Abstract
Purpose
Several synthetic materials have been used for cardiac reconstruction in patients with complex congenital heart defects. These materials are not viable, do not grow with children, and may necessitate reoperation. We report here on the cardiac implantation of a recently developed, degradable porous material designed to facilitate cellular ingrowth during the healing process.
Description
An elastomeric, biodegradable polyester urethane urea (PEUU) was processed into circular scaffolds and used to replace a surgical defect in the right ventricular outflow tract of adult rats. Control rats were implanted with expanded polytetrafluoroethylene. Histologic evaluation was performed at 4, 8, and 12 weeks postsurgery.
Evaluation
All animals survived the surgery. Both PEUU and expanded polytetrafluoroethylene were encapsulated with fibrous tissue and had complete endothelialization on the endocardial surface with no aneurysm formation or thrombus noted. With PEUU patching, fibroblast ingrowth occurred at 4 weeks, increasing with time. In contrast, expanded polytetrafluoroethylene patches exhibited no ingrowth and elicited local inflammation that moderated with time.
Conclusions
The PEUU patch demonstrated suitable mechanical properties and biocompatible characteristics in the right ventricular outflow tract replacement model, permitting cellular integration and endocardial endothelialization with minimal inflammation. Future application of this material as a cardiovascular scaffold seems promising.
Reconstruction of tissue deficiencies in the cardiovascular system is necessary for a variety of congenital conditions. Often this reconstruction requires the implantation of synthetic or xenotypic material such as polyethylene terepthalate fabric (Dacron [C. R. Bard Inc, Murray Hill, NJ]), expanded polytetrafluoroethylene (ePTFE) (eg, Gore-Tex [W. L. Gore & Associates, Flagstaff, AZ] or IMPRA [IMPRA Inc, Tempe, AZ]), or glutaraldehyde-fixed bovine pericardium. The limitations of such materials are well known (ie, the implant becomes a permanent foreign body that has the potential to serve as an infection nidus), and in constrained geometries this foreign body will prevent tissue growth and remodeling [1].
It is notable that the primary polymeric materials used in reconstructive procedures, Dacron, and ePTFE are polymers developed for a broad array of commercial applications prior to adoption by the medical community. With advances in our understanding of biocompatibility and polymer science in the decades since these materials were first applied, it is surprising that materials molecularly designed and processed specifically for surgical applications have not replaced these generic polymers. One approach that has received considerable interest of late has been to use biodegradable polymers with or without cell seeding to generate viable, tissue-engineered structures [2]. However the polymeric materials used as tissue-engineering scaffolds in most studies have similarly come from a limited set of polyesters including polyglycolic acid, polylactic acid, polycaprolac-tone, and their copolymers. More recently, investigators have begun exploring a broader array of polymers that may better fit the requirements of particular clinical applications, with emphasis on appropriate mechanical properties, biodegradation rates, drug delivery potential, and cytocompatibilty.
We have been specifically interested in developing polymeric materials that would be suited for application in cardiovascular tissue engineering and have reported on the synthesis and processing of biodegradable polyester urethane ureas (PEUUs) that possess high elasticity and strength and that are readily processed into porous scaffolds. These materials have been shown to be cytocompatible in vitro, and when processed into scaffolds they are amenable to cellularization in vitro [3, 4]. We hypothesized that these materials would support cell in-growth after implantation in vivo.
Our objective in this study was to assess the performance of a PEUU scaffold in the reconstruction of a transmural defect created in the right ventricular outflow tract (RVOT) of the rat. After implantation periods of 4, 8, and 12 weeks, the RVOT was histologically examined to assess material degradation, inflammation, host cell migration, and endocardial endothelialization. For control purposes, an ePTFE patch of the same dimensions was implanted in a separate group of animals and assessed at the same time points. This is the first report on the implantation of this material.
Technology and Technique
Experimental Animals
Adult male syngeneic Lewis rats (Harlan Sprague Dawley Inc, Indianapolis, IN) weighing 300 g to 350 g were used for the RVOT replacement procedure. The research protocol followed the National Institutes of Health guidelines for animal care and was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Material Synthesis and Preparation
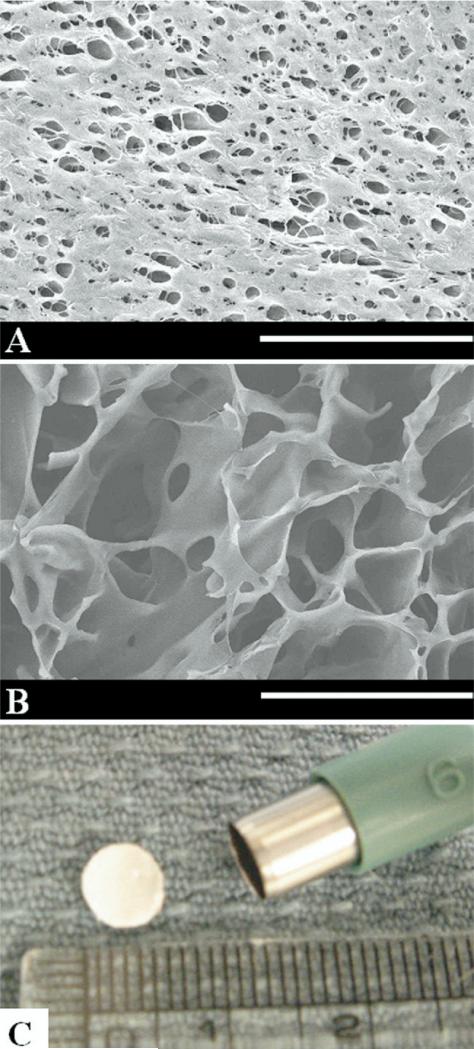
The PEUU was synthesized from butyl diisocyanate, polycaprolactone (2,000 MW), and putrescine [3] and processed according to the methods of previous reports in the authors’ laboratory [4]. Briefly a thermally induced phase separation technique was used for processing, wherein 10 weight % PEUU in dimethyl sulfoxide was quenched at –80°C. This resulted in a scaffold with 85% overall porosity, interconnected pores, and a relatively smaller pore size on the surface skin (Figs 1A, 1B). The PEUU scaffold was created in a mold that provided a material thickness of 0.4 mm. For control purposes, the ePTFE (Impra-Bard, Tempe, AZ), also with a thickness of 0.4 mm, was used. Both materials were cut into 6-mm diameter circular patches using a biopsy punch (Fig 1C). Patches were sterilized by immersion in 70% ethanol for 6 hours, followed by immersion in phosphate buffer saline solution, and exposure to an ultraviolet light source in a laminar flow hood for 3 hours. After rinsing thoroughly with phosphate buffer saline solution, patches were implanted in the rat RVOT as described below.
Fig 1.
Electron micrographs of polyester urethane urea (PEUU) (A) scaffold surface (scale bar, 50 μm) and (B) cross-section (scale bar, 100 μm), and of (C) the punched 6-mm diameter PEUU patch.
RVOT Replacement Procedure
The surgical procedure was based on the method previously reported by Sakai and colleagues [5]. Briefly, rats were anesthetized by intramuscular injection of ketamine hydro-chloride (22 mg/kg) and then intraperitoneal injection of sodium pentobarbital (30 mg/kg). Intubation was performed with ventilation at 60 cycles/min and a tidal volume of 2.0 mL under room air. The heart was exposed through a median sternotomy and a pursestring suture was placed in the RVOT free wall with 7-0 polypropylene to form a perimeter greater than 6 mm diameter (Ethicon, Somerville, NJ). Both suture ends were then passed through a 24-gauge plastic vascular cannula (Becton Dickinson, Franklin Lakes, NJ), which was used as a tourniquet. With tourniquet tightening, the RVOT wall inside the pursestring stitching was distended and resected to create a defect just under 6 mm in diameter. At that point the tourniquet was released slightly to demonstrate pulsatile bleeding, assuring trans-mural defect creation. A PEUU or ePTFE patch (n = 12, each group) was sutured along the margin of the purse-string suture with an over-and-over method with 7-0 polypropylene to cover the defect. The tourniquet was then released and the pursestring suture was removed, leaving a whole wall thickness defect just under 6 mm in diameter covered by one of the two 6-mm patch types. The chest incision was closed in layers with running sutures of 4-0 Vicryl (Ethicon). The first 3 days after surgery, buprenorphine (1 mg/kg) and cefuroxime (100 mg/kg) were administered intramuscularly twice a day. No anticoagulation therapy was administered.
Cardiac Explantation
At each scheduled explant time point (4, 8, and 12 weeks), animals were administered 500 units of heparin and were then euthanized (n = 4 per group) by intraperitoneal injection of an overdose of pentobarbital (100 mg/kg). The heart was exposed through a repeated median sternotomy. After macroscopic photography of the heart in situ, it was harvested and frozen in 2-methylbutane, which was pre-cooled in liquid nitrogen.
Histology and Immunohistochemistry
The frozen heart tissue was serially cryosectioned into 10 μm-thick specimens and processed for hematoxylin and eosin or immunohistochemical evaluation. To assess the extracellular matrix, specimens were stained with the Masson Modified IMEB Trichrome Stain Kit (IMEB, Inc, San Marcos, CA). Specimens for immunohistochemistry were reacted with an antibody against factor VIII (polyclonal 1:300, DAKO [Dako North America Inc, Carpinteria, CA]) to identify endothelial cells. Nuclei were stained with 4′,6-diamidino-2-phenyindole, DAPI (1:10,000, Sigma [Sigma-Aldrich, St. Louis, MO]).
The tissue response (presence of macrophages, neovascularization, and cellular infiltration, respectively) was rated by 3 persons according to a relative scoring system: 0 = no observation to +++ = severe. The sections were also examined for the formation of a fibrous capsule around the patches.
Results
Postoperative Course and Gross Observations
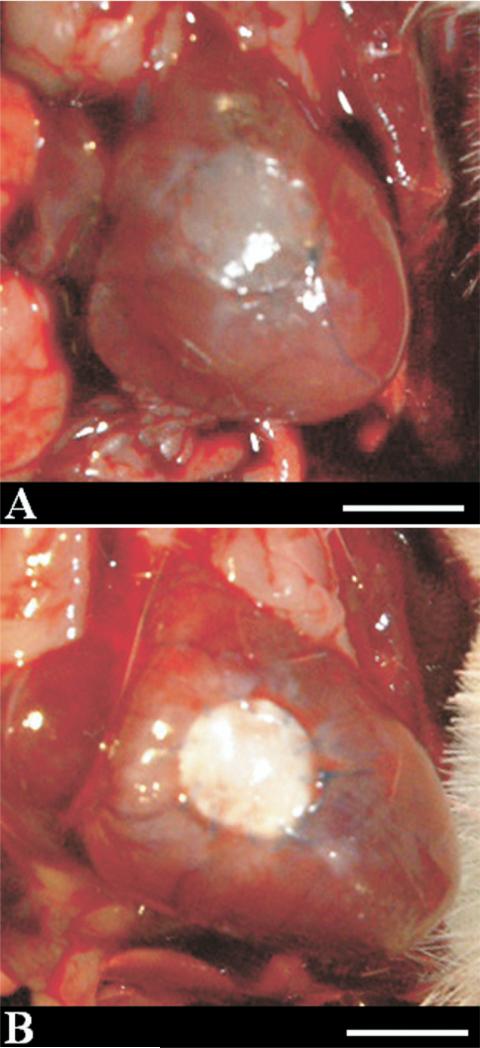
No deaths occurred during the postoperative course in either group and no gross evidence of thrombosis was present in any of the animal explants. At the time of explantation, all rat hearts exhibited minimal thoracic adhesions with no recognizable pattern of adhesive tissue present in ePTFE or PEUU implanted animals. Neither group showed any dehiscence or aneurysm formation at the site of the implanted patch in the RVOT at each time point. Looking at macroscopic images of the RVOT 12 weeks after surgery, the replacement of the PEUU patch with native tissue is apparent (Fig 2). This effect is not seen with the ePTFE patch.
Fig 2.
Representative images at 12-week explantation for (A) polyester urethane urea and (B) expanded polytetrafluoroethylene; (scale bar, 5 mm).
Histology and Immunohistochemistry
FIBROUS CAPSULE AND VASCULARIZATION
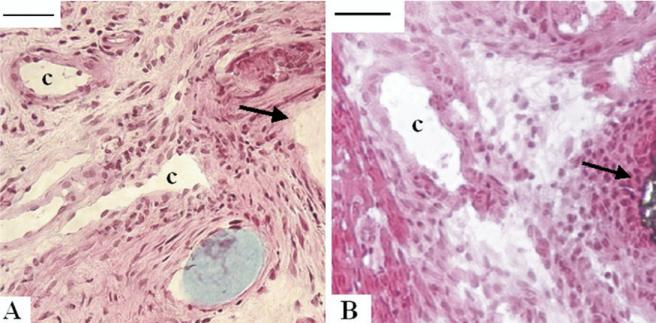
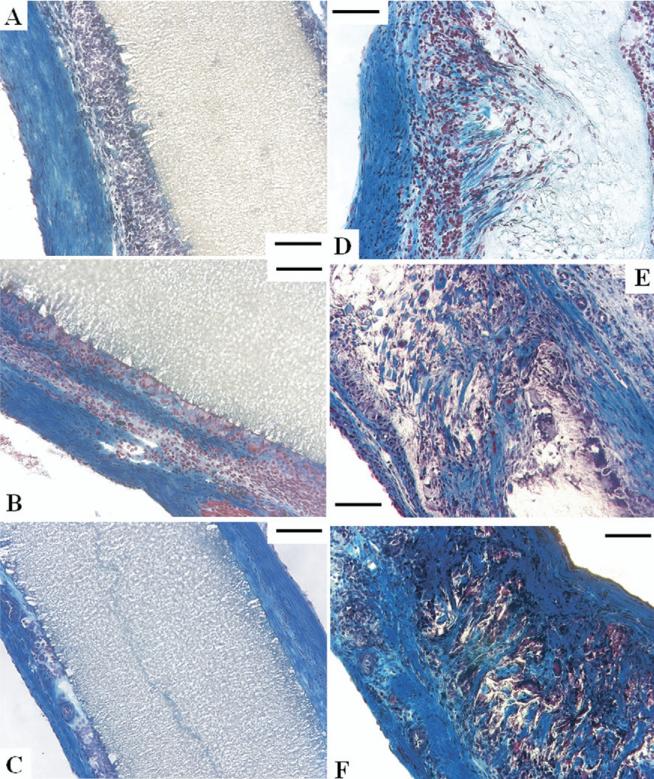
Both patches were surrounded by layered fibrous tissue at each time point. The fibrous capsule around both patches had capillaries mainly in the peripheral region between the patches and the native right ventricular muscle (Fig 3). However at 12 weeks in the PEUU group, capillary formation was also noted in the endocardial fibrous tissue (Fig 4F).
Fig 3.
In the fibrous tissue transition region between the implanted (A) polyester urethane urea or (B) the expanded polytetrafluoroethylene material and the native right ventricular muscle, microvasculature was noted (marked with “c,” scale bar, 20 μm). Sections were obtained 4 weeks after right ventricular outflow tract implantation and stained with hematoxylin and eosin. Black arrows show implanted material edge.
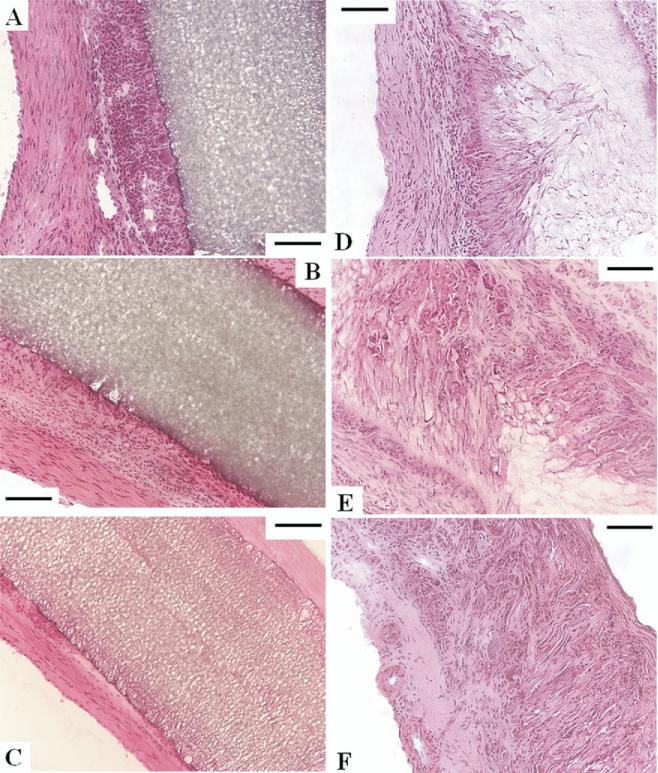
Fig 4.
Time course of the healing response for expanded polytetrafluoroethylene (ePTFE) at (A) 4 weeks, (B) 8 weeks, and (C) 12 weeks, and (D) polyester urethane urea (PEUU) at 4 weeks, (E) 8 weeks, and (F) 12 weeks, with hematoxylin and eosin staining (scale bars, 100 μm). The right ventricular cavity is to the left or lower left for all images. (A–C) No cellular ingrowth was observed with ePTFE, where the foreign body response was most exuberant with surface macrophages at 4 weeks, decreasing to thin fibrous tissue at 12 weeks. (D–F) PEUU scaffolds experienced cellular ingrowth and macrophage activity consistent with cellular infiltration and material degradation. (F) At 12 weeks the PEUU scaffolds were almost completely absorbed with microvasculature apparent on the endocardial side.
CELLULAR INGROWTH AND INFLAMMATION
No cellular in-growth was noted in ePTFE at any time points. The entire surface of the ePTFE was surrounded by a fibrous tissue with foreign body reaction composed of macrophages. The foreign body reaction was most exuberant at 4 weeks, decreased gradually, and became slight at 12 weeks (Figs 4A–4C and 5A–5C). In contrast, PEUU experienced cellular ingrowth consisting of macrophages and fibroblasts. Macrophage infiltration, which was verified with CD68 immunostaining (results not shown), was mild throughout the course. The fibroblasts proliferated in the PEUU patch and were active in synthesizing collagen. The PEUU patches at 12 weeks were almost completely absorbed (Figs 4D–4F, 5D–5F) by the putative actions of hydrolysis and phagocytosis. Histologic assessment is summarized in Table 1.
Fig 5.
Masson trichrome staining of sections indicating collagen (blue), fibrous cells (red), and nuclei (black) for expanded polytetrafluoroethylene at (A) 4 weeks, (B) 8 weeks, and (C) 12 weeks, and polyester urethane urea (PEUU) at (D) 4 weeks, (E) 8 weeks, and (F) 12 weeks (all scale bars, 100 μm). (D–F) Cellular ingrowth into the PEUU scaffolds was consistent with macrophage and fibro-blast infiltration accompanied by collagen secretion.
Table 1.
Tissue Reaction to Implanted Materials
| Expanded Polytetrafluoroethylenea | Polyester Urethane Ureaa | |||||
|---|---|---|---|---|---|---|
| Weeks after implantation | 4 | 8 | 12 | 4 | 8 | 12 |
| Macrophages | +++ | ++ | ± | ++ | ++ | ++ |
| Neovascularization | +b | +b | +b | +b | +b | ++c |
| Cellular infiltration into material | – | – | – | + | ++ | +++ |
Fibrous capsule formation around the patches and endothelialization of the endocardial side were present for each group.
present only in transition part.
present in transition and endocardial part.
± to +++ = sporadic to severe; – = not present.
ENDOTHELIALIZATION
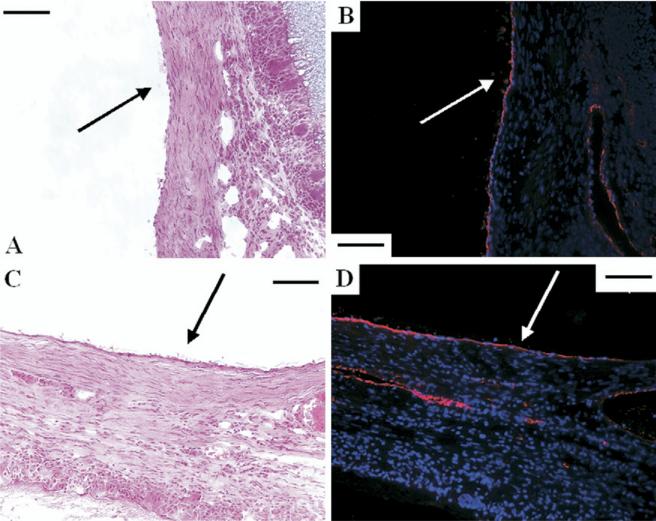
At each time point after implantation, all the patches had complete endothelialization on the endocardial surface in the RVOT free wall. There was no thrombus observed on the endocardial surface of the patches in either group at any time point (Fig 6).
Fig 6.
The endocardial surfaces (arrows) of (A, B) both expanded polytetrafluoroethylene and (C, D) polyester urethane urea were completely endothelialized at 4 weeks as evidenced by (A, C) hematoxylin and eosin and (B, D) immunohistochemical stain against vWF factor displaying endothelial cells (red) with nuclear staining in blue.
Comment
The results with the PEUU patch represent the first report on the implantation of this material as a step toward more extensive in vivo studies in the cardiovascular system. This material has theoretical advantages over nondegradable materials used in reconstructive cardiovascular procedures in that it seems capable of mechanically performing early in the implant period while allowing tissue ingrowth that takes over this mechanical role by 3 months in this model. As with other tissue-engineering approaches, complications associated with a permanent foreign body that is incapable of growth with the patient are avoided.
There are several other reports wherein scaffolds, with or without seeded cells, have been evaluated for cardiac tissue replacement. Most notable are the works of Hibino and colleagues [6] who have now reported on the clinical implementation of the tissue-engineering approach with seeded cells and a scaffold comprised of polylactic acid and polycaprolactone. Also notable are the reports of Sakai and colleagues [5], who specifically investigated replacement of the rat RVOT with both synthetic and natural scaffolding materials and whose experimental approach served as a guide for this work [7]. Potential advantages of the PEUU material over polyester and protein scaffolds used in these earlier investigations are the elastic properties inherent to the biodegradable polyurethane as well as the ability to alter the polyurethane chemistry to prescribe the degradation rate and susceptibility to enzymatic degradation [8]. The PEUU may also be variably processed to control scaffold porosity, to incorporate proteins such as growth factors for controlled release, and to create microfibrous scaffolds incorporating cells into an elastic tissue construct.
Several limitations to this report should be mentioned. First, this short-term study in the rat model, while appropriate for an initial study, did not allow evaluation of the material in a growing animal model, nor was long-term dilation resistance evaluated. Scaling effects in moving to a larger animal could become an issue as the stresses to which the patch would be exposed increase. We also did not evaluate the effect of seeding the PEUU patch with cells, which may lead to more rapid cellularization of the scaffold. However, from a clinical perspective, avoiding cell seeding while maintaining an attractive result would be preferred.
In conclusion, this report presents the first in vivo evaluation of a recently described biodegradable and elastic polymer, PEUU, which may ultimately find application in the reconstruction of cardiovascular tissues. A porous PEUU patch replacing a full-wall thickness RVOT defect was replaced during a 12-week period with fibrous tissue, had endocardial endothelialization at 4 weeks, and had a moderate inflammatory reaction with no evidence of acute thrombosis. Further studies of longer implant duration, in a variety of cardiovascular sites, and in larger animals seem to be warranted.
Disclosures and Freedom of Investigation
This work was supported by the National Institutes of Health (HL #069368). The polyester urethane urea material was synthesized and processed by the authors, who also had full control of the study design, methods used, outcome measurements, data analysis, and production of the written report.
Footnotes
Disclaimer
The Society of Thoracic Surgeons, the Southern Thoracic Surgical Association, and The Annals of Thoracic Surgery neither endorse nor discourage use of the new technology described in this article.
References
- 1.Jonas RA, Freed MD, Mayer JE, Jr, Castaneda AR. Long-term follow-up of patients with synthetic right heart conduits. Circulation. 1985;72:II77–83. [PubMed] [Google Scholar]
- 2.Tang GH, Fazel S, Weisel RD, Van Arsdell GS, Li RK. Cardiovascular tissue engineering therapy: so near, so far? Ann Thorac Surg. 2005;79:1831–3. doi: 10.1016/j.athoracsur.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 3.Guan J, Sacks MS, Beckman EJ, Wagner WR. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J Biomed Mater Res. 2002;61:493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 4.Guan J, Fujimoto KL, Sacks MS, Wagner WR. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials. 2005;26:3961–71. doi: 10.1016/j.biomaterials.2004.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakai T, Li RK, Weisel RD, Mickle DA, Kim ET, Jia ZQ, Yau TM. The fate of a tissue-engineered cardiac graft in the right ventricular outflow tract of the rat. J Thorac Cardiovasc Surg. 2001;121:932–42. doi: 10.1067/mtc.2001.113600. [DOI] [PubMed] [Google Scholar]
- 6.Hibino N, Shin'oka T, Kurosawa H. Long-term histologic findings in pulmonary arteries reconstructed with autologous pericardium. N Engl J Med. 2003;348:865–7. doi: 10.1056/NEJM200302273480922. [DOI] [PubMed] [Google Scholar]
- 7.Ozawa T, Mickle DA, Weisel RD, et al. Optimal biomaterial for creation of autologous cardiac grafts. Circulation. 2002;106:I176–82. [PubMed] [Google Scholar]
- 8.Guan J, Wagner WR. Synthesis, characterization and cytocompatibility of polyurethaneurea elastomers with designed elastase sensitivity. Biomacromolecules. 2005;6:2833–42. doi: 10.1021/bm0503322. [DOI] [PMC free article] [PubMed] [Google Scholar]