Abstract
Objective
The contribution of cytochrome P-450 (CYP) metabolites of arachidonic acid: epoxyeicosatrienoic acids (EETs) and 20-hydroxyeicosatetraenoic acid (20-HETE) in the regulation of nonclipped kidney function in two-kidney, one-clip (2K1C) Goldblatt hypertensive rats during the phases of initial and stable hypertension (7 or 27 days after clipping, respectively) were investigated.
Methods
Male Hannover-Sprague Dawley rats had the right renal artery clipped or had a sham-operation. Urinary excretion of EETs, their inactive metabolites (DHETEs), and 20-HETE were measured. Intrarenal CYP protein expression and the activities of epoxygenase, ω-hydroxylase and soluble epoxide hydrolase (sEH) were also determined.
The responses of renal hemodynamics and electrolyte excretion of the non-clipped kidney to left renal artery infusions of inhibitors of EETs or 20-HETE formation (MS-PPOH and DDMS, respectively) were measured.
Results
In 2K1C rats the urinary EETs excretion was lower and 20-HETE excretion was higher than in sham-operated animals. Intrarenal inhibition of EETs significantly decreased renal hemodynamics and sodium excretion in sham-operated but not in 2K1C rats. Intrarenal inhibition of 20-HETE decreased sodium excretion in sham-operated rats but elicited increases in renal hemodynamics and sodium excretion in 2K1C rats.
Conclusions
The results indicate that the nonclipped kidney of Goldblatt 2K1C rats in the phase of sustained hypertension exhibits decreased intrarenal EETs and elevated 20-HETE levels as compared with the kidney of sham-operated animals. This suggests that altered production and action of CYP-derived metabolites in this phase contributes to the mechanism of Goldblatt 2K1C hypertension.
Keywords: two-kidney one-clip Goldblatt hypertension, cytochrome P-450 metabolites, epoxyeicosatrienoic acids, 20-hydroxyeicosatetraenoic acid, renin-angiotensin system, renal function
Introduction
The two-kidney, one-clip (2K1C) Goldblatt model of hypertension resembles human renovascular hypertension in many respects. A prominent common feature is the enhanced activity of the renin-angiotensin system (RAS) and its critical role in the mechanism of the increase in blood pressure [1–3]. In 2K1C rats plasma angiotensin II (ANG II) is elevated during the initial phase whereas in the phase of sustained hypertension the values return to levels observed in normotensive controls. This indicates that enhanced activity of the systemic RAS is not the causative factor responsible for the persistence of hypertension in this model [1–7]. According to the concept originally proposed by Guyton and associates [8] and supported and further developed by many other groups, the kidney’s pressure-natriuresis mechanism plays a predominant role in the long-term control of blood pressure (BP). In theory, in the 2K1C Goldblatt model the presumably intact nonclipped kidney should respond to pressure elevation by increasing sodium excretion and normal BP should be restored. However, recent studies have shown that the nonclipped kidney of 2K1C hypertensive rats exhibits a compromised ability to maintain normal sodium excretion at normal BP and impaired natriuretic responsiveness to BP elevations. Most probably this contributes to the maintenance of hypertension in this model [1,2], however, the mechanism(s) underlying impaired pressure-natriuresis relationship in the nonclipped kidney of 2K1C Goldblatt hypertensive rats during the phase of sustained hypertension are still poorly understood.
It is now well recognized that cytochrome P-450 (CYP) metabolites of arachidonic acid, such as 20-hydroxyeicosatetraenoic acid (20-HETE) and epoxyeicosatrienoic acids (EETs), play an important role in the regulation of renal tubular ion transport and renal and systemic vascular tone [9–11]. Moreover, a close functional interplay has been shown between ANG II and 20-HETE. An increased generation and/or action of 20-HETE augments the vasoconstrictor responses to ANG II [12,13] whereas chronic inhibition of 20-HETE formation attenuates the course of ANG II-dependent forms of hypertension [12,14]. On the other hand, EETs have been shown to be potent vasodilators involved in the action of the endothelium-derived hyperpolarizing factor (EDHF), and in the kidney they inhibit tubular reabsorption of sodium and water in the proximal tubule and collecting duct. Both these actions could contribute to potential antihypertensive properties of EETs [15–19]. In addition, it has been recently shown that intrarenal deficiency of EETs contributes to impaired renal sodium excretion and the development of hypertension in rats transgenic for the mouse Ren-2 renin gene (TGR) and in ANG II-infused rats [15,20,21]. In view of these findings, we hypothesized that in 2K1C Goldblatt model altered formation and/or activity of 20-HETE and EETs may at least partially account for the impaired ability of the nonclipped kidney to maintain normal rates of sodium excretion at normotensive BP level and also to contribute to the maintenance of high BP during the phase of sustained hypertension.
To test this hypothesis, we first assessed the intrarenal availability of biologically active CYP-derived eicosanoids, such as 20-HETE, EETs and, in addition, dihydroxyeicosatrienoic acids (DHETEs), the biologically inactive metabolites of EETs, in the nonclipped kidney of 2K1C rats during the initial (7 days after clip placement) and sustained (27 days after clip placement) phases of hypertension. The results were compared with those in age-matched normotensive sham-operated rats. In addition, the urinary excretion of these eicosanoids was determined in 2K1C hypertensive and sham-operated normotensive rats.
Furthermore, to gain a more detailed insight into the role of intrarenal CYP-derived metabolites in the regulation of the function of the nonclipped kidney of 2K1C and sham-operated rats [22,23], we investigated the protein expression of the CYP4A enzyme and the activity of ω-hydroxylases in the renal cortex, which account for most of the renal formation of 20-HETE, as well as the protein expression of the CYP2C3 enzyme and the renal cortical activity of epoxygenase, which is the enzyme predominantly responsible for EETs formation in the kidney. In addition, the protein expression of the soluble epoxide hydrolase (sEH) and its activity, which is responsible for the generation of DHETEs from EETs, were assessed.
Finally, the responses were determined of the renal hemodynamics and sodium excretion to selective intrarenal inhibition of EETs and 20-HETE formation in the nonclipped kidney of 2K1C rats during the initial (7 days after clip placement) and sustained (27 days after clip placement) phases of hypertension, as well as in age-matched normotensive sham-operated rats.
Methods
The studies were performed in accordance with guidelines and practices established by the Animal Care and Use Committee of the Institute for Clinical and Experimental Medicine. Hannover-Sprague Dawley (HanSD) rats used in the present study were bred at the Department for Experimental Medicine.
General surgical and preparatory procedures: preparation of 2K1C Goldblatt hypertensive rats
Male HanSD rats weighing 170 to 210 g were anesthetized with thiopental sodium (60 mg/kg i.p.). They were placed on a thermoregulated table to maintain body temperature at 37–37.5°C. A tracheostomy was performed to maintain a patent airway and the exterior end of the tracheal cannula was placed inside a small plastic chamber into which a humidified 95% O2/5% CO2 mixture was continuously passed. The right jugular vein was catheterized with a PE-50 tubing for infusion of solutions. For renal function studies (Series 4, see below), the right femoral artery was cannulated to allow continuous monitoring of arterial BP and blood sampling. Mean arterial pressure (MAP) was monitored with a pressure transducer (model MLT 1050) and recorded on the computer using a computerized data-acquisition system (PowerLab/4SP, AD Instruments, UK). The left kidney was exposed via a flank incision, isolated from the surrounding tissue, and placed in a lucite cup. A tapered PE-10 catheter was inserted into the left renal artery via the left femoral artery for selective intrarenal arterial (i.r.) administration of the vehicle or drugs. This catheter was kept patent by a continuous infusion of heparinized isotonic saline at a rate of 4 µl/min throughout the experiment. A PE-10 catheter was also placed in the left ureter. During surgery, an isotonic saline solution containing bovine serum albumin (6%) (Sigma Chemical Co., Prague, Czech Republic) was infused at a rate of 20 µl/min.
For kidney clipping, the right renal artery was isolated through a flank incision and, as described previously [2], a silver clip (0.25 mm in internal diameter) was placed on the renal artery. Sham-operated rats that underwent the same surgical procedure except for placement of the renal artery clip served as controls.
Experimental protocols
Series 1: Assessment of urinary excretion of 20-HETE, EETs, DHETEs and plasma and kidney ANG II levels
Experiments were performed to determine the excretion by the nonclipped (left) kidney of 20-HETE, EETs and DHETEs, and ANG II levels in plasma and nonclipped kidney tissue, in 2K1C Goldblatt hypertensive and sham-operated normotensive rats at days 7 and 27 after clip placement or sham-operation (n = 9 in each group).
After a 50-min equilibration period, a 60-min urine collection was performed. Urine samples were stored at −80° C. The 20-HETE, EETs and DHETEs concentrations in the urine samples were measured by ELISA using commercially available kits, according to the manufacturer’s instructions (Detroit R&D Inc., Detroit, MI, USA). Subsequently, the nonclipped kidney was immediately excised, drained, weighed and homogenized in chilled methanol. Immediately after removal of the kidney, arterial blood was collected for ANG II concentration. Plasma and kidney tissue ANG II levels were determined by radioimmunoassay as described in detail in our previous studies [7, 24].
Series 2: Western blot analysis for the quantification of renal cortical CYP-450 protein expression
Seven and 27 days after clip placement, respectively, and after sham-operation (n = 5 in each group), 2K1C and sham-operated rats were anesthetized with thiopental sodium and kidneys were removed and placed in ice-cold isotonic saline solution. The renal cortex was separated and Western blot analysis for the protein expression of CYP2C3, CYP4A and sEH enzyme was performed as described in detail previously [25–28]. Detection was accomplished using enhanced chemiluminescence Western blotting (ECL, Amersham Corp.); blots were exposed to X-ray film. Band intensity was measured densitometrically and the values were normalized for β-actin.
Series 3: Activities of arachidonic acid metabolism in the kidney
Evaluation of epoxygenase and ω-hydroxylase activities was performed as described previously [27,28] and employed and validated in our recent study [21]. Briefly, renal cortex homogenate (500 µg) isolated from 2K1C rats 7 and 27 days after clip placement, or after sham-operation (n=6 in each group) were incubated with [1-14C]-arachidonic acid (0.4 µCi, 7 nmol/L) and NADPH (1 mmol/L, pH 7.4) containing 10 mmol/L MgCl2 for 30 minutes at 37°C. The reaction was terminated by acidification to pH 3.5 – 4.0 with 2 mol/L formic acid, and arachidonic acid metabolites were extracted with ethyl acetate. The ethyl acetate was evaporated under nitrogen, and the metabolites were resuspended in 50 µL of methanol and injected onto the HPLC column. The activity of the formation of these metabolites was estimated on the basis of the specific activity of the added [1-14C]-arachidonic acid, and was expressed as picomoles per milligram of protein per minute.
Evaluation of sEH activity was performed as described recently [29]. Briefly, [3H]-trans-diphenylpropene oxide (tDPPO) was used as a substrate and the rate of hydration of tDPPO was determined by liquid scintillation spectroscopy after differential extraction of the epoxide and diol. Cytosolic or peroxisomal fractions were incubated at 37°C in 100 nL incubation mixtures containing sodium phosphate buffer (90 mmol/L, pH 7.4) and tDPPO (1 to 50 µmol/L in 1 µL dimethylformamide). Incubations were stopped after 5 minutes by addition of 60 µL methanol and 200 µL isooctane. Zero-time and zero-protein incubations served as blanks. Incubations were vortexed vigorously to extract the substrate into the isooctane (the diol metabolite remains in the aqueous phase). A known aliquot of the aqueous phase was removed and added to 1 mL scintillation cocktail for scintillation counting.
Series 4: Renal function studies
Animals were prepared as described in General surgical and preparatory procedures. (see above). After surgery, isotonic saline solution containing bovine serum albumin (0.6%), p-aminohippurate sodium (PAH, Merck, Sharp & Dohme West Point, USA) (1.5%), and polyfructosan (Inutest, Laevosan, Austria) (7.5%) was infused at a rate of 20 µl/min. An equilibration period of 50 minutes was first allowed to establish steady state before initiating two 30-minute clearance periods to assess control renal function. Thereafter, a continuous i.r. infusion of either MS-PPOH, an EETs inhibitor (4.65 µg/hour) or DDMS, a 20-HETE inhibitor (1.09 µg/hour) was administered i.r. and continued throughout the remaining clearance periods. These doses of MS-PPOH and DDMS were chosen based on our previous study evaluating the role of EETs and 20-HETE and in the regulation of renal function. It has been shown that the doses selectively and sufficiently block the intrarenal production of EETs or 20-HETE. [21]. After 15 minutes of drug or vehicle infusion, two 30-min experimental urine collections followed. Coincident with the urine collections, blood samples for PAH and polyfructosan were drawn to allow estimation of whole kidney glomerular filtration rate (GFR) and renal plasma flow (RPF). The following experimental groups of rats were examined:
Sham-operated rats, 7 days after surgery + i.r. infusion of saline vehicle (7-Sham-operated + i.r. saline, n = 11).
Sham-operated rats, 7 days after surgery + i.r. infusion of MS-PPOH (7-Sham-operated + i.r. MS-PPOH, n = 16).
Sham-operated rats, 7 days after surgery + i.r. infusion of DDMS (7-Sham-operated + i.r. DDMS, n = 15).
Sham-operated rats, 27 days after surgery + i.r. infusion of saline vehicle (27-Sham-operated + i.r. saline, n = 11).
Sham-operated rats, 27 days after surgery + i.r. infusion of MS-PPOH (27-Sham-operated + i.r. MS-PPOH, n = 16).
Sham-operated rats, 27 days after surgery + i.r. infusion of DDMS (27-Sham-operated + i.r. DDMS, n = 16).
2K1C Goldblatt hypertensive rats, 7 days after clip placement + i.r. infusion of saline vehicle (7-2K1C + i.r. saline, n = 11).
2K1C Goldblatt hypertensive rats, 7 days after clip placement + i.r. infusion of MS-PPOH (7-2K1C + i.r. MS-PPOH, n = 16).
2K1C Goldblatt hypertensive rats, 7 days after clip placement + i.r. infusion of DDMS (7-2K1C + i.r. DDMS, n = 16).
2K1C Goldblatt hypertensive rats, 27 days after clip placement + i.r. infusion of saline vehicle (27-2K1C + i.r. saline, n = 11).
2K1C Goldblatt hypertensive rats, 27 days after clip placement + i.r. infusion of MS-PPOH (27-2K1C + i.r. MS-PPOH, n = 15).
2K1C Goldblatt hypertensive rats, 27 days after clip placement + i.r. infusion of DDMS (27-2K1C + i.r. DDMS, n = 15).
General Analytical Methods
Urine volume was measured gravimetrically. Sodium and potassium concentrations were determined by flame photometry. Inulin and PAH concentrations were measured colorimetrically. Inulin and PAH clearances were used as indices of the glomerular filtration rate (GFR) and renal plasma flow (RPF), respectively. Both values were calculated per gram of kidney weight. Fractional sodium and potassium excretion rates were calculated using standard formulas.
Statistics
Statistical analysis of data was performed using Graph Pad Prism (Graph Pad Software, San Diego, California, USA). Data are expressed as means ± SEM. Statistical comparisons within groups were conducted using ANOVA for repeated measurements, followed by Newman-Keuls test. One-way ANOVA was used when appropriate. Values exceeding the 95% probability limits (P<0.05) were considered statistically significant.
Results
Experimental series 1: plasma and kidney ANG II levels and urinary excretion of 20-HETE, EETs and DHEETs
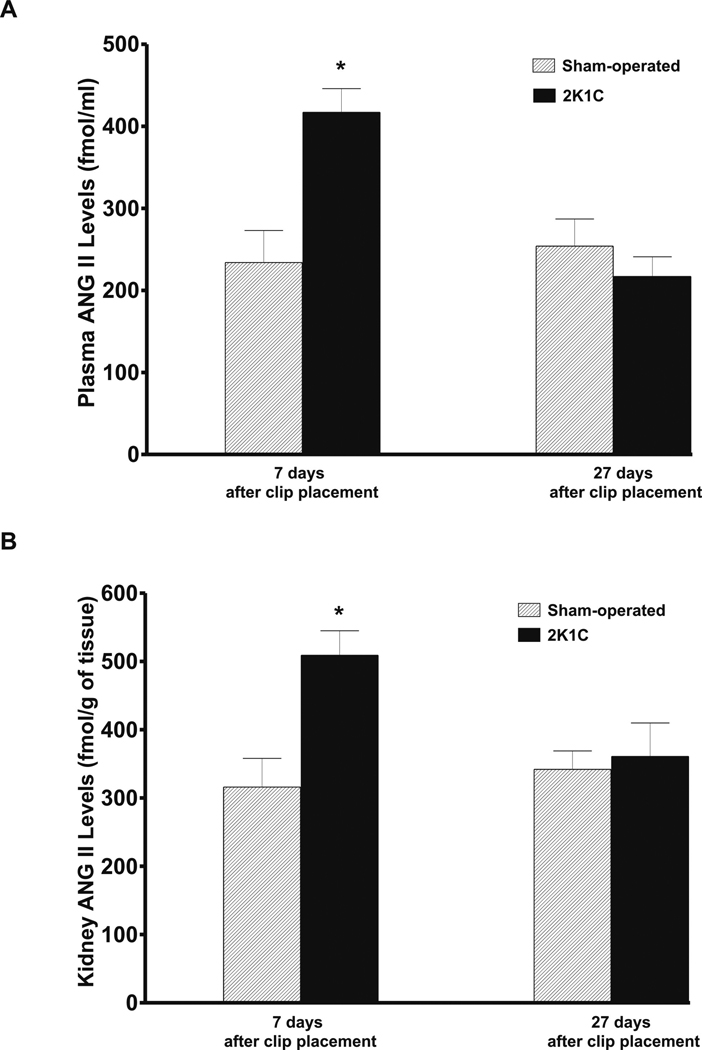
As shown in Figure 1, on day 7 after clip placement the plasma and whole kidney tissue ANG II levels were significantly higher than in sham-operated rats. By the day 27, the differences between 2K1C and sham-operated groups have disappeared.
Figure 1.
Plasma (A) and nonclipped kidney tissue (B) angiotensin II (ANG II) levels in sham-operated rats and in two-kidney, one-clip Goldblatt hypertensive (2K1C) rats 7 and 27 days after clip placement. * P<0.05 compared with sham-operated rats.
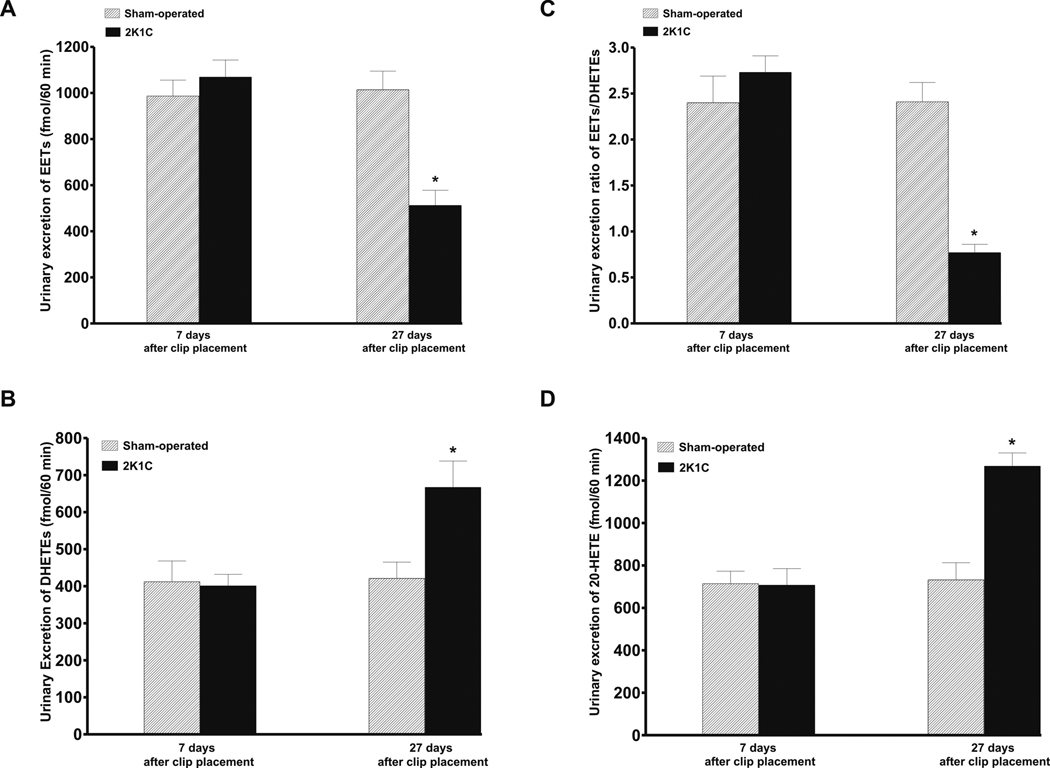
Figure 2A shows that on day 7 after clip placement the basal values of urinary excretion of EETs did not significantly differ between sham-operated and 2K1C rats. In contrast, on the day 27 the basal EETs excretion was significantly lower in 2K1C than in sham-operated rats (512 ± 66 fmol/60 min vs 1014 ± 81., p<0.05). As shown in figure 2B, on day 7 after clip placement the urinary excretion of DHETEs, the biologically inactive metabolites of EETs, did not significantly differ between sham-operated and 2K1C rats. However, on the day 27 the urinary DHETEs excretion was significantly higher in 2K1C than in sham-operated rats (667 ± 71 fmol/60 min vs 421 ± 44., p<0.05). Figure 2C summarizes the data on the intrarenal availability of biologically active epoxygenase metabolites when expressed as the EETs/DHETEs excretion ratio. On day 7 after clip placement this ratio did not significantly differ between 2K1C and sham-operated rats. However, on the day 27 the ratio was significantly lower in 2K1C than in sham-operated rats (0.77 ± 0.09 vs 2.41 ± 0.21., p<0.05). As shown in figure 2D, on day 7 after clip placement the basal urinary excretion of 20-HETE did not significantly differ between 2K1C and sham-operated rats. In contrast, on the day 27 the urinary excretion of 20-HETE was significantly higher in 2K1C than in sham-operated rats (1268 ± 62 vs 732 ± 81. fmol/60 min, p<0.05).
Figure 2.
Basal urinary excretion of EETs (A), and DHEETs (B), as well as the basal urinary EETs to DHETEs excretion ratio (C), and 20-HETE excretion (D) in sham-operated and in two-kidney, one-clip Goldblatt hypertensive (2K1C) rats 7 and 27 days after clip placement. * P<0.05 compared with sham-operated rats.
Experimental series 2: Western blot analysis of renal cortical CYP-450 protein expression
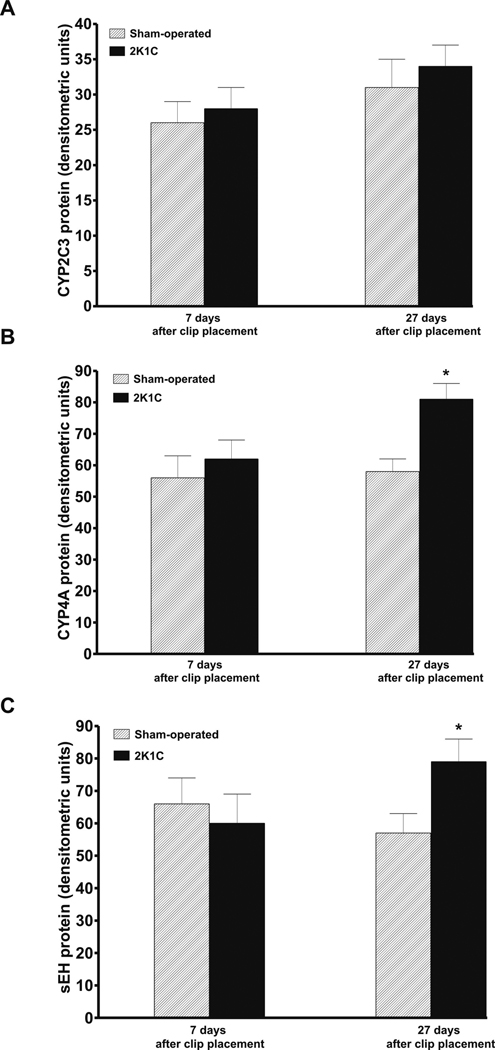
As shown in figure 3A, densitometric analysis normalized for β-actin revealed that there were no significant differences in CYP2C3 protein expression in the renal cortex between sham-operated and 2K1C rats on day 7 and day 27 after clip placement. In contrast, CYP4A and sEH protein expression in the renal cortex were significantly lower in sham-operated than in 2K1C rats on day 27 after clip placement (58 ± 4 vs. 81 ± 5 and 57 ± 6 vs. 79 ± 7 arbitrary units, p<0.05 in both cases) (figures 3B and 3C).
Figure 3.
Renal cortical protein expression of CYP2C3 (A), CYP4A (B) cytochromes and of soluble epoxide hydrolase, sEH, (C) in sham-operated and in two-kidney, one-clip Goldblatt hypertensive (2K1C) rats 7 and 27 days after clip placement. * P<0.05 compared with sham-operated rats.
Experimental series 3: Enzyme activities of arachidonic acid metabolism in the kidney
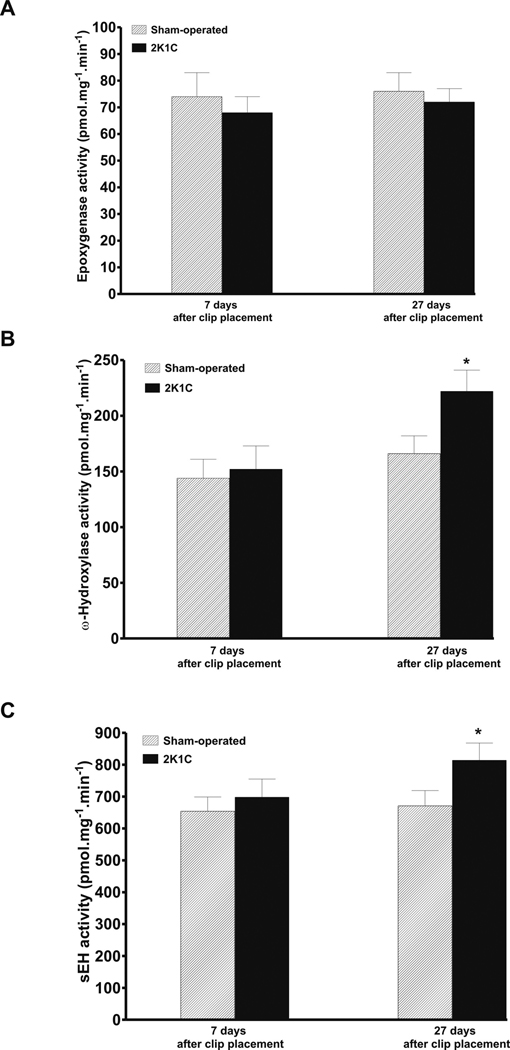
As shown in figure 4A, there were no significant differences in the renal cortical activity of epoxygenase between sham-operated and 2K1C rats at days 7 and 27 after clip placement. In contrast, ω-hydroxylase and sEH activities were significantly lower in sham-operated than in 2K1C rats on day 27 after clip placement (166 ± 16 vs. 222 ± 19 and 671 ± 48 vs. 814 ± 54 pmol.mg−1.min−1, p<0.05) (figures 4B and 4C).
Figure 4.
Renal cortical activity of epoxygenase (A), ω-hydroxylase (B) and sEH (C) in sham-operated and in two-kidney, one-clip Goldblatt hypertensive (2K1C) rats 7 and 27 days after clip placement. * P<0.05 compared with sham-operated rats.
Series 4: Renal function studies
The basal values (averages from two control clearance periods) of MAP, renal hemodynamics and electrolyte excretion are summarized in table 1. It is seen that 2K1C rats exhibited significantly higher MAP compared to sham-operated normotensive rats. The rise in BP was more pronounced at 27 days than at 7 days after clip placement. The basal renal hemodynamics and electrolyte excretion rate did not significantly differ between sham-operated and 2K1C rats, however, some inter-group variability was visible. Thus, to analyse more accurately the renal function responses to experimental manipulations, we compared percent rather than absolute differences between control and experimental periods. Since the responses of renal hemodynamics and electrolyte excretion to experimental manipulations were not significantly different between experimental periods 1 and 2, in fig 5 and fig 6 we show, for clarity of presentation, only the values based on the data for the experimental period 2.
Table 1.
Basal values of mean arterial pressure, renal hemodynamics and electrolyte excretion of the left kidney in sham-operated rats and in nonclipped kidneys of 2K1C rats.
| Group | n | MAP mmHg |
GFR ml.min−1.g−1 |
RPF ml.min−1.g−1 |
UNaV µmol.min−1.g−1 |
FENa % |
V µl.min−1.g−1 |
|---|---|---|---|---|---|---|---|
| 7-Sham-operated + i.r. saline | 11 | 119 ± 3 | 0.79 ± 0.07 | 2.34 ± 0.24 | 1.13 ± 0.14 | 0.97 ± 0.19 | 12.6 ± 0.8 |
| 7-Sham-operated + i.r. MS-PPOH | 16 | 123 ± 3 | 0.81 ± 0.08 | 2.28 ± 0.21 | 1.18 ± 0.19 | 0.95 ± 0.18 | 12.9 ± 0.6 |
| 7-Sham-operated + i.r. DDMS | 15 | 122 ± 2 | 0.76 ± 0.09 | 2.31 ± 0.19 | 1.21 ± 0.17 | 0.94 ± 0.09 | 12.7 ± 0.7 |
| 27-Sham-operated + i.r. saline | 11 | 121 ± 4 | 0.82 ± 0.08 | 2.22 ± 0.18 | 1.15 ± 0.21 | 0.96 ± 0.12 | 11.6 ± 0.8 |
| 27-Sham-operated + i.r. MS-PPOH | 16 | 123 ± 4 | 0.77 ± 0.06 | 2.36 ± 0.25 | 1.19 ± 0.26 | 0.88 ± 0.11 | 11.9 ± 0.7 |
| 27-Sham-operated + i.r. DDMS | 16 | 120 ± 3 | 0.82 ± 0.07 | 2.28 ± 0.21 | 1.21 ± 0.29 | 0.84 ± 0.14 | 12.1 ± 0.8 |
| 7-2K1C + i.r. saline | 11 | 145 ± 4* | 0.75 ± 0.07 | 2.14 ± 0.14 | 1.41 ± 0.24 | 1.01 ± 0.17 | 12.4 ± 0.5 |
| 7-2K1C + i.r. MS-PPOH | 16 | 147 ± 5* | 0.76 ± 0.05 | 2.19 ± 0.18 | 1.38 ± 0.28 | 1.09 ± 0.16 | 12.8 ± 0.6 |
| 7-2K1C + i.r. DDMS | 16 | 148 ± 4* | 0.77 ± 0.06 | 2.17 ± 0.20 | 1.42 ± 0.18 | 1.07 ± 0.25 | 12.2 ± 0.7 |
| 27-2K1C + i.r. saline | 11 | 162 ± 3# | 0.74 ± 0.08 | 2.27 ± 0.22 | 1.39 ± 0.19 | 1.03 ± 0.19 | 12.9 ± 0.9 |
| 27-2K1C + i.r. MS-PPOH | 15 | 161 ± 3# | 0.75 ± 0.06 | 2.32 ± 0.19 | 1.27 ± 0.16 | 1.07 ± 0.26 | 11.8 ± 0.7 |
| 27-2K1C + i.r. DDMS | 15 | 162 ± 4# | 0.77 ± 0.07 | 2.21 ± 0.18 | 1.31 ± 0.28 | 1.01 ± 0.15 | 11.9 ± 0.6 |
Abbreviations are: 2K1C, 2-kidney, 1-clip Goldblatt hypertensive rats; , 7, 27 - seven or 27 days after clip placement (or sham operation) MAP, mean arterial pressure; GFR, glomerular filtration rate; RPF, renal plasma flow; UNaV, absolute sodium excretion; FENa, fractional sodium excretion; V, urine flow.
P<0.05 versus the corresponding values in sham-operated rats ;
P<0.05 versus the corresponding values in 7day-2K1C rats and in sham-operated rats
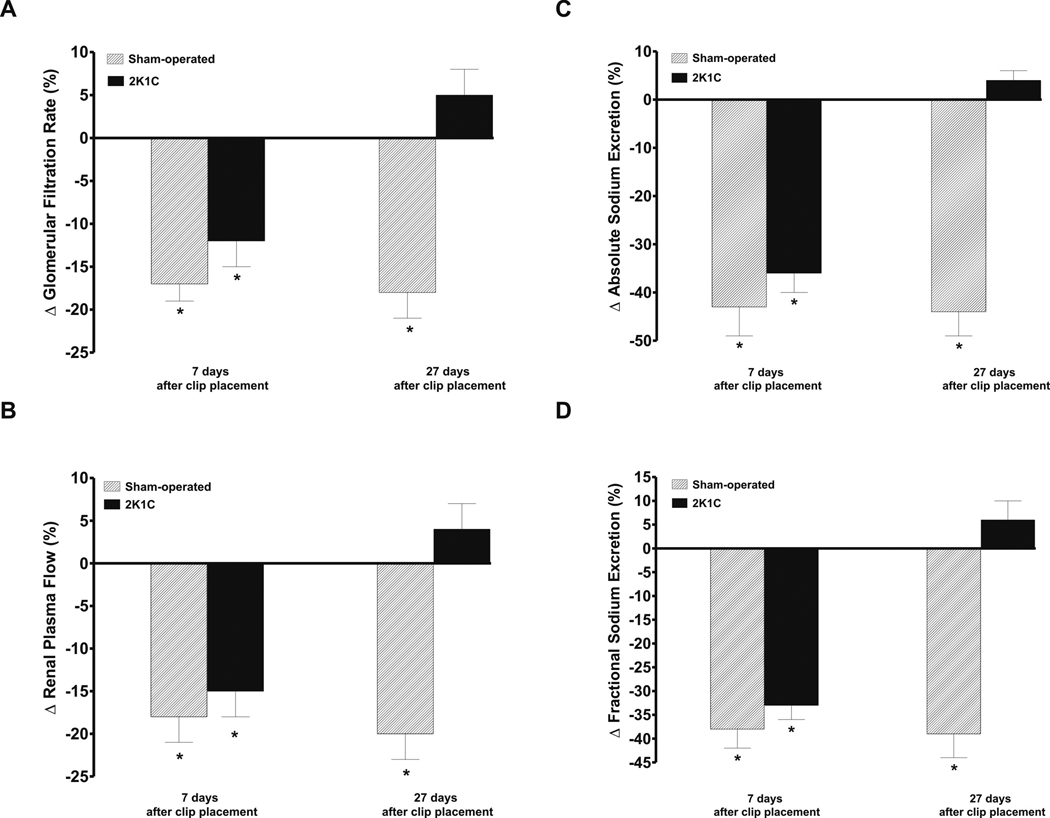
Figure 5.
Responses of the glomerular filtration rate (A), renal plasma flow (B), and absolute (C) and fractional sodium excretion (D) to left renal artery infusion of MS-PPOH in sham-operated animals or 2K1C rats studied 7 or 27 days after clipping of the right kidney * P<0.05 compared with the basal pre-infusion values.
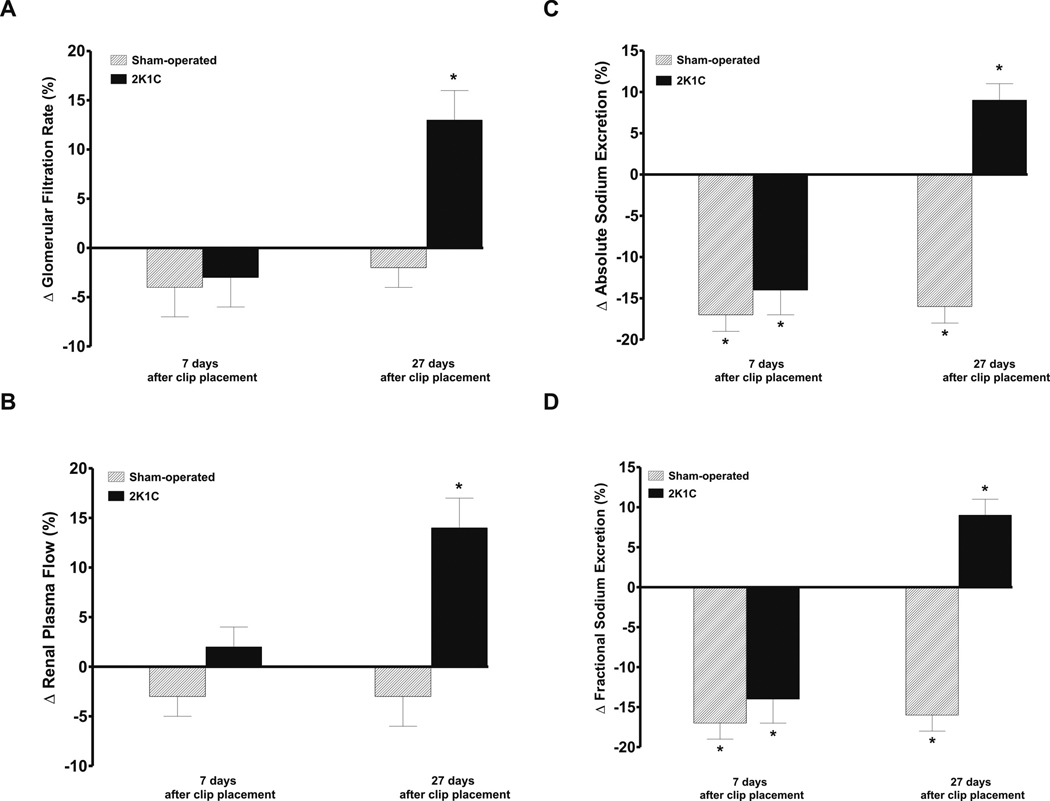
Figure 6.
Responses of the glomerular filtration rate (A), renal plasma flow (B), and absolute (C) and fractional sodium excretion (D) to left renal artery infusion of DDMS in sham-operated animals or 2K1C rats studied 7 or 27 days after clipping of the right kidney. * P<0.05 compared with the basal pre-infusion values.
As shown in figures 5A and 5B, renal artery (i.r.) infusion of MS-PPOH decreased GFR and RPF, significantly and to a similar extent, in sham-operated and 2K1C rats on day 7 after clip placement. In contrast, on the day 27 i.r. infusion of MS-PPOH significantly decreased GFR and RPF in sham-operated rats (−18 ± 3 and −20 ± 3 %, respectively, p<0.05), but did not alter GFR and RPF in 2K1C animals (+5 ± 3 and +4 ± 3 %, respectively).
Figures 5C and 5D show that i.r. infusion of MS-PPOH elicited significant and similar decreases in absolute and fractional sodium excretion in sham-operated animals and 2K1C rats studied on day 7 after clip placement. Likewise, i.r. infusion of MS-PPOH caused significant decreases in urine flow in sham-operated and 2K1C rats studied on the day 7 (not shown in the figure, −22 ± 5 and −24 ± 6 %, respectively, p<0.05). However, these percent decreases were distinctly smaller than those observed for the absolute and fractional sodium excretion. When studied on day 27 after clip placement, i.r. infusion of MS-PPOH did not change absolute or fractional sodium excretion in 2K1C rats but elicited significant decreases in these parameters in sham-operated rats (−44 ± 5 and −39 ± 5, respectively, p<0.05).
As shown in figures 6A and 6B, i.r. infusion of DDMS did not change GFR and RPF in rats studied on days 7 and 27 after sham-operation and in 2K1C rats studied on day 7 after clip placement. In contrast, i.r. infusion of DDMS significantly increased GFR and RPF in 2K1C rats studied on the day 27 (+13 ± 3 and +14 ± 3 %, respectively, p<0.05).
Figures 6C and 6D shows that i.r. infusion of DDMS caused significant decreases in absolute and fractional sodium excretion in rats studied on days 7 and 27 after sham-operation and in 2K1C rats on day 7 after clip placement. In contrast, i.r. infusion of DDMS in 2K1C rats studied on day 27 after clip placement elicited significant increases in absolute and fractional sodium excretion (+9 ± 2 and +10 ± 2 %, respectively, p<0.05).
In time control experiments sham-operated and 2K1C rats given i.r. infusion of saline vehicle did not induce any significant changes in renal hemodynamics or sodium excretion.
Discussion
A major finding of this study is that during the phase of sustained hypertension (data obtained on day 27 after clipping), the nonclipped kidney of 2K1C Goldblatt hypertensive rats exhibits significantly lower excretion of EETs compared to that of sham-operated normotensive rats. This occurs in spite of an apparently normal renal cortical generation of EETs, as indicated by normal protein expression of the CYP2C3 enzyme and normal epoxygenase activity in the renal cortex. In contrast, the urinary excretion of DHETEs, and protein expression of sEH and its activity were significantly higher in the nonclipped kidney of 2K1C Goldblatt rats with sustained hypertension than in sham-operated normotensive rats. Evidently, during the stable phase of hypertension the formation of EETs in the nonclipped kidney of 2K1C Goldblatt hypertensive rats is not altered, however, the conversion of EETs to DHETEs, which are devoid of vasodilator and natriuretic activities [9–11,16,20,22,23] is enhanced. Thus, the intrarenal availability of biologically active epoxygenase products, assessed as the urinary EETs/DHETEs excretion ratio, is reduced in 2K1C rats studied 27 days after clip placement to approximately 30 % of that observed in sham-operated rats, indicating that during the phase of sustained hypertension, 2K1C Goldblatt hypertensive rats exhibit a net deficiency of the biologically active epoxygenase products. This interpretation is further supported by the results of our renal function studies. They show that during the initial phase of hypertension in 2K1C rats, the selective intrarenal inhibition of EETs formation resulted in significant decreases in renal hemodynamics and in absolute and fractional sodium excretion, similar as in sham-operated normotensive rats studied in parallel. In contrast, during the sustained phase of hypertension, an intrarenal inhibition of EETs production did not alter renal hemodynamics or sodium excretion in 2K1C hypertensive whereas it led to significant decreases in both these parameters in sham-operated rats. This suggests that during the phase of sustained 2K1C Goldblatt hypertension an intrarenal deficit of biologically active EETs participates in the regulation of renal vascular tone and tubular sodium transport.
Mechanisms of EETs action involve stimulation of the large-conductance, calcium-activated potassium channels (KCa) and hyperpolarization of the smooth muscle cell membrane, leading to an inhibition of voltage-activated calcium channel activity, which reduces calcium entry and results in vasodilatation [16,17]. In addition, EETs participate in the bradykinin-induced, endothelium-dependent vasodilatation of the glomerular afferent arteriole and, in general, preferentially dilate the renal preglomerular vasculature [30,31]. Based on vast evidence, EETs have been identified as the endothelium-derived hyperpolarizing factor (EDHF) that mediates nitric oxide- and prostaglandin-independent vasodilatation [32]. In addition, EETs have been found to oppose the vasoconstrictor action of ANG II [17,25,33].
In addition to altering the vascular tone, EETs inhibit proximal tubular sodium reabsorption by blocking the sodium-hydrogen exchanger [18], and inhibit sodium reabsorption and potassium secretion in the cortical collecting duct by blocking the epithelial sodium channels [19]. Most of available evidence indicates that the EETs´ antihypertensive properties are mainly associated with the action on sodium excretion [9–11]. It has been demonstrated that either intrarenal deficiency of EETs or an inability to properly increase intrarenal EETs´ levels are implicated in the pathophysiology of certain forms of ANG II-dependent and salt-sensitive hypertension [25,27,34,35]. It is therefore conceivable that net intrarenal deficiency of EETs in the nonclipped kidney of 2K1C Goldblatt hypertensive rats contributes to the impairment of the pressure-natriuresis relationship and, consequently, to the maintenance of hypertension in this model.
Another major finding of this study is that during the phase of sustained hypertension 2K1C Goldblatt hypertensive rats showed increased urinary excretion of 20-HETE, increased kidney protein expression of CYP4A, and increased renal cortical activity of ω-hydroxylase. These data provide strong evidence that intrarenal 20-HETE formation is elevated during the sustained hypertension phase in 2K1C Goldblatt hypertensive rats. In addition, we found that during the initial phase of hypertension, selective intrarenal inhibition of 20-HETE formation elicited similar decreases in sodium excretion in 2K1C and in sham-operated normotensive rats. In contrast, during sustained hypertension, the inhibition of 20-HETE substantially increased GFR, RPF and absolute and fractional sodium excretion in 2K1C rats, whereas in sham-operated rats it decreased absolute and fractional sodium excretion without altering GFR or RPF.
It has been well documented that 20-HETE inhibits sodium reabsorption in the proximal tubule by blocking sodium-potassium-ATPase activity [36], and in the thick ascending limb of the loop of Henle (TALH) by inhibiting the Na, K, 2Cl co-transporter [37] and also, secondarily, by inhibiting Na,K-ATPase. [38]. Thus, in 2K1C rats in the phase of sustained hypertension the inhibition of 20-HETE formation under conditions of elevated intrarenal 20-HETE levels should cause a decrease rather than an increase in sodium excretion. Our results indicate that unlike in normotensive rats and 2K1C rats during the initial phase of hypertension, in the sustained hypertension 20-HETE - despite its elevated intrarenal levels – is not natriuretic. Possibly, its transport inhibitory action at the tubule level is superseded by antinatriuresis secondary to vasoconstriction and a decrease in renal hemodynamics. Thus, the increase in sodium excretion after inhibition of 20-HETE could depend on an increase in GFR and RPF. Likely, there was also an increase in the medullary blood flow which could per se promote natriuresis.
The vasoconstrictor action of 20-HETE involves inhibition of the opening of large-conductance, calcium-activated potassium channels [39]; an increase in calcium channel conductance leads to depolarization of the arteriolar vascular smooth muscle [40,41], and activation of Rho-kinase causes sensitization of the contractile apparatus to calcium [42]. In addition, 20-HETE produced in the renal vasculature augments vascular reactivity to vasoconstrictor agents [43–45],e.g. plays a critical role as a second messenger for ANG II-mediated vasoconstriction [12,13]. It has been postulated that enhanced formation of 20-HETE in the vasculature contributes to exaggerated systemic and renal vascular responsiveness to vasoconstrictors in ANG II-dependent models of hypertension and thereby contributes to the development and maintenance of hypertension in these models [12,14,15].
With these observations in mind it is conceivable that elevated intrarenal 20-HETE levels during the phase of sustained hypertension in Goldblatt 2K1C rats might contribute importantly to the increased renal vascular resistance and to the compromised ability of the kidney to respond to elevations of arterial blood pressure with appropriate increases in sodium excretion. This notion is supported by our results from renal function studies showing that selective intrarenal inhibition of 20-HETE formation resulted in significant increases in renal hemodynamics.
Interestingly in our 2K1C hypertensive rats, plasma and nonclipped kidney ANG II concentrations were elevated during the initial phase of hypertension and returned to basal levels in the sustained phase. These findings are in agreement with previous studies evaluating the role of the RAS in the pathophysiology of 2K1C Goldblatt hypertension in rats and mice. They confirm the notion that the sustained phase hypertension cannot be simply ascribed to an increased activity of the systemic RAS but is rather the consequence of a compromised pressure-natriuresis relationship in the nonclipped kidney of 2K1C rats [1–7].
In summary, our present findings indicate that during the phase of sustained hypertension, the nonclipped kidney of 2K1C Goldblatt hypertensive rats exhibits a reduced availability of biologically active EETs. Simultaneously, the intrarenal 20-HETE concentration is elevated, which prevents appropriate pressure-dependent natriuresis and potentiates the renal vasoconstrictor responses. Moreover, our data demonstrate that the nonclipped kidney of 2K1C rats is unable to suppress intrarenal ANG II content to make it adequate to the elevated blood pressure. Collectively, our findings indicate that altered production and/or action of CYP-derived metabolites in the nonclipped kidney is combined with kidney tissue ANG II levels inappropriately high for hypertensive rats. Both these factors could importantly contribute to the derangement of the pressure-natriuresis relationship of the nonclipped kidneys of 2K1C Goldblatt hypertensive rats and to play a crucial role in the pathophysiology of sustained hypertension in this model.
Acknowledgments
Source of Support: This study was supported by grant No. 305/08/J006 awarded to L.Č. by Czech Science Foundation (GAČR). Z.H. is supported by the grant awarded by Czech Science Foundation (GAČR) No. 305/08/P053. A.W. was partly supported from the Center for Cardiovascular Research (1M6798582302) and by the institutional financial support of the Institute for Clinical and Experimental Medicine (MZO 00023001). Z.V. was partly supported by grant No. NS/10499-3 awarded to L.Č. by the Internal Grant Agency of the Ministry of Health of the Czech Republic. J.D. Imig is supported by grants from the National Institutes of Health (HL59699 and DK38226) and Advancing a Healthier Wisconsin. H.J. Kramer is supported by grants from the German Research Foundation (DFG), Bonn (Kra 433/14-2 and 436 TSE 113/50/0-1).
Footnotes
Previous presentations: NONE
Conflict of Interest: NONE
References
- 1.Navar LG, Zou L, Von Thun A, Wang CT, Imig JD, Mitchell KD. Unraveling the mystery of Goldblatt hypertension. News Physiol Sci. 1998;13:170–176. doi: 10.1152/physiologyonline.1998.13.4.170. [DOI] [PubMed] [Google Scholar]
- 2.Cervenka L, Wang CT, Mitchell KD, Navar LG. Proximal tubular angiotensin II levels and renal functional responses to AT1 receptor blockade in nonclipped kidneys of Goldblatt hypertensive rats. Hypertension. 1999;33:102–107. doi: 10.1161/01.hyp.33.1.102. [DOI] [PubMed] [Google Scholar]
- 3.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 4.Von Thun AM, Vari RC, El-Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol. 1994;266:F120–F128. doi: 10.1152/ajprenal.1994.266.1.F120. [DOI] [PubMed] [Google Scholar]
- 5.Guan S, Fox K, Mitchell, Navar LG. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one-clip hypertensive rats. Hypertension. 1992;20:763–767. doi: 10.1161/01.hyp.20.6.763. [DOI] [PubMed] [Google Scholar]
- 6.El-Dahr SS, Dipp S, Guan S, Navar LG. Renin, angiotensinogen, and kallikrein gene expression in two-kidney Goldblatt hypertensive rats. Am J Hypertens. 1993;6:914–919. doi: 10.1093/ajh/6.11.914. [DOI] [PubMed] [Google Scholar]
- 7.Červenka L, Vaněčková I, Husková Z, Vaňourková Z, Erbanová M, Thumová M, et al. Pivotal role of AT1A receptors in the development of two-kidney, one-clip hypertension: study in AT1A receptor knockout mice. J of Hypertens. 2008;26:1379–1389. doi: 10.1097/HJH.0b013e3282fe6eaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guyton AC, Hall JE, Coleman TG, Manning RD., Jr . The dominant role of the kidneys in the long term regulation of arterial pressure in normal and hypertensive states. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis and Management. New York, Ny: Raven Press, Publishers; 1990. pp. 1029–1052. [Google Scholar]
- 9.Capdevila JH, Falck JR, Imig JD. Role of the cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Int. 2007;72:683–689. doi: 10.1038/sj.ki.5002394. [DOI] [PubMed] [Google Scholar]
- 10.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 11.Sarkis A, Lopez B, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids in hypertension. Curr Opin Nephrol Hypertens. 2004;13:205–214. doi: 10.1097/00041552-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol. 2002;283:R60–R68. doi: 10.1152/ajpregu.00664.2001. [DOI] [PubMed] [Google Scholar]
- 13.Joly E, Seqqat R, Flamion B, Caron N, Michal A, Imig JD, Kramp R. Increased renal vascular reactivity to angiotensin II after unilateral nephrectomy in the rat involves 20-HETE. Am J Physiol. 2006;291:R977–R986. doi: 10.1152/ajpregu.00401.2005. [DOI] [PubMed] [Google Scholar]
- 14.Muthalif MM, Karzoun NA, Gaber L, Khandekar Z, Benter IF, Saeed AF, Parmentier JH, Estes A, Malik KU. Angiotensin II-induced hypertension: contribution of Ras GTPase/mitogen-activated protein kinase and cytochrome P-450 metabolites. Hypertension. 2000;36:604–609. doi: 10.1161/01.hyp.36.4.604. [DOI] [PubMed] [Google Scholar]
- 15.Čertíková Chábová V, Kramer HJ, Vaněčková I, Vernerová Z, Eis V, Tesař V, et al. Effects of chronic cytochrome P-450 inhibition on the course of hypertension and end-organ damage in Ren-2 transgenic rats. Vascular Pharmacol. 2007;47:145–159. doi: 10.1016/j.vph.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 17.Imig JD, Deichmann PC. Afferent arteriolar responses to ANG II involve activation of PLA2 and modulation by lipoxygenase and P-450 pathways. Am J Physiol. 1997;273:F274–F282. doi: 10.1152/ajprenal.1997.273.2.F274. [DOI] [PubMed] [Google Scholar]
- 18.Madhun ZT, Goldthwait DA, McKay D, Hopfer U, Douglas JG. An epoxygenase metabolite of arachidonic acid mediates angiotensin II-induced rises in cytosolic calcium in rabbit proximal tubule epithelial cells. J Clin Invest. 1991;88:456–461. doi: 10.1172/JCI115325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakairi Y, Jacobson HR, Noland DT, Capdevila JH, Falck JR, Breyer MD. 5,6-EET inhibits ion transport in collecting duct by stimulating endogenous prostaglandin synthesis. Am J Physiol. 1995;268:F931–F939. doi: 10.1152/ajprenal.1995.268.5.F931. [DOI] [PubMed] [Google Scholar]
- 20.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 21.Čertíková Chábová V, Kramer HJ, Vaněčková I, Thumová M, Škaroupková P, Tesař V, et al. The roles of intrarenal 20-hydroxyeicosatetraenoic and epoxyeicosatrienoic acids in the regulation of renal function in hypertensive Ren-2 transgenic rats. Kidney Blood Press Res. 2007;30:335–346. doi: 10.1159/000107710. [DOI] [PubMed] [Google Scholar]
- 22.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 23.Imig JD. Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors. Cardiovas Drug Rev. 2006;24:169–188. doi: 10.1111/j.1527-3466.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 24.Husková Z, Kramer HJ, Vaňourková Z, Červenka L. Effects of changes in sodium balance on plasma and kidney angiotensin II levels in anesthetized and conscious Ren-2 transgenic rats. J Hypertens. 2006;24:517–527. doi: 10.1097/01.hjh.0000209988.51606.c7. [DOI] [PubMed] [Google Scholar]
- 25.Imig JD, Zhao X, Falck JR, Wei S, Capdevila JH. Enhanced renal mircovascular reactivity to angiotensin II in hypertension is ameliorated by the sulfonimide analog of 11,12-epoxyeicosatrienoic acid. J Hypertens. 2001;19:983–992. doi: 10.1097/00004872-200105000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Ai D, Fu Z, Guo D, Tanaka H, Wang N, Tang C, et al. Angiotensin II up/regulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Proc Natl Acad Sci. 2007;104:9018–9023. doi: 10.1073/pnas.0703229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liclican EL, McGiff JC, Falck JR, Carroll MA. Failure to upregulate the adenosine2A receptor-epoxyeicosatrienoic acid pathway contributes to the development of hypertension in Dahl salt-sensitive rats. Am J Physiol. 2008;295:F1696–F1704. doi: 10.1152/ajprenal.90502.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang H, Morisseau C, Wang JF, Yang T, Falck JR, Hammock BD, Wang MH. Increasing or stabilizing renal epoxyeicosatrienoic acid production attenuates abnormal renal function and hypertension in obese rats. Am J Physiol. 2007;293:F342–F349. doi: 10.1152/ajprenal.00004.2007. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, et al. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab. 2007;27:1931–1940. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imig JD, Falck JR, Wei S, Capdevila JH. Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilatation in response to bradykinin. J Vasc Res. 2001;38:247–255. doi: 10.1159/000051053. [DOI] [PubMed] [Google Scholar]
- 31.Wang D, Borrego-Conde LJ, Falck JR, Sharma KK, Wilcox CS, Umans JG. Contribution of nitric oxide, EDHF, and EETs to endothelium-dependent relaxation in renal afferent arterioles. Kidney Int. 2003;63:2187–2193. doi: 10.1046/j.1523-1755.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 32.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49:590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- 33.Kohagure K, Endo Y, Ito O, Arima S, Omata K, Ito S. Endogenous nitric oxide and epoxyeicosatrienoic acids modulate angiotensin II-induced constriction in the rabbit afferent arteriole. Acta Physiol Scand. 2000;168:107–112. doi: 10.1046/j.1365-201X.2000.00638.x. [DOI] [PubMed] [Google Scholar]
- 34.Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim HI, Watanabe T, Hammock BD. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension. 2005;46:975–981. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, et al. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol. 2004;15:1244–1253. [PubMed] [Google Scholar]
- 36.Quigley R, Baum M, Reddy KM, Griener JC, Falck JR. Effects of 20-HETE and 19(S)-HETE on rabbit proximal straight tubule volume transport. Am J Physiol. 2000;278:F949–F953. doi: 10.1152/ajprenal.2000.278.6.F949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Escalante B, Erlij D, Falck JR, McGiff JC. Cytochrome P-450 arachidonate metabolites affect ion fluxes in rabbit medullary thick ascending limb. Am J Physiol. 1994;266:C1775–C1782. doi: 10.1152/ajpcell.1994.266.6.C1775. [DOI] [PubMed] [Google Scholar]
- 38.Yu M, Lopez B, Dos Santos EA, Falck JR, Roman RJ. Effects of 20-HETE on Na+-K+- ATPase activity in the thick ascending loop of Henle. Am J Physiol. 2007;292:R2400–R2405. doi: 10.1152/ajpregu.00791.2006. [DOI] [PubMed] [Google Scholar]
- 39.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. 20-HETE is an endogenous inhibitor of the large-conductance Ca2+-activated K+ channel in renal arterioles. Am J Physiol. 1996;270:R228–R237. doi: 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]
- 40.Randriamboavonjy V, Kiss L, Falck JR, Busse R, Fleming I. The synthesis of 20-HETE in small porcine coronary arteries antagonizes EDHF-mediated relaxation. Cardiovasc Res. 2005;65:487–494. doi: 10.1016/j.cardiores.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 41.Gebremedhin D, Lange AR, Narayanan J, Aebly MR, Jacobs ER, Harder DR. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol. 1998;507:771–781. doi: 10.1111/j.1469-7793.1998.771bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Randriamboavonjy V, Busse R, Fleming I. 20-HETE-induced contraction of small coronary arteries depends on the activation of Rho-Kinase. Hypertension. 2003;41:801–806. doi: 10.1161/01.HYP.0000047240.33861.6B. [DOI] [PubMed] [Google Scholar]
- 43.Kaide J, Wang MH, Wang JS, Zhang F, Gopal VR, Falck JR, Nasjletti A, Laniado-Schwartzman M. Transfection of CYP4A1 cDNA increases vascular reactivity in renal interlobar arteries. Am J Physiol. 2003;284:F51–F56. doi: 10.1152/ajprenal.00249.2002. [DOI] [PubMed] [Google Scholar]
- 44.Imig JD, Pham BT, LeBlanc EA, Reddy KM, Falck JR, Inscho EW. Cytochrome P450 and cyclooxygenase metabolites contribute to the endothelin-1 afferent arteriolar vasoconstrictor and calcium responses. Hypertension. 2000;35:307–312. doi: 10.1161/01.hyp.35.1.307. [DOI] [PubMed] [Google Scholar]
- 45.Oyekan AO, McGiff JC. Cytochrome p-450-derived eicosanoids participates in the renal functional effects of ET-1 in the anaesthetized rat. Am J Physiol. 1998;274:R52–R61. doi: 10.1152/ajpregu.1998.274.1.R52. [DOI] [PubMed] [Google Scholar]