Abstract
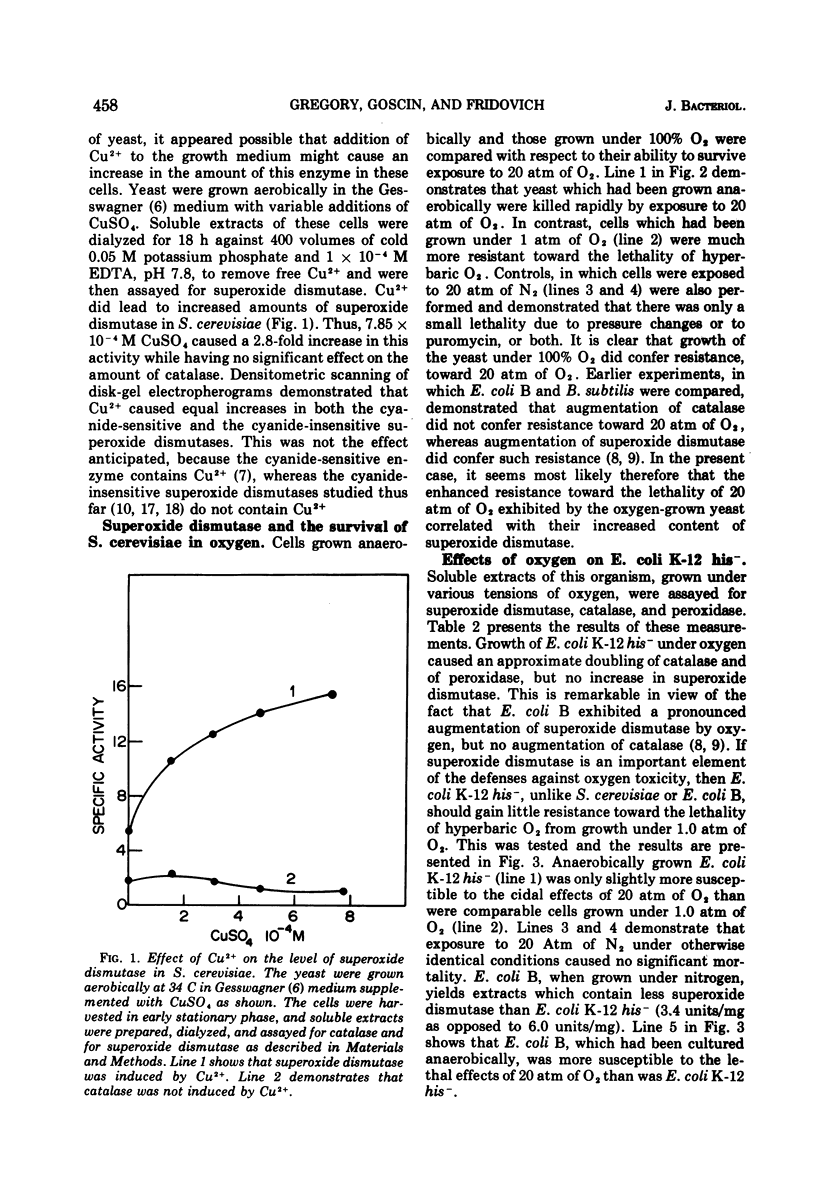
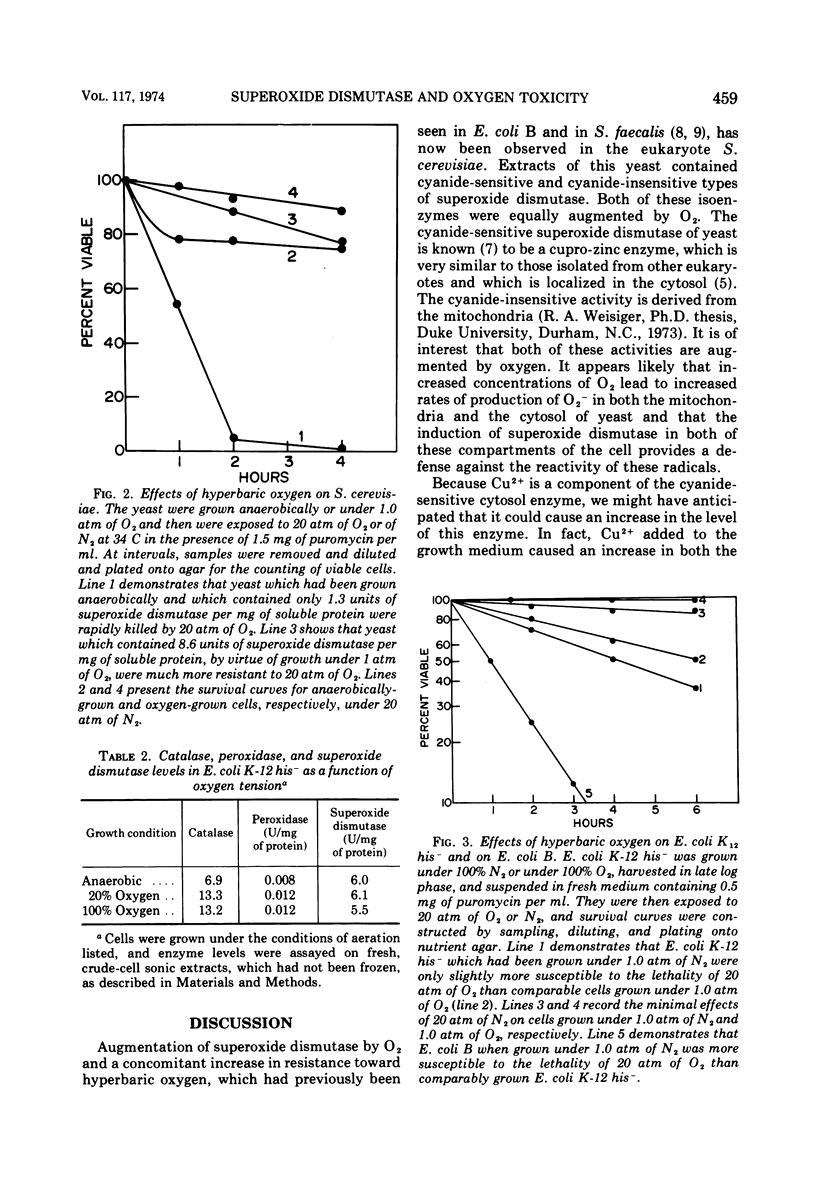
Saccharomyces cerevisiae var. ellipsoideus contained 6.5 times more superoxide dismutase and 2.3 times more catalase when grown under 100% O2 than when grown anaerobically. Growth under oxygen caused equal increases in both the cyanide-sensitive and the cyanide-insensitive superoxide dismutases of this organism. Experience with other eukaryotes has shown that cyanide sensitivity is a property of the cupro-zinc superoxide dismutase of the cytosol, whereas cyanide insensitivity is a property of the corresponding mangani-enzyme found in mitochondria. Cu2+, which has been shown to increase the radioresistance of yeast, also caused an increase of both of the superoxide dismutases of S. cerevisiae. Yeast which had been grown under 1 atm of O2 were more resistant toward the lethal effects of 20 atm of O2 than were yeast which had been grown in the absence of O2. Escherichia coli K-12 his− responded to growth under 1 atm of O2 by increasing its content of catalase and of peroxidase, but not of superoxide dismutase. This contrasts with E. coli B, which was previously shown to respond to O2 by a striking increase in superoxide dismutase. E. coli K-12 his− did not gain resistance toward 20 atm of O2 because of having been grown under 1 atm of O2. Once again, this contrasts with the behavior of E. coli B. These data indicate that, in both prokaryotes and in eukaryotes, superoxide dismutase is an important component of the defenses against oxygen toxicity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Beauchamp C. O., Fridovich I. Isozymes of superoxide dismutase from wheat germ. Biochim Biophys Acta. 1973 Jul 12;317(1):50–64. doi: 10.1016/0005-2795(73)90198-0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Gesswagner D., Altmann H., Szilvinyi A. V., Kaindl K. The relationship of Cu2+ content to the radioresistance of yeast. Int J Appl Radiat Isot. 1968 Feb;19(2):152–153. doi: 10.1016/0020-708x(68)90083-5. [DOI] [PubMed] [Google Scholar]
- Goscin S. A., Fridovich I. The purification and properties of superoxide dismutase from Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Dec 7;289(2):276–283. doi: 10.1016/0005-2744(72)90078-2. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973 May;114(2):543–548. doi: 10.1128/jb.114.2.543-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 1973 Jun;114(3):1193–1197. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- Koch A. L. Turbidity measurements of bacterial cultures in some available commercial instruments. Anal Biochem. 1970 Nov;38(1):252–259. doi: 10.1016/0003-2697(70)90174-0. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKS L. W., STARR P. R. A relationship between ergosterol and respiratory competency in yeast. J Cell Comp Physiol. 1963 Feb;61:61–65. doi: 10.1002/jcp.1030610107. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973 Jul 10;248(13):4793–4796. [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973 May 25;248(10):3582–3592. [PubMed] [Google Scholar]



