs can be regulated [10,11]. FABP has affinity with EETs and this binding decreases the conversion of EETs to their corresponding diols [12]. FABP binding of EETs could serve as a source for EETs and could be a means for regulating EETs biological actions. Several studies have also demonstrated that EETs are metabolized to short chain epoxides by β-oxidation [13,14]. Fang et al. [13] reported that 11,12-EET is converted to a short chain epoxide, 7,8-dihydroxy-hexadecadienoic acid in porcine vascular smooth muscle cells and this chain shortened epoxide possesses coronary artery vasoactive properties [14]. Although these fates of EETs have potential biological consequences, the major pathway for metabolism of EETs is by sEH.
The primary pathway for EET metabolism to dihydroxyeicosatrienoic acids (DHETs) is through hydration by sEH (Figure 1). The sEH enzyme is widely distributed in mammalian tissues, red blood cells, and blood vessels [15-17]. The presence of DHETs in many organs confirms that this pathway occurs in vivo [18-20]. The 14,15-EET regioisomer is the preferred substrate for sEH followed by 11,12-EET and 8,9-EET. On the other hand, 5,6-EET is a poor substrate for this enzyme [21]. 14,15-EET is converted to 14,15-DHET by near 100% over a six-hour period in human coronary artery and aorta [22]. Likewise, porcine aortic endothelial cells, canine and bovine coronary arteries convert 14,15-EET to 14,15-DHET [14,23,24]. EET metabolism by sEH depends on regioisomeric as well as stereoselective properties. Zeldin et al. [21] showed that EET hydration by sEH was stereoselective for 14(R),15(S)-EET, 11(S),l2(R)-EET, and 8(S),9(R)-EET enantiomers. Interestingly, sEH inhibition increases the synthesis of several short chain β-oxidation products in porcine coronary endothelial cells suggesting a shift in EET metabolism [14]. In general, the conversion of EETs to their corresponding diols by sEH diminishes the biological activity of epoxides. 14,15-DHET is less potent in respect to dilation than 14,15-EET in the bovine coronary arteries [6,24]. Imig et al. [25] reported that 11,12-EET induces afferent arteriolar relaxation but 11,12-DHET had no effect in renal arterioles. The metabolism of EETs is very important since sEH inhibitors are currently in phase II clinical trials for the treatment of cardiovascular diseases.
PHYSIOLOGICAL ROLE OF EETs IN VASCULAR SYSTEM
Modulation of Vascular Tone
One of the most important cardiovascular effects of EETs is inducing vasodilation. EETs are endothelium derived hyperpolarization factors (EDHFs) that are released from the endothelium and relax the vascular smooth muscle cells in a paracrine manner. EETs relax preconstricted mesenteric arteries, renal arteries, cerebral arteries, and coronary arteries [25-33]. EET-induced vasodilation occurs through the activation of large-conductance calcium-activated K+ (BKCa) channels [1,5,7,27]. Activation of K+ channels results in K+ efflux from the vascular smooth muscle cell and subsequent membrane hyperpolarization. Investigations have implicated several cell signaling pathways in EET-induced activation of K+ channels (Figure 2A). 11,12-EET increases cAMP levels and activates protein phosphatase 2A (PP2A) in mesenteric resitance arteries and renal microvessels and these signaling pathways contribute to activation of the BKCa channel and vasodilation [27,34-36]. Weston et al. [37] reported that 11,12-EET activates porcine coronary vascular smooth muscle cell BKCa channel along with endothelial cell small (SKCa) and intermediate (IKCa) conductance calcium-activated K+ channels. On the other hand, 5,6-EET and 8,9-EET have been demonstrated to activate transient receptor potential vanilloid 4 channels in mouse endothelial cells [38]. Activation of this vanilloid channel produces Ca2+ influx, endothelial K+ channel activation, and hyperpolarizes the endothelium that subsequently results in relaxation of the adjacent vascular smooth muscle. The potency and actions of EET regioisomers and the cell signaling pathways utilized are not the same in all vascular tissues. This variability in cell signaling and vasoactivity for the regioisomeric EETs provides the impetus for developing agonists and antagonists that selectively inhibit or mimic the activities of various EETs.
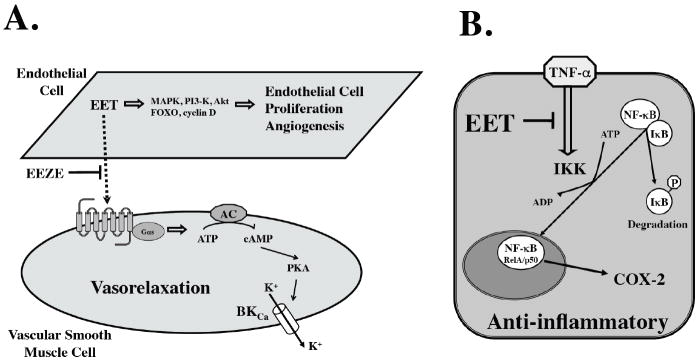
Figure 2.
Epoxyeicosatrienoic acid (EET) activate vascular (panel A) and anti-inflammatory (panel B) cell signaling mechanisms. Panel A: Endothelial cell proliferation and angiogensis involves activation of p38 mitogen-activated protein (MAPK), phosphatidylinositol 3-kinase (PI3-K), kinase Akt, forkhead factors (FOXO) and cyclin D. Vasorelaxation involves activation G protein (Gαs), adenylyl cyclase (AC) generation of cAMP, protein kinase A (PKA) and opening of large-conductance calcium-activated potassium channels (BKCa). Panel B: EET anti-inflammatory action involves inhibition of tumor necrosis factor-α(TNF-α) activation of the IK kinase (IKK). IKK induces phosphorylation of the NFκB inhibitor IκB that results in ubiquitination and degradation IκB. NFκB dimmers (RelA/p50) translocate to the nucleus and activate pro-inflammatory genes such as cyclooxygenase-2 (COX-2).
Anti-inflammatory Actions
Because inflammation plays an important role in the progression of cardiovascular diseases, recent studies have focused on the connection between inflammation and EETs. EETs exhibit anti-inflammatory properties in the vasculature. Kessler et al. [39] demonstrated that pro-inflammatory mediators like cytokines and lipopolysaccharide decrease the formation of EETs and endothelial epoxygenase enzyme expression. Activated nuclear factor-κB (NF-κB) is a critical cell-signaling step for the induction of numerous inflammatory mediators in the caridovascular system. NF-κB activity is essential for the up-regulation of genes encoding vascular cell adhesion molecule (VCAM), inter-cellular adhesion molecule and E-selectin which contribute to the progression of cardiovascular diseases [40]. EETs or CYP2J2 overexpression was found to be anti-inflammatory and decreased tumor necrosis factor (TNF)-α induced endothelial VCAM-1 expression, and EETs prevented leukocyte adhesion to the vascular wall [41]. Other evidence reports that sEH inhibitors have anti-inflammatory effects by decreasing plasma levels of pro-inflammatory cytokines in a mouse model of acute inflammation [42]. Several studies demonstrated that inhibiting NF-κB activation is the primary signaling pathway by which epoxides exert their anti-inflammatory effects (Figure 2B) [39,41,43]. Additionally, endothelial cell shear stress can increase EET production and EETs can subsequently bind and activate the peroxisome proliferator-activated receptor α (PPARα) to provide anti-inflammatory effects on blood vessels [44]. Taken together these experimental findings suggest that EETs or EET agonists could be effective as anti-inflammatory agents.
Migration and Proliferation in Vascular Cells
EETs have migratory and proliferative effects in vascular endothelial and smooth muscle cells. The migration and proliferation of endothelial and vascular smooth muscle cells play an important role in cardiovascular diseases. Previous studies have reported that EETs and CYP2C9 overexpression induce endothelial cell proliferation in murine and human endothelial cells [45-47]. Several signaling pathways have been implicated in the endothelial cell proliferation including the phosphotidyl inositol-3-kinase (PI3K)/Akt pathway, the mitogen-activated protein kinase (MAPK) pathway and the cAMP/protein kinase A (PKA) pathway (Figure 2A). CYP2C9 overexpression in human umbilical vein endothelial cells (HUVECs) activates p38MAP kinase and inactivates c-Jun N-terminal kinase (JNK) resulting in increased expression of cyclin D1 and endothelial cell proliferation [45]. It was further reported that in HUVECs, 11,12-EET-induced proliferation involved the down-regulation of the cyclin D1 inhibitor, P27kip1, and PI3K/Akt-regulated inactivation of a forkhead transcription factor [46]. In addition, a recent study reported that 11,12-EET induces endothelial cell proliferation through activation of sphingosine kinase-1 and the subsequent activation of Akt kinase and epidermal growth factor receptors [47]. On the other hand, EETs have anti-migratory effects in vascular smooth muscle cells. 11,12-EET, 14,15-EET, and 5,6-EET inhibit platelet-derived growth factor induced migration of rat aortic smooth muscle cells [48]. This report also demonstrated that CYP2J overexpression inhibited vascular smooth muscle cell migration by activating cAMP-dependent PKA. Other experimental studies have provided evidence that sEH inhibitors decrease cyclin D expression and inhibit proliferation of human vascular smooth muscle cells [49]. Epoxygenase metabolites have been demonstrated to contribute to vascular endothelial growth factor-induced angiogensis by activating Akt and MAPK endothelial cell signaling pathways [50,51]. Taken together, these results suggest that EETs can significantly effect migration and proliferation of endothelial and vascular smooth muscle cells and that modulation of EETs in cardiovascular diseases has potential therapeutic value.
Anti-platelet Aggregation Actions
Platelet aggregation in cardiovascular diseases contributes to the development of acute thrombotic events and can result in the development of atherosclerosis. Studies have demonstrated that epoxides have antiaggregatory properties in platelets [52-54]. Fitzpatrick et al. [52] showed that 11,12-EET or 14,15-EET inhibits arachidonic acid stimulation of platelet aggregation. 11,12-EET, 8,9-EET and 14,15-EET cause membrane hyperpolarization of human platelets through activation of BKCa channels that subsequently inhibits platelet adhesion to endothelial cells [54]. Vascular thrombosis is regulated in part by the tissue-type plasminogen activator (t-PA) and plasminogen activator inhibitor 1 [55]. Node et al. [56] demonstrated that 11,12-EET increases the expression and activity of t-PA in human endothelial cells through activation of the G-protein, GαS and PKA. Thus EETs decrease platelet aggregation through a membrane hyperpolarization and enhanced expression of endothelial fibrolytic enzymes. Overall these findings suggest that epoxygenase metabolites or EET agonists have the potential to decrease thrombolytic events associated with cardiovascular diseases.
Overview
There are a variety of vascular actions that have been attributed to EETs and these actions can occur through various cell-signaling pathways. Additionally, EET regioisomers have different activities or potencies depending on the organ system or cell type. Vascular actions have been mostly attributed to 11,12-EET and 14,15-EET while 5,6-EET activities have been demonstrated in fewer experimental settings [17,57]. This type of variability in EET regioisomer activities, potencies, and cell-signaling would suggests that EETs act through one or multiple receptors. The development of EET agonist and antagonists are major steps towards identifying EET binding sites and receptors.
EET AGONIST AND ANTAGONIST ACTIONS
Importance of Synthesizing EET Analogs
EETs are rapidly taken up into the cells when applied exogenously and EETs can be metabolized via β-oxidation or sEH, esterified by phospholipids, or conjugated to glutathione or FABP [2,57-59]. The use of EETs is also complicated by difficulties with synthesizing regioisomeric pure EETs, limited solubility, and storage issues. At present, the synthesis of radiolabelled EETs from arachidonic acid is expensive and inefficient because they metabolize into their corresponding diols; the final yield does not exceed 50% and the chemical methods produce all regioisomeric EETs in varying amounts [60,61]. Another complicating issue is that there is a rapid decrease in epoxygenase enzymes in cell culture systems. The loss of the epoxygenase pathway in cell culture has hampered attempts to identify EET receptors. Although there is ample evidence to suggest that EET receptors exist, the identity of these receptors remains elusive. Thus synthetic EET analogs designed to resist metabolism and possess improved solubility will facilitate the identification of the structure-function relationships, cardiovascular cell-signaling mechanisms and have the potential to discover EET receptors. To date, EET analogs have been shown to exert vascular protective effects like vasodilation and anti-inflammation [6,34,43,62]. The following sections will discuss the effects of 11,12-EET, 14,15-EET and 5,6-EET analogs as well as EET antagonists on vascular function and the structural requirements necessary for this function.
Agonistic Activity of 14,15-EET Analogs
14,15-EET analogs have progressed from the first generation of methyl esters and sulfonimide carboxylic acid, to second-generation analogs that eliminated specific olefin bonds. These substitutions were designed to resist β-oxidation and improve solubility. The next generation of EET analogs involved substitution of the epoxide with sulphur or ether moieties that resist metabolism by sEH. Another modification that has been evaluated is the shortening and elongation of carbon chain length. These variations of 14,15-EET analogs have provided significant information concerning the structure activity relationship for 14,15-EET with regards to cardiovascular function.
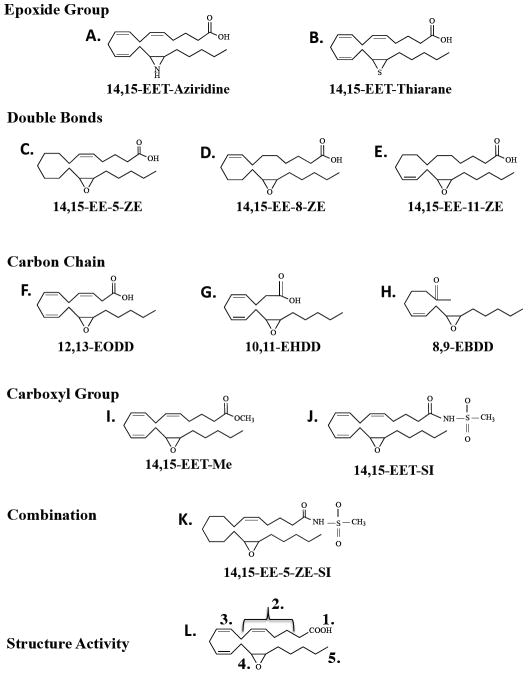
Experimental findings with 14,15-EET analogs have provided significant insight concerning cardiovascular actions. Falck et al. [6] and Gauthier et al. [62] found that the 14,15-EET analogs have a vasodilatory effect in bovine coronary arteries and described the structural requirements in the 14,15-EET induced vasodilation using synthetic analogs. Several successful analogs have been synthesized (Figure 3) with changes in the epoxide group (A-B), the position of double bonds (C-E), carbon chain length (F-H), carboxyl group (I-J) and combinational changes (K) [6,62]. The first generation analogs, 14,15-EET-Me and 14,15-EET-SI, have full agonist activity. Subsequent generations of 14,15-EET analogs also resulted in vasodilation and provided additional insight into the structure activity relationship for 14,15-EET. Moreover, it was found that the 14,15-(cis)-EET conformation and 14(S),15(R)-EET enantiomers with an epoxide group are important for full the agonistic effect. Taken together, these findings indicate that the acidic carboxyl group in carbon 1, the Δ8 double bond, 20-carbon chain length and 14(S),15(R)-(cis)-epoxide are required for full vasodilator activity of 14,15-EET (L) [6,62]. In addition, Yang et al. [63] demonstrated that the three synthetic sulfonamide derivatives of 14,15-EET analogs relaxed bovine coronary arteries and that the vasodilation was inhibited by K+ channel blockade. This and other studies demonstrate that 14,15-EET analogs utilize the same cell-signaling pathways as the endogenous 14,15-EET. The characterization of the structural requirements of 14,15-EET provides a framework from which potential therapeutic analogs for treating cardiovascular diseases could be designed.
Figure 3.
Chemical structures of 14,15-epoxyeicosatrienoic acid (EET) analogs with alterations in the epoxide group (A-B), the position of double bonds (C-E), carbon chain length (F-H), carboxyl group (I-J), and combinational changes (K). A structure activity domain map for 14,15-EET (L) includes 5 regions: 1.) ionic attraction at carbon-1; 2.) lipophilic region; 3.) 8,9-olefin double bond; 4.) 14,15-epoxide; 5.) terminal lipophilic pocket.
14,15-EET analogs have also been used for studying the structure-function relationship for an EET binding site. Snyder et al. [58] evaluated the possibility for an EET receptor in the plasma membrane by measuring cAMP-induced aromatase enzyme activity in vascular smooth muscle cells. 14,15-EET and the stable analog 14,15-EET-SI, inhibited aromatase activity, whereas, the corresponding diol, 14,15-DHET did not effect aromatase activity. In addition, 14,15-EET has been suggested to effect aromatase activity via a cell membrane event and the 14,15-EET-SI inhibition of aromatase activity persisted when the analog was covalently tethered to silica beads that would restrict it from entering the cells [58,64]. This finding indicated that the EETs produce their biological effect on aromatase activity by possibly acting on an EET receptor present in the plasma membrane. In addition, Fang et al. [65] studied the involvement of 14,15-EET and 14,15-DHET on activation of PPARγ in COS-7 cells. 14,15-EET has a less potent effect than 14,15-DHET in respect to activation of PPARγ and the stable and non-hydrolyzed 14,15-EET analog, 14-hexyloxytetradec-5(Z)-enoic acid did not activate PPARγ. This result indicates that the 14,15-DHET produced from 14,15-EET by sEH could function as an endogenous activator for PPARγ in COS-7 cells [65]. Thus 14,15-EET analogs can also be utilized to determine whether or not a biological action is due to 14,15-EET or 14,15-DHET.
Agonistic Activity of 11,12-EET Analogs
Like 14,15-EET, 11,12-EET has potent biological effects that contribute to cardiovascular function. EET anti-inflammatory actions have been relatively selective for the 11,12-EET regioisomer. Likewise, 11,12-EET is the predominant biologically active EET regioisomer when examining epithelial transport in the kidneys [66,67]. Several studies have investigated the structural requirements and cell-signaling mechanisms for 11,12-EET actions using 11,12-EET analogs.
Experimental findings provide initial support to the idea that 11,12-EET analogs could have beneficial actions in cardiovascular diseases [34,43]. Recent studies have determined the key structures of 11,12-EET that are essential for vasodilation. Several 11,12-EET analogs were synthesized with better solubility that resisted auto-oxidation and metabolism by sEH. The 11,12-EET analogs, 11,12-epoxyeicosatrienoic acid sulfonamide (11,12-EET-SI), 11-nonyloxy-undec-8(Z)-enoic acid (11,12-ether-EET-8-ZE), and 11,12-trans-oxidoeicosa-8(Z)-enoic acid (11,12-tetra-EET-8-ZE) were found to fulfill those requirements [34]. All these analogs caused concentration dependent vasodilation in rat mesenteric resistance arteries and renal afferent arterioles and provided information on the structure activity relationship for 11,12-EET [27,34]. A recognition/binding domain map for 11,12-EET mediated vasodilation indicates that there are certain key elements: the ionic attraction of the carboxylate at carbon-1, the Δ8,6-olefin double bond, an epoxide positioned between C(11) and C(12) and a terminal C20 lipophilic pocket [34]. The Δ5,6-olefin region also makes a minor contribution to recognition. 11,12-EET analogs were also used to identify the signal transduction pathways involved in vasodilation. These studies provide evidence that 11,12-EET utilizes cAMP and PP2A dependent pathways to activate BKCa channels in mesenteric and renal arteries [27,34]. As for possible beneficial actions in cardiovascular diseases, 11,12-EET analogs have been used to a limited extent. One of the first findings in a cardiovascular disease was that 11,12-EET-SI ameliorated the enhanced renal microvascular responsiveness to angiotensin in an animal model of hypertension [68]. A more recent in vivo study demonstrated that another 11,12-EET analog lowers the blood pressure in spontaneously hypertensive rats (Figure 4) [69,70]. Another recent study demonstrated that the EET analog (S)-2-(11-(nonyloxy)undec-8(Z)-enamido)succinic acid (NUDSA) ameliorated the metabolic syndrome phenotype in heme-oxygenase 2 gene deficient mice and prevented adiposity-mediated vascular and renal damage [71]. This is a potential breakthrough for the use of EET analogs as therapy for cardiovascular diseases.
Figure 4.
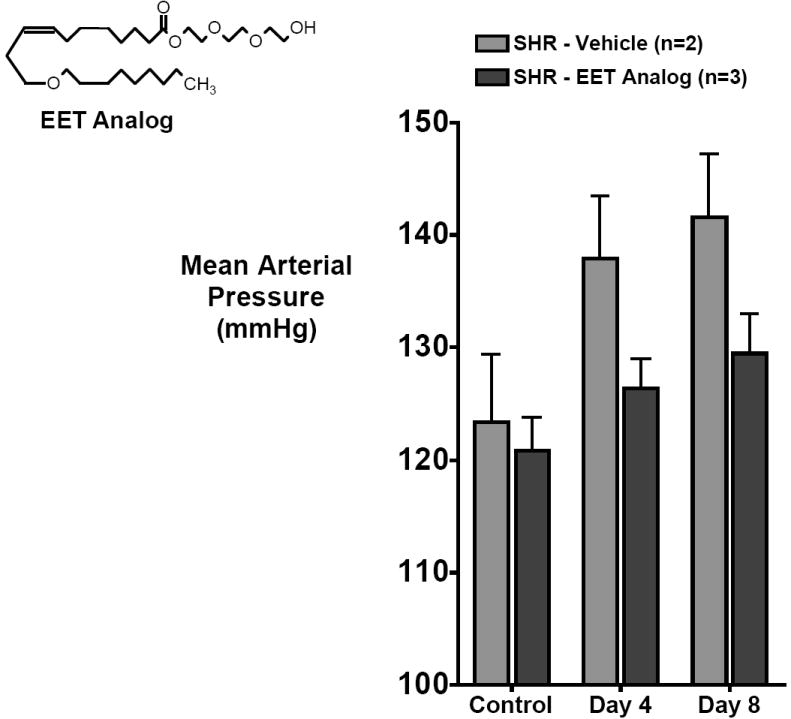
EET analogs have anti-hypertensive actions. Decreased blood pressure in spontaneously hypertensive rats (SHR) treated for one week with an 11-nonyloxy-undec-8(Z)-eonic acid (6 mg/kg/d i.p.).
The 11,12-EET analogs also have vascular anti-inflammatory and renal actions that could be beneficial for cardiovascular diseases. Falck et al. [43] demonstrated that 11,12-EET analogs inhibit vascular smooth muscle cell TNF-α-induced VCAM-1 expression and based on a series of 11,12-EET analogs described the recognition/binding domain map for this anti-inflammatory action. A recent study reported that another analog, 11,12-epoxyeicosa-8(Z)-enoic acid (11,12-EEZE) has a greater potency on the activation of PPARγ than 11,12-EET or 14,15-EET [72]. Other studies demonstrated that 11,12-EET analogs stimulate mitogenesis in renal epithelial cells through activation of the epidermal growth factor signal transduction pathway [73-75]. More recently, 11,12-EET has been demonstrated to inhibit epithelial sodium reabsorption and possibly contribute to blood pressure regulation in response to high dietary sodium [66,67]. Thus these initial promising findings suggest that the therapeutic potential for 11,12-EET analogs needs to be explored further.
Agonistic Activity of 5,6-EET Analogs
Another regioisomer, 5,6-EET that has a short half-life has also been shown to have a vasodilatory effect [76-79]. Previous studies reported that 5,6-EET is metabolized by COX to 5,6-epoxy-prostaglandins and that COX metabolism contributes to 5,6-EET mediated biological actions [25,78]. Yang et al. [79] described the vascular activity of 5,6-EET and the structural requirement for this activity by using analogs. The methyl derivative of 5,6-EET analog, 5,6-EET-Me causes dose-dependent relaxation in bovine coronary arteries [79]. The esterification of 5,6-EET carboxyl group protects the epoxide from hydration by blocking the catalytic effect of the C-1 carboxyl group. The stable analog, 5-(pentadeca-3(Z),6(Z),9(Z)-trienyloxy)pentanoic acid (PTPA) also induces vasorelaxation and this effect is abolished by COX or BKCa inhibition [79]. The absence of an 8,9 or 11,12 double bond decreased the vasorelaxation when compared to PTPA. Inhibition of the BKCa attenuated the vasorelaxation response of all the 5,6-EET analogs; whereas only PTPA induced vasorelaxation was affected by COX inhibition [79]. This provides further evidence that COX metabolism contributes to the vascular actions of 5,6-EET. Future studies with 5,6-EET analogs will be required to determine cell signaling mechanisms and structure function relationships for this epoxide.
EET Antagonists
The development of EET agonists led to the discovery and characterization of EET antagonists. 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE; Figure 2C) and 14,15-EEZE methylsulfonamide (14,15-EEZE-SI; Figure 2K) have very low agonist activity and were determined to antagonize EET-induced relaxation [6,62]. 14,15-EEZE inhibits the relaxation to all regioisomeric EETs whereas 14,15-EEZE-SI inhibits 14,15-EET and 5,6-EET-induced vasorelaxation [32,33]. These studies also demonstrated that prostaglandin independent vasodilation in response to arachidonic acid was inhibited by 14,15-EEZE. Neither 14,15-EEZE nor 14,15-EEZE-SI alters the vasodilator responses to the prostacyclin analog iloprost, the nitric oxide (NO) donor sodium nitroprusside or the K+ channel openers bimakalim and NS1619 [32,33]. These results clearly suggests that these analogs are selective EET antagonist.
These EET antagonists have been utilized as a pharmacological means to identify the biological roles for EETs in vascular tissues. In bovine coronary arteries pretreated with nitric oxide synthase (NOS) and COX inhibitors, both bradykinin and methacholine induce dose dependent relaxation in small coronary arteries. 14,15-EEZE inhibited the NOS and COX independent vasodilation and bradykinin-induced vascular smooth muscle cell hyperpolarization [32]. Gauthier et al. [33] also reported that 14,15-EEZE-SI could inhibit the vascular relaxation induced by bradykinin and methacholine in bovine coronary arteries but this effect was less than 14,15-EEZE. Bradykinin and acetylcholine induced dilation of human mammary arteries in the presence of COX and NOS inhibitors were eliminated by 14,15-EEZE [80]. These findings provided strong evidence to support a role for EETs in mediating the NO and COX independent vasodilation to bradykinin, methacholine and acetylcholine via vascular smooth muscle cell hyperpolarization. In addition, the contribution of EETs to the adenosine 5’-N-ethylcarboxamide, an adenosine analog relaxation in mice aorta was assessed. 14,15-EEZE was utilized and it was determined that EETs contributed to the relaxation mediated by adenosine analogs that activate the A2A receptors [81]. Cardiac protective EET actions in ischemic reperfusion injury can be eliminated by 14,15-EEZE [82]. Interestingly, 14,15-EEZE also reduces the erectile response in rats indicating that EETs along with NO are required for normal erectile function [83]. These findings indicate that the EET antagonists are important pharmacological tools to identify the biological actions of EETs.
CONCLUSIONS
There is strong evidence that EETs importantly contribute to vascular function and that this contribution is dysfunctional in cardiovascular diseases. However, difficulty in investigating EETs and manipulating these eicosanoids has hampered progress towards evaluating EETs possible therapeutic potential. Synthesis and development of stable EET analogs have enhanced our understanding of EET actions and their contribution to vascular function. EET analogs have also aided in determining the cell signaling pathways activated by EETs and how EET cell signaling mechanisms are altered in cardiovascular disease states. Interestingly, initial studies have demonstrated that EET analogs possess cardiovascular protective effects. EET antagonists have also aided in our understanding of the vascular function of EETs. The impact of these EET agonists and antagonists on epoxygenase expression and activity remains unknown and should be explored in future experimental studies.
Evaluation of EET analogs and antagonists have also contributed significantly to our understanding of the structural requirements for EETs and have given hints as to the identity of possible EET receptors. Although many investigators have attempted to identify EET receptors their identity remains elusive. Hopefully, EET analogs and antagonists and those in development will speed the identity of the EET receptors. A recent study has identified a high affinity radioligand that was used to characterize an EET antagonist-binding site [84]. Moreover, a newer generation of 14,15-EET analogs has recently been described that possess vascular relaxation and sEH inhibition [85]. Overall, the future development of EET analogs and antagonist for the treatment of cardiovascular disease remains upbeat.
Acknowledgments
This work was supported by NIH grants HL59699 and DK38226 and Advancing a Healthier Wisconsin.
ABBREVIATIONS
- BKca
large-conductance calcium-activated potassium channel
- COX
cyclooxygenase
- CYP
cytochrome P450
- DHET
dihydroxyeicosatrienoic acid
- EET
epoxyeicosatrienoic acid
- FABP
fatty acid binding protein
- HUVEC
human umbilical vein endothelial cell
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen activated protein kinase
- NF-κB
nuclear factor-κB
- NOS
nitric oxide synthase
- PKA
protein kinase A
- PP2A
phosphoprotein phosphatase 2A
- PPAR
peroxisome proliferator-activated receptor
- sEH
soluble epoxide hydrolase
- TNF-α
tumor necrosis factor α
- t-PA
tissue-type plasminogen activator
- VCAM
vascular cell adhesion molecule
References
- 1.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H491–9. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–62. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 3.Daikh BE, Lasker JM, Raucy JL, Koop DR. Regio- and stereoselective epoxidation of arachidonic acid by human cytochromes P450 2C8 and 2C9. J Pharmacol Exp Ther. 1994;271:1427–33. [PubMed] [Google Scholar]
- 4.Zeldin DC, Plitman JD, Kobayashi J, Miller RF, Snapper JR, Falck JR, Szarek JL, Philpot RM, Capdevila JH. The rabbit pulmonary cytochrome P450 arachidonic acid metabolic pathway: characterization and significance. J Clin Invest. 1995;95:2150–60. doi: 10.1172/JCI117904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K(+)-channel activity. Am J Physiol. 1996;270:F822–32. doi: 10.1152/ajprenal.1996.270.5.F822. [DOI] [PubMed] [Google Scholar]
- 6.Falck JR, Krishna UM, Reddy YK, Kumar PS, Reddy KM, Hittner SB, Deeter C, Sharma KK, Gauthier KM, Campbell WB. Comparison of vasodilatory properties of 14,15-EET analogs: structural requirements for dilation. Am J Physiol Heart Circ Physiol. 2003;284:H337–49. doi: 10.1152/ajpheart.00831.2001. [DOI] [PubMed] [Google Scholar]
- 7.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–23. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 8.Karara A, Dishman E, Falck JR, Capdevila JH. Endogenous epoxyeicosatrienoyl-phospholipids. A novel class of cellular glycerolipids containing epoxidized arachidonate moieties. J Biol Chem. 1991;266:7561–9. [PubMed] [Google Scholar]
- 9.Chen J, Capdevila JH, Zeldin DC, Rosenberg RL. Inhibition of cardiac L-type calcium channels by epoxyeicosatrienoic acids. Mol Pharmacol. 1999;55:288–95. doi: 10.1124/mol.55.2.288. [DOI] [PubMed] [Google Scholar]
- 10.Richieri GV, Ogata RT, Zimmerman AW, Veerkamp JH, Kleinfeld AM. Fatty acid binding proteins from different tissues show distinct patterns of fatty acid interactions. Biochemistry. 2000;39:7197–204. doi: 10.1021/bi000314z. [DOI] [PubMed] [Google Scholar]
- 11.Luxon BA, Milliano MT. Cytoplasmic transport of fatty acids in rat enterocytes: role of binding to fatty acid-binding protein. Am J Physiol. 1999;277:G361–6. doi: 10.1152/ajpgi.1999.277.2.G361. [DOI] [PubMed] [Google Scholar]
- 12.Widstrom RL, Norris AW, Van Der Veer J, Spector AA. Fatty acid-binding proteins inhibit hydration of epoxyeicosatrienoic acids by soluble epoxide hydrolase. Biochemistry. 2003;42:11762–7. doi: 10.1021/bi034971d. [DOI] [PubMed] [Google Scholar]
- 13.Fang X, Kaduce TL, Weintraub NL, VanRollins M, Spector AA. Functional implications of a newly characterized pathway of 11,12-epoxyeicosatrienoic acid metabolism in arterial smooth muscle. Circ Res. 1996;79:784–93. doi: 10.1161/01.res.79.4.784. [DOI] [PubMed] [Google Scholar]
- 14.Fang X, Kaduce TL, Weintraub NL, Harmon S, Teesch LM, Morisseau C, Thompson DA, Hammock BD, Spector AA. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J Biol Chem. 2001;276:14867–74. doi: 10.1074/jbc.M011761200. [DOI] [PubMed] [Google Scholar]
- 15.Newman JW, Morisseau C, Hammock BD. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res. 2005;44:1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Yu Z, Davis BB, Morisseau C, Hammock BD, Olson JL, Kroetz DL, Weiss RH. Vascular localization of soluble epoxide hydrolase in the human kidney. Am J Physiol Renal Physiol. 2004;286:F720–6. doi: 10.1152/ajprenal.00165.2003. [DOI] [PubMed] [Google Scholar]
- 17.Imig JD. Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors. Cardiovasc Drug Rev. 2006;24:169–88. doi: 10.1111/j.1527-3466.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 18.Zeldin DC, DuBois RN, Falck JR, Capdevila JH. Molecular cloning, expression and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch Biochem Biophys. 1995;322:76–86. doi: 10.1006/abbi.1995.1438. [DOI] [PubMed] [Google Scholar]
- 19.Zeldin DC, Moomaw CR, Jesse N, Tomer KB, Beetham J, Hammock BD, Wu S. Biochemical characterization of the human liver cytochrome P450 arachidonic acid epoxygenase pathway. Arch Biochem Biophys. 1996;330:87–96. doi: 10.1006/abbi.1996.0229. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39:2073–8. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeldin DC, Kobayashi J, Falck JR, Winder BS, Hammock BD, Snapper JR, Capdevila JH. Regio- and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase. J Biol Chem. 1993;268:6402–7. [PubMed] [Google Scholar]
- 22.Fang X, Weintraub NL, McCaw RB, Hu S, Harmon SD, Rice JB, Hammock BD, Spector AA. Effect of soluble epoxide hydrolase inhibition on epoxyeicosatrienoic acid metabolism in human blood vessels. Am J Physiol Heart Circ Physiol. 2004;287:H2412–20. doi: 10.1152/ajpheart.00527.2004. [DOI] [PubMed] [Google Scholar]
- 23.Oltman CL, Weintraub NL, VanRollins M, Dellsperger KC. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res. 1998;83:932–9. doi: 10.1161/01.res.83.9.932. [DOI] [PubMed] [Google Scholar]
- 24.Campbell WB, Deeter C, Gauthier KM, Ingraham RH, Falck JR, Li PL. 14,15-Dihydroxyeicosatrienoic acid relaxes bovine coronary arteries by activation of K(Ca) channels. Am J Physiol Heart Circ Physiol. 2002;282:H1656–64. doi: 10.1152/ajpheart.00597.2001. [DOI] [PubMed] [Google Scholar]
- 25.Imig JD, Navar LG, Roman RJ, Reddy KK, Falck JR. Actions of epoxygenase metabolites on the preglomerular vasculature. J Am Soc Nephrol. 1996;7:2364–70. doi: 10.1681/ASN.V7112364. [DOI] [PubMed] [Google Scholar]
- 26.Sacerdoti D, Bolognesi M, Di Pascoli M, Gatta A, McGiff JC, Schwartzman ML, Abraham NG. Rat mesenteric arterial dilator response to 11,12-epoxyeicosatrienoic acid is mediated by activating heme oxygenase. Am J Physiol Heart Circ Physiol. 2006;291:H1999–2002. doi: 10.1152/ajpheart.00082.2006. [DOI] [PubMed] [Google Scholar]
- 27.Dimitropoulou C, West L, Field MB, White RE, Reddy LM, Falck JR, Imig JD. Protein phosphatase 2A and Ca2+-activated K+ channels contribute to 11,12-epoxyeicosatrienoic acid analog mediated mesenteric arterial relaxation. Prostaglandins Other Lipid Mediat. 2007;83:50–61. doi: 10.1016/j.prostaglandins.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, Weldon SM, da Costa Goncalves Ach, Huang Y, Luft FC, Gollasch M. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler Thromb Vasc Biol. 2009;29:54–60. doi: 10.1161/ATVBAHA.108.171298. [DOI] [PubMed] [Google Scholar]
- 29.Cheng MK, Doumad AB, Jiang H, Falck JR, McGiff JC, Carroll MA. Epoxyeicosatrienoic acids mediate adenosine-induced vasodilation in rat preglomerular microvessels (PGMV) via A2A receptors. Br J Pharmacol. 2004;141:441–8. doi: 10.1038/sj.bjp.0705640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellis EF, Police RJ, Yancey L, McKinney JS, Amruthesh SC. Dilation of cerebral arterioles by cytochrome P-450 metabolites of arachidonic acid. Am J Physiol. 1990;259:H1171–7. doi: 10.1152/ajpheart.1990.259.4.H1171. [DOI] [PubMed] [Google Scholar]
- 31.Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol. 1992;263:H519–25. doi: 10.1152/ajpheart.1992.263.2.H519. [DOI] [PubMed] [Google Scholar]
- 32.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res. 2002;90:1028–36. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- 33.Gauthier KM, Jagadeesh SG, Falck JR, Campbell WB. 14,15-epoxyeicosa-5(Z)-enoic-mSI: a 14,15- and 5,6-EET antagonist in bovine coronary arteries. Hypertension. 2003;42:555–61. doi: 10.1161/01.HYP.0000091265.94045.C7. [DOI] [PubMed] [Google Scholar]
- 34.Imig JD, Dimitropoulou C, Reddy DS, White RE, Falck JR. Afferent arteriolar dilation to 11, 12-EET analogs involves PP2A activity and Ca2+-activated K+ Channels. Microcirculation. 2008;15:137–50. doi: 10.1080/10739680701456960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imig JD, Inscho EW, Deichmann PC, Reddy KM, Falck JR. Afferent arteriolar vasodilation to the sulfonimide analog of 11, 12-epoxyeicosatrienoic acid involves protein kinase A. Hypertension. 1999;33:408–13. doi: 10.1161/01.hyp.33.1.408. [DOI] [PubMed] [Google Scholar]
- 36.Carroll MA, Doumad AB, Li J, Cheng MK, Falck JR, McGiff JC. Adenosine2A receptor vasodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am J Physiol Renal Physiol. 2006;291:F155–61. doi: 10.1152/ajprenal.00231.2005. [DOI] [PubMed] [Google Scholar]
- 37.Weston AH, Félétou M, Vanhoutte PM, Falck JR, Campbell WB, Edwards G. Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: involvement of potassium channel activation and epoxyeicosatrienoic acids. Br J Pharmacol. 2005;145:775–84. doi: 10.1038/sj.bjp.0706256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97:908–15. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 39.Kessler P, Popp R, Busse R, Schini-Kerth VB. Proinflammatory mediators chronically downregulate the formation of the endothelium-derived hyperpolarizing factor in arteries via a nitric oxide/cyclic GMP-dependent mechanism. Circulation. 1999;99:1878–84. doi: 10.1161/01.cir.99.14.1878. [DOI] [PubMed] [Google Scholar]
- 40.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 41.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:276–9. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci U S A. 2005;102:9772–7. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falck JR, Reddy LM, Reddy YK, Bondlela M, Krishna UM, Ji Y, Sun J, Liao JK. 11,12-epoxyeicosatrienoic acid (11,12-EET): structural determinants for inhibition of TNF-alpha-induced VCAM-1 expression. Bioorg Med Chem Lett. 2003;13:4011–4. doi: 10.1016/j.bmcl.2003.08.060. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, Spector AA, Gill S, Morisseau C, Hammock BD, Shyy JY. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci U S A. 2005;102:16747–52. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potente M, Michaelis UR, Fisslthaler B, Busse R, Fleming I. Cytochrome P450 2C9-induced endothelial cell proliferation involves induction of mitogen-activated protein (MAP) kinase phosphatase-1, inhibition of the c-Jun N-terminal kinase, and up-regulation of cyclin D1. J Biol Chem. 2002;277:15671–6. doi: 10.1074/jbc.M110806200. [DOI] [PubMed] [Google Scholar]
- 46.Potente M, Fisslthaler B, Busse R, Fleming I. 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J Biol Chem. 2003;278:29619–25. doi: 10.1074/jbc.M305385200. [DOI] [PubMed] [Google Scholar]
- 47.Yan G, Chen S, You B, Sun J. Activation of sphingosine kinase-1 mediates induction of endothelial cell proliferation and angiogenesis by epoxyeicosatrienoic acids. Cardiovasc Res. 2008;78:308–14. doi: 10.1093/cvr/cvn006. [DOI] [PubMed] [Google Scholar]
- 48.Sun J, Sui X, Bradbury JA, Zeldin DC, Conte MS, Liao JK. Inhibition of vascular smooth muscle cell migration by cytochrome p450 epoxygenase-derived eicosanoids. Circ Res. 2002;90:1020–7. doi: 10.1161/01.res.0000017727.35930.33. [DOI] [PubMed] [Google Scholar]
- 49.Davis BB, Thompson DA, Howard LL, Morisseau C, Hammock BD, Weiss RH. Inhibitors of soluble epoxide hydrolase attenuate vascular smooth muscle cell proliferation. Proc Natl Acad Sci U S A. 2002;99:2222–7. doi: 10.1073/pnas.261710799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang S, Wei S, Pozzi A, Capdevila JH. The arachidonic acid epoxygenase is a component of the signaling mechanisms responsible for VEGF-stimulated angiogenesis. Arch Biochem Biophys. 2009 doi: 10.1016/j.abb.2009.05.006. (in press). PMID 194464254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pozzi A, Macias-Perez I, Abair T, Wei S, Su Y, Zent R, Falck JR, Capdevila JH. Characterization of 5,6- and 8,9-epoxyeicosatrienoic acids (5,6- and 8,9-EET) as potent in vivo angiogenic lipids. J Biol Chem. 2005;280:27138–46. doi: 10.1074/jbc.M501730200. [DOI] [PubMed] [Google Scholar]
- 52.Fitzpatrick FA, Ennis MD, Baze ME, Wynalda MA, McGee JE, Liggett WF. Inhibition of cyclooxygenase activity and platelet aggregation by epoxyeicosatrienoic acids. Influence of stereochemistry. J Biol Chem. 1986;261:15334–8. [PubMed] [Google Scholar]
- 53.Heizer ML, McKinney JS, Ellis EF. 14,15-Epoxyeicosatrienoic acid inhibits platelet aggregation in mouse cerebral arterioles. Stroke. 1991;22:1389–93. doi: 10.1161/01.str.22.11.1389. [DOI] [PubMed] [Google Scholar]
- 54.Krötz F, Riexinger T, Buerkle MA, Nithipatikom K, Gloe T, Sohn HY, Campbell WB, Pohl U. Membrane-potential-dependent inhibition of platelet adhesion to endothelial cells by epoxyeicosatrienoic acids. Arterioscler Thromb Vasc Biol. 2004;24:595–600. doi: 10.1161/01.ATV.0000116219.09040.8c. [DOI] [PubMed] [Google Scholar]
- 55.Collen D. On the regulation and control of fibrinolysis. Edward Kowalski Memorial Lecture. Thromb Haemost. 1980;43:77–89. [PubMed] [Google Scholar]
- 56.Node K, Ruan XL, Dai J, Yang SX, Graham L, Zeldin DC, Liao JK. Activation of Galpha s mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. J Biol Chem. 2001;276:15983–9. doi: 10.1074/jbc.M100439200. [DOI] [PubMed] [Google Scholar]
- 57.Imig JD. epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2005;289:F496–503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 58.Snyder GD, Krishna UM, Falck JR, Spector AA. Evidence for a membrane site of action for 14,15-EET on expression of aromatase in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2002;283:H1936–42. doi: 10.1152/ajpheart.00321.2002. [DOI] [PubMed] [Google Scholar]
- 59.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50:S52–56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corey EJ, Niwa H, Falck JR. Selective epoxidation of Eicosa-cis-5,8,11,14-Tetraenoic (Arachidonic) acid and Eicosa-cis-8,1,14-Trienoic acid. J Am Chem Soc. 1979;101:1586–7. [Google Scholar]
- 61.Rosolowsky M, Campbell WB. Role of PGI2 and epoxyeicosatrienoic acids in relaxation of bovine coronary arteries to arachidonic acid. Am J Physiol. 1993;264:H327–35. doi: 10.1152/ajpheart.1993.264.2.H327. [DOI] [PubMed] [Google Scholar]
- 62.Gauthier KM, Falck JR, Reddy LM, Campbell WB. 14,15-EET analogs: characterization of structural requirements for agonist and antagonist activity in bovine coronary arteries. Pharmacol Res. 2004;49:515–24. doi: 10.1016/j.phrs.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 63.Yang W, Holmes BB, Gopal VR, Kishore RV, Sangras B, Yi XY, Falck JR, Campbell WB. Characterization of 14,15-epoxyeicosatrienoyl-sulfonamides as 14,15-epoxyeicosatrienoic acid agonists: use for studies of metabolism and ligand binding. J Pharmacol Exp Ther. 2007;321:1023–31. doi: 10.1124/jpet.107.119651. [DOI] [PubMed] [Google Scholar]
- 64.Sacerdoti D, Bolognesi M, Di Pascoli M, Gatta A, McGiff JC, Schwartzman ML, JR, Spector AA. Evidence for a membrane site of action for 14,15-EET on expression of aromatase in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2002;283:H1936–42. doi: 10.1152/ajpheart.00321.2002. [DOI] [PubMed] [Google Scholar]
- 65.Fang X, Hu S, Xu B, Snyder GD, Harmon S, Yao J, Liu Y, Sangras B, Falck JR, Weintraub NL, Spector AA. 14,15-Dihydroxyeicosatrienoic acid activates peroxisome proliferator-activated receptor-alpha. Am J Physiol Heart Circ Physiol. 2006;290:H55–63. doi: 10.1152/ajpheart.00427.2005. [DOI] [PubMed] [Google Scholar]
- 66.Sun P, Lin DH, Wang T, Babilonia E, Wang Z, Jin Y, Kemp R, Nasjletti A, Wang WH. Low Na intake suppresses expression of CYP2C23 and arachidonic acid-induced inhibition of ENaC. Am J Physiol Renal Physiol. 2006;291:F1192–1200. doi: 10.1152/ajprenal.00112.2006. [DOI] [PubMed] [Google Scholar]
- 67.Nakagawa K, Holla VR, Wei Y, Wang WH, Gatica A, Wei S, Mei S, Miller CM, Cha DR, Price E, Jr, Zent R, Pozzi A, Breyer MD, Guan Y, Falck JR, Waterman MR, Capdevila JH. Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest. 2006;116:1696–1702. doi: 10.1172/JCI27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Imig JD, Zhao X, Falck JR, Wei S, Capdevila JH. Enhanced renal microvascular reactivity to angiotensin II in hypertension is ameliorated by the sulfonimide analog of 11,12-epoxyeicosatrienoic acid. J Hypertens. 2001;19:983–92. doi: 10.1097/00004872-200105000-00020. [DOI] [PubMed] [Google Scholar]
- 69.Imig JD, Falck JR. Compositions and methods for the treatment of rnela and cardiovascular disease. 7,550,617. US Patent. 2009
- 70.Elmarakby AA, Falck JR, Imig JD. Ether epoxyeicosatrienoic acid (EET) analogs lower blood pressure when administered in-vivo to the spontaneouly hypertensive rats. FASEB J. 2008;22:479.44. (abstract) http://www.fasebj.org/cgi/content/meeting_abstract/22/1_MeetingAbstracts/479.44.
- 71.Sodhi K, Inoue K, Gotlinger KH, Canestraro M, Vanella L, Kim DH, Manthati VL, Koduru SR, Falck JR, Schwarzman ML, Abraham NG. Epoxyeicosatrienoic acid agonist rescues metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther. 2009;331:906–16. doi: 10.1124/jpet.109.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ng VY, Huang Y, Reddy LM, Falck JR, Lin ET, Kroetz DL. Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor alpha. Drug Metab Dispos. 2007;35:1126–34. doi: 10.1124/dmd.106.013839. [DOI] [PubMed] [Google Scholar]
- 73.Burns KD, Capdevila J, Wei S, Breyer MD, Homma T, Harris RC. Role of cytochrome P-450 epoxygenase metabolites in EGF signaling in renal proximal tubule. Am J Physiol. 1995;269:C831–40. doi: 10.1152/ajpcell.1995.269.4.C831. [DOI] [PubMed] [Google Scholar]
- 74.Chen JK, Falck JR, Reddy KM, Capdevila J, Harris RC. Epoxyeicosatrienoic acids and their sulfonimide derivatives stimulate tyrosine phosphorylation and induce mitogenesis in renal epithelial cells. J Biol Chem. 1998;273:29254–61. doi: 10.1074/jbc.273.44.29254. [DOI] [PubMed] [Google Scholar]
- 75.Chen JK, Capdevila J, Harris RC. Overexpression of C-terminal Src kinase blocks 14, 15-epoxyeicosatrienoic acid-induced tyrosine phosphorylation and mitogenesis. J Biol Chem. 2000;275:13789–92. doi: 10.1074/jbc.275.18.13789. [DOI] [PubMed] [Google Scholar]
- 76.Carroll MA, Garcia MP, Falck JR, McGiff JC. 5,6-epoxyeicosatrienoic acid, a novel arachidonate metabolite. Mechanism of vasoactivity in the rat. Circ Res. 1990;67:1082–8. doi: 10.1161/01.res.67.5.1082. [DOI] [PubMed] [Google Scholar]
- 77.Fulton D, Falck JR, McGiff JC, Carroll MA, Quilley J. A method for the determination of 5,6-EET using the lactone as an intermediate in the formation of the diol. J Lipid Res. 1998;39:1713–21. [PubMed] [Google Scholar]
- 78.Carroll MA, Balazy M, Margiotta P, Falck JR, McGiff JC. Renal vasodilator activity of 5,6-epoxyeicosatrienoic acid depends upon conversion by cyclooxygenase and release of prostaglandins. J Biol Chem. 1993;268:12260–6. [PubMed] [Google Scholar]
- 79.Yang W, Gauthier KM, Reddy LM, Sangras B, Sharma KK, Nithipatikom K, Falck JR, Campbell WB. Stable 5,6-epoxyeicosatrienoic acid analog relaxes coronary arteries through potassium channel activation. Hypertension. 2005;45:681–6. doi: 10.1161/01.HYP.0000153790.12735.f9. [DOI] [PubMed] [Google Scholar]
- 80.Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim D, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation. 2003;107:769–76. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- 81.Nayeem MA, Poloyac SM, Falck JR, Zeldin DC, Ledent C, Ponnoth DS, Ansari HR, Mustafa SJ. Role of CYP epoxygenases in A2A AR-mediated relaxation using A2A AR-null and wild-type mice. Am J Physiol Heart Circ Physiol. 2008;295:H2068–78. doi: 10.1152/ajpheart.01333.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gross GJ, Gauthier KM, Moore J, Campbell WB, Falck JR, Nithipatikom K. Evidence for role of epoxyeicosatrienoic acids in mediating ischemic preconditioning and postconditioning in dog. Am J Physiol Heart Circ Physiol. 2009;297:H47–52. doi: 10.1152/ajpheart.01084.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jin L, Foss CE, Zhao X, Mills TM, Wang MH, McCluskey LP, Yaddanapud GS, Falck JR, Imig JD, Webb RC. Cytochrome P450 epoxygenases provide a novel mechanism for penile erection. FASEB J. 2006;20:539–41. doi: 10.1096/fj.05-4341fje. [DOI] [PubMed] [Google Scholar]
- 84.Chen Y, Falck JR, Tuniki VR, Campbell WB. 20-125Ido-14,15-epoxyeicosa-5(Z)-enoic acid: a high affinity radioligand used to charaterize the epoxyeicosatrienoic acid antagonist binding site. J Pharmacol Exp Ther. 2009;331:1137–45. doi: 10.1124/jpet.109.157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Falck JR, Kodela R, Manne R, Atcha R, Puli N, Dubasi N, Manthati VL, Capdevila JH, Yi XY, Goldman DH, Morisseau C, Hammock BD, Campbell WB. 14,15-Epoxyeicosa-5,8,11-trienoic acid (14,15-EET) surrogates containing epoxide bioisosteres: influence upon vascular relaxation and soluble epoxide hydrolase inhibition. J Med Chem. 2009;52:5069–75. doi: 10.1021/jm900634w. [DOI] [PMC free article] [PubMed] [Google Scholar]