Abstract
Inflammatory signaling plays a key role in tumor progression, and the pleiotropic cytokine interleukin-6 (IL-6) is an important mediator of protumorigenic properties. Activation of the aryl hydrocarbon receptor (AHR) with exogenous ligands coupled with inflammatory signals can lead to synergistic induction of IL6 expression in tumor cells. Whether there are endogenous AHR ligands that can mediate IL6 production remains to be established. The indoleamine-2,3-dioxygenase pathway is a tryptophan oxidation pathway that is involved in controlling immune tolerance, which also aids in tumor escape. We screened the metabolites of this pathway for their ability to activate the AHR; results revealed that kynurenic acid (KA) is an efficient agonist for the human AHR. Structure-activity studies further indicate that the carboxylic acid group is required for significant agonist activity. KA is capable of inducing CYP1A1 messenger RNA levels in HepG2 cells and inducing CYP1A-mediated metabolism in primary human hepatocytes. In a human dioxin response element–driven stable reporter cell line, the EC25 was observed to be 104nM, while in a mouse stable reporter cell line, the EC25 was 10μM. AHR ligand competition binding assays revealed that KA is a ligand for the AHR. Treatment of MCF-7 cells with interleukin-1β and a physiologically relevant concentration of KA (e.g., 100nM) leads to induction of IL6 expression that is largely dependent on AHR expression. Our findings have established that KA is a potent AHR endogenous ligand that can induce IL6 production and xenobiotic metabolism in cells at physiologically relevant concentrations.
Keywords: aryl hydrocarbon receptor, kynurenic acid, IDO, TCDD, IL6
The aryl hydrocarbon receptor (AHR) is a member of the basic helix-loop-helix Per/ARNT/Sim family of transcription factors. The AHR is the only known member of this family that is a ligand-activated transcription factor, regulated through the binding of structurally diverse endogenous and exogenous ligands. The prototypic high-affinity ligand, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), mediates its toxicity through continual prolonged activation of the AHR. This hyperactivation of the AHR leads to a myriad of transcriptional responses resulting in a wide range of toxicities. AHR-mediated liver toxicity requires that the AHR bind to its cognate response element (Bunger et al., 2008). The AHR resides in the cytoplasm of the cell bound to a dimer of HSP90 and the X-associated protein 2 (Meyer et al., 1998; Perdew, 1988). Following ligand binding, the AHR translocates into the nucleus and dimerizes with ARNT. This heterodimer then binds to dioxin responsive elements (DRE), leading to altered gene expression. The most studied group of genes directly regulated by the AHR are involved in xenobiotic metabolism and includes CYP1A1, CYP1A2, and CYP1B1 (Beischlag et al., 2008). A number of other genes regulated by the AHR are involved in an array of distinct pathways. For example, the genes Snai 2, Ereg, and Cdkn1b are directly induced through AHR/ARNT binding to DRE sites. DNA microarray experiments that examine gene regulation following activation of the AHR reveal that, among the gene expression levels that are altered, a significant number of genes are both induced and repressed (Sato et al., 2008). Recently, the AHR has been shown to repress acute-phase gene expression in a DRE-independent manner, probably through protein-protein interactions (Patel et al., 2009). This observation underscores the complexity of gene regulation mediated by the AHR.
An important question that remains to be resolved is the identity of key endogenous ligands for the AHR. A number of endogenous compounds have been shown to be AHR ligands, including indirubin, bilirubin, 7-ketocholesterol, and canthaxanthin (for review, see Denison and Nagy, 2003). Recently, arachidonic acid derivatives, including 12(R)HETE, have been shown to activate the AHR (Chiaro et al., 2008a,b).Whether these compounds are present at the physiological levels that would lead to a significant amount of AHR activation remains unclear. While indirubin was originally identified in human urine as a potent activator of the AHR, its rapid metabolism leads to it only being present in urine at a low concentration of 0.2nM (Adachi et al., 2001). The other endogenous ligands mentioned above are relatively weak ligands and are not likely to significantly activate the AHR under normal physiological homeostasis. However, during jaundice, bilirubin levels are likely to be elevated sufficiently to activate the AHR in liver, resulting in increased metabolism (Phelan et al., 1998; Togawa et al., 2008).
We have recently observed that the combination of AHR ligands and an inflammatory signal leads to synergistic induction of interleukin-6 (IL-6) expression in MCF-7 cells (Hollingshead et al., 2008). While potent exogenous AHR ligands can mediate this activity, it has not been established whether there are endogenous AHR ligands at physiological levels that can similarly mediate IL6 induction in tumor cells. Our rationale for assessing tryptophan derivatives follows previous studies, which demonstrated that this amino acid may be a common precursor for the synthesis of human AHR ligands. A number of tryptophan metabolites and indole derivatives exhibit ligand-binding activity toward the human AHR, including indirubin and 6-formylindolo[3,2-b]carbazole (FICZ). Such products can elicit AHR signaling, as determined by induction of the AHR target gene CYP1A1. However, while detectable in humans, both indirubin and FICZ are generated in a spontaneous nonregulated fashion through acid condensation or as an ultraviolet-mediated photoproduct, respectively. Such unregulated synthesis is unlikely to be compatible with the coordinated physiological regulation of AHR signaling. Thus, we opted to examine endogenous tryptophan derivatives that arise from regulated synthesis and demonstrate physiological activity, which may correlate with AHR-dependent signaling. Products generated by the indoleamine-2,3-dioxygenase (IDO) pathway are consistent with this criteria. Tryptophan oxidation through the kynurenine pathway results in tryptophan depletion as well as the production of a number of metabolites, the biological activity of which are poorly understood (King and Thomas, 2007). The first step of this pathway is mediated by IDO or tryptophan 2,3-dioxygenase, resulting in kynurenine formation. IDO activation has been studied in the context of its role in T-cell function as it relates to tumor progression and tumor escape from immune surveillance during chronic inflammation (Lob and Konigsrainer, 2008; Muller et al., 2008). One metabolite of this pathway, kynurenic acid (KA), was found to be a ligand for the human AHR and was an efficient activator of IL6 production in MCF-7 cells. The discovery of KA as a physiologically relevant AHR ligand has wide ranging implications in determining how the AHR may be activated in various cell types.
MATERIALS AND METHODS
Reagents.
TCDD was a gift from Steve Safe (Texas A&M University, College Station, TX). All other chemicals used were obtained from Sigma (St Louis, MO) at the highest grade available, unless otherwise noted.
Cell culture.
HepG2 40/6 and H1L1.1c2 cells were maintained in alpha minimum essential medium, supplemented with 8% fetal bovine serum (Hyclone Laboratory, Logan, UT), 1000 U/ml penicillin, and 0.1 mg/ml streptomycin (Sigma). MCF-7 breast tumor cells were maintained as previously described (Hollingshead et al., 2008).
Primary human hepatocytes.
Female primary human hepatocytes from two different donors were obtained from the University of Pittsburgh, through the Liver Tissue Cell Distribution System, National Institutes of Health Contract #N01-DK-7-0004/HHSN267200700004C. Culture details have been reported previously (Olsavsky et al., 2007). Forty-eight hours following Matrigel additions, cells were exposed to ligands.
Stable reporter luciferase assay.
Assays were performed using the human hepatoma HepG2 40/6 and mouse hepatoma H1L1.1c2 stable reporter cell lines, as described previously (Chiaro et al., 2008b; Garrison et al., 1996). Each cell line had the DRE-driven reporter vector pGudLuc 6.1 or pGudLuc 1.1 stably integrated, respectively.
Ligand-binding assay and transgenic mice.
AHR ligand–binding assays were performed as described previously, as was the development of the AHRTtrCreAlbAhrfx/fx mouse model from which liver cytosol was used as a source for human AHR (Flaveny et al., 2009). In this mouse line, the mouse AHR has been deleted from hepatocytes, and the human AHR is expressed from the transgene driven by a hepatocyte-specific promoter.
RNA isolation and real-time PCR.
Real-time reverse transcriptase-PCR was performed as previously described (Chiaro et al., 2008b). Primers used for real-time RT-PCR analysis are listed in Supplementary table 1.
Gene silencing.
AHR protein levels were decreased using the Dharmacon On-Target plus small interfering RNA (siRNA) oligo (Dharmacon; J004990-07). Electroporation was performed using the Amaxa nucleofection system. Briefly, cells were washed and suspended at a concentration of 3 × 106 per 100 μl of nucleofection solution. The siRNA was added to the sample for a final concentration of 2μM. Cells were electroporated using the manufacturer’s high efficiency MCF-7 program and plated into six-well plates.
Protein electrophoresis and immunoblot analysis.
Whole-cell extracts were prepared and resolved by tricine-SDS-polyacrylamide gel electrophoresis and transferred to membrane. Immunoblotting was performed as previously described (Hollingshead et al., 2004).
Cytochrome P450 activity assay.
The P450-Glo CYP1A1 Assay was purchased from Promega Corporation (Madison, WI). Primary human hepatocytes were grown in 12-well plates. KA, 1nM TCDD, or carrier solvent was added to each well (0.1% dimethyl sulfoxide [DMSO]). Following 18 h of treatment, cells were washed once with PBS and fresh media, containing luciferin-CEE reagent (1:100 dilution), was added to the wells (500 μl per well). Cells were incubated for an additional 20 h before luciferase readings were performed. Samples were normalized for total protein concentration.
Electrophoretic mobility shift assay.
Electrophoretic mobility shift assays (EMSAs) were performed as previously described (Chiaro et al., 2008a). Briefly, pCI-hAHR, pcDNA3-mAHR, or pCI-ARNT were in vitro translated with the TNT-coupled rabbit reticulocyte lysate sytem (Promega Corporation) as described by the manufacturer, except with the addition of 1.5mM sodium molybdate. AHR and ARNT (4 μl each) were combined in the presence of 1.5 μl of HEDG buffer (25mM 4-(2-hydroxethyl)-1-piperazineethane-sulfonic acid, 1mM EDTA, 10mM sodium molybdate, and 10% glycerol, pH 7.5) along with the indicated treatments for 30 min at room temperature. Then, 32P-labeled DRE-containing probe was added to each reaction and incubated for an additional 15 min. Samples were resolved on 6% DNA retardation gels (Invitrogen) and visualized by autoradiography.
Statistical analysis.
Treatments were compared to control values. One-way ANOVA was followed by Tukey’s multiple comparison test to compare samples for all analyses, including luciferase reporter assays and qRT-PCR using GraphPad Prism v5.0.
RESULTS
Screening of Tryptophan Oxidation Products Reveals that KA Can Activate AHR-Mediated Transcription
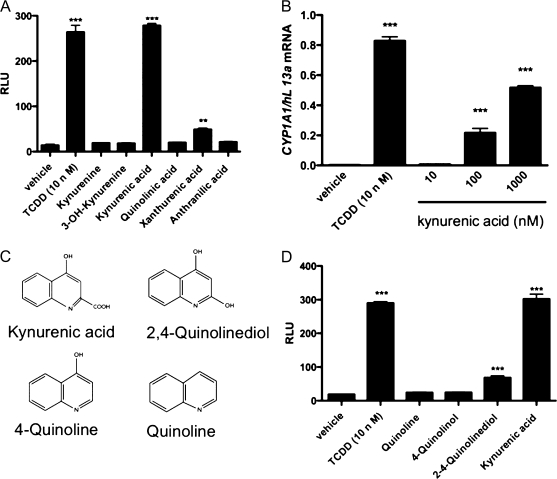
Recent evidence has revealed that the human AHR is capable of binding indirubin with high affinity (Flaveny et al., 2009). This result has led to screening of tryptophan oxidation products generated through the IDO pathway for their potential to activate the human AHR (Supplementary fig. 1). Six products of the IDO pathway were tested in a DRE-driven luciferase reporter stable cell line HepG2 40/6; results showed that of which 10μM xanthurenic acid and 10μM KA exhibited a significant increase in reporter activity (Fig. 1A). In comparison, 10nM TCDD provides the maximal level of induction that we have observed with an AHR ligand. Considering that KA is more potent and apparently of greater physiological significance, we continued to characterize the role of KA in AHR activation. The ability of KA to alter transcription of an AHR target gene was examined in HepG2 cells, and it was found that 100nM KA induced CYP1A1 mRNA levels ∼66-fold (Fig. 1B). These results suggest that KA is the primary metabolite in the IDO pathway that can activate the AHR.
FIG. 1.
KA is the primary tryptophan oxidation product that exhibits potent AHR agonist activity. (A) TCDD and the primary products of the IDO pathway were tested at 10μM for their ability after 4 h to activate the AHR in HepG2 40/6 reporter cell line. (B) Induction of CYP1A1 mRNA levels in HepG2 cells after 3-h exposure to increasing concentrations of KA. (C) Structures of the compounds utilized to determine the structural requirements necessary to mediate AHR activity. (D) Luciferase reporter assays were used to test the ability of quinoline and several derivatives at 10μM to activate the AHR in HepG2 40/6 cells. Experiments were performed in triplicate; error bars denote SD. ** or ***Significance at p < 0.01 or p < 0.001, as compared to respective control-treated samples.
Structure-Activity Analysis of KA-Mediated Activation of the AHR
The structural feature(s) of KA that is responsible for AHR activation were assessed utilizing a series of compounds shown in Figure 1C. These compounds were tested for their ability to activate AHR-mediated transcriptional activity in the HepG2 40/6 reporter cell line (Fig. 1D). The results reveal that the carboxylic acid moiety is critical to obtaining maximal transcriptional activity. The concept that a polar charged group is required for efficient KA binding to the AHR goes against the dogma in the AHR field established over the past 20 years. In general, investigators had demonstrated that planar hydrophobic neutral polycyclic structures were ideal attributes of a high-affinity AHR ligand.
KA Increases CYP1A1/2-Mediated Metabolism
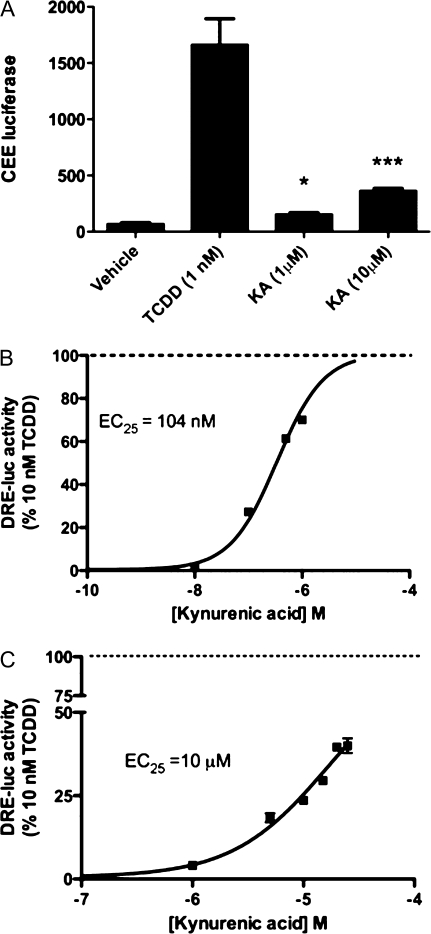
The ability of KA to induce CYP1A1/2 protein production and subsequent enzymatic activity was examined in primary human hepatocytes (Fig. 2A). A significant increase in CYP1A1/2 metabolism was observed at 1 and 10μM KA, confirming that KA exposure can lead to altered xenobiotic metabolism through AHR activation. This experiment was repeated with hepatocytes from another individual and essentially the same data were obtained. The relatively high level of KA needed to observe CYP1A1/2 metabolic activity is probably due to the 18-h exposure time frame utilized and the ability of hepatocytes to metabolize KA. In addition, whether KA might directly inhibit CYP1A1/2 activity has not been tested, but this experiment definitely established that KA can induce CYP1A1/2 metabolic activity.
FIG. 2.
KA induces CYP1A1/2 metabolism and differentially activates the human AHR relative to the mouse AHR in reporter cell lines. (A) CYP1A1/2 metabolic activity was measured by CEE luciferase in primary human hepatocytes treated with KA, TCDD, or DMSO for 18 h. Experiment was performed in triplicate; error bars denote SD. * or ***Significance at p < 0.05 or p < 0.001, as compared to respective control-treated samples. (B) HepG2 40/6 and (C) H1L1.1c2 cell lines were exposed to increasing concentrations of KA for 4 h. The EC25 values are defined as the dose of KA that yields 25% of the luciferase activity obtained with 10nM TCDD.
KA Selectively Activates the Human AHR and Fails to Activate CAR or PXR
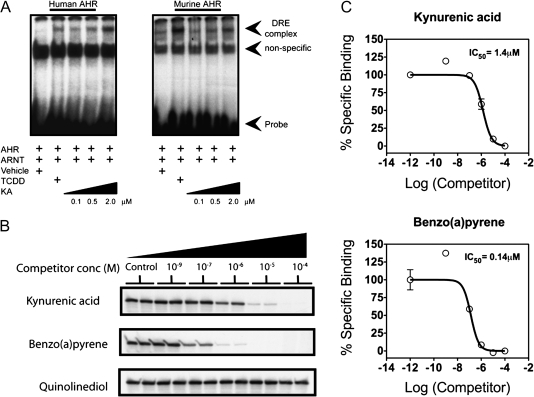
The ability of KA to induce AHR-mediated transcriptional activity in a species- and dose-dependent manner was examined in HepG2 40/6 and H1L1.1c2 reporter cell lines. KA induced considerable transcriptional activity at 1μM in the HepG2 40/6 reporter cell line (Fig. 2B). In contrast, KA only induced a modest level of transcriptional activity in the mouse H1L1.1c2, even at 25μM (Fig. 2C). Each dose-response curve was expressed as percentage of activity relative to 10nM TCDD, which represents maximal transcriptional activity. For comparative purposes, a concentration that yields 25% of the maximal response was calculated (EC25). KA was able to induce AHR-mediated transcriptional activity in a human cell line at a 100-fold lower concentration compared to the mouse cell line. To test whether this response is due to the ability of KA to directly activate the AHR, EMSAs were performed utilizing mouse and human AHR expressed in reticulocyte lysate. It is important to note that the mouse and human AHR exhibit their own unique stability and transformation potential; for this reason, only a comparison relative to a high dose of TCDD was made with each receptor. KA was able to more efficiently transform the human AHR, relative to the response obtained from TCDD exposure, while the opposite was observed with the mouse AHR (Fig. 3A). These results support the overall conclusion that the human AHR is selectively activated by KA. The ability of KA to activate other xenobiotic receptors was also tested, and KA failed to transcriptionally activate CAR or PXR (Supplementary fig. 2).
FIG. 3.
KA selectively induces human AHR/ARNT binding to its cognate response element and binds directly to the ligand-binding pocket of the AHR. (A) In vitro translated AHR and ARNT were mixed and treated with ligand as indicated and then subjected to an EMSA analysis. (B) Photoaffinity ligand competition assays were performed with increasing concentrations of ligands and KA and benzo(a)pyrene; both effectively reduced radioligand binding. (C) Quantitative graphic illustration of the results from panel B. The IC50 is defined as the concentration of ligand that results in 50% reduction of photoaffinity ligand binding to the human AHR.
KA Is a Direct AHR Ligand
To establish that KA is an AHR activator that binds to its ligand-binding pocket, a radioligand competition assay was performed. The photoaffinity ligand, 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin, was added at a saturating concentration to liver cytosol isolated from human AHR-expressing mice. Increasing concentrations of KA, benzo(a)pyrene, or quinolinediol were added to test for their ability to block photoaffinity ligand binding to the human AHR. Both benzo(a)pyrene and KA were able to completely block binding of the photoaffinity ligand to the AHR (Figs. 3B and 3C). In contrast, quinolinediol largely failed to compete with the photoaffinity ligand. This is consistent with the weak agonist activity exhibited by quinolinediol seen in Figure 1 and further underscores the importance of the carboxylic acid group on KA and its role in mediating binding to the human AHR.
KA and Interleukin-1β Synergistically Induce IL6 Expression and Require AHR Expression
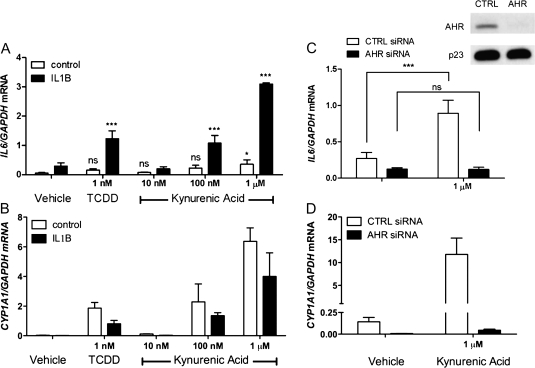
Previously, we have shown that the combination of an exogenous AHR ligand (e.g., TCDD, benzo(a)pyrene) and interleukin-1β (IL-1B) leads to synergistic induction of IL6 secretion in MCF-7 or ECC-1 tumor cell lines (Hollingshead et al., 2008). Also, CYP1A1 induction by an AHR agonist is inhibited by IL1B treatment. Therefore, the ability of KA in the presence of IL1B to induce IL6 in MCF-7 cells was examined. Induction of CYP1A1 expression, which is almost entirely dependent on AHR-mediated activity, was compared to induction of IL6 expression. Both genes were induced to significant levels by 100nM KA treatments in the presence of IL1B (Figs. 4A and 4B), while treatment of MCF-7 cells with 1μM KA significantly induced IL6 regardless of IL1B exposure. Assessment of combinational treatment pairs for synergistic IL6 induction using two-way ANOVA was performed on sample replicates, with or without IL1B and with or without TCDD or KA, as described (Slinker, 1998). A p value <0.05 for the interaction of the two treatments was defined as statistical synergy as random sampling of either treatment alone is not able to account for the increase when the treatments are combined. Thus, the combination of KA and IL1B treatment of MCF-7 cells synergistically induced IL6. TCDD at 1nM yielded maximal induction of IL6 production in the presence of IL1B and was used as a reference point. An siRNA knockdown approach was used to establish that the observed increase in CYP1A1 and IL6 mRNA is mediated by the AHR after KA treatment (Figs. 4C and 4D). The Western blot in Figure 4C indicates that an almost total repression of AHR expression was achieved with siRNA targeting AHR expression. Overall, these results indicate that IL6 mRNA expression is highly dependent on AHR expression. Furthermore, KA could play an important role in mediating IL6 production in tumors through human AHR activation.
FIG. 4.
KA and IL1B synergistically induce IL6 gene expression and requires AHR expression. (A and B) MCF-7 cells were treated with increasing doses of KA for 2 h with or without IL1B and subjected to quantitative real-time PCR for IL6 and CYP1A1. (C) MCF-7 cells were electroporated with control or AHR siRNA and analyzed 48 h later after a 3-h exposure to IL1B in the presence or absence of KA. Western blot analyses of whole-cell extract, AHR, and p23 protein levels were assessed. (C and D) Real-time PCR analysis was performed for IL6 and CYP1A1 following 2-h KA treatment 48 h after electroporation. Experiments were performed in triplicate; error bars denote SD. * or ***Significance at p < 0.05 or p < 0.001, as compared to respective control-treated samples. Statistical analysis in panel A was performed relative to their respective vehicle controls.
DISCUSSION
Since its initial identification and characterization as the dioxin receptor, regulator of phase I/II metabolism, and mediator of xenobiotic toxicity, studies have demonstrated the increasingly pleiotropic nature of the AHR. The AHR is an evolutionary conserved factor, expressed from less complex invertebrates through to mammals (Beischlag et al., 2008). Interestingly, invertebrate AHR is incapable of binding xenobiotic ligands, substantiating the long-held belief that the AHR contributes to physiological processes, which extend beyond its extensively characterized function as a xenobiotic sensor and suggest physiological function(s) in the absence of exogenous ligand. The first experimental indications that the mammalian AHR integrates into extrametabolic physiological roles were gleaned from null rodent models. Ahr knockout mice are largely resistant to the well-characterized toxic effects of xenobiotic AHR ligands yet exhibit profound physiological abnormalities, including reduced body weight, limited fecundity, immune dysfunction, and aberrant vascular development. Such observations clearly indicate that the AHR contributes to normal physiological development and functions independently of exogenous ligands. Subsequent studies have expanded upon these observations to reveal that the AHR directly influences hormonal signaling, adipocyte differentiation, inflammatory acute-phase signaling, hematopoiesis, and immune cell programming (Ho and Steinman, 2008; Patel et al., 2009; Singh et al., 2009). Of particular interest has been the identification that AHR is intimately involved in the progression of CD4+ T cells into both anti-inflammatory TReg and proinflammatory TH17 cells (Veldhoen et al., 2008, 2009). In addition, a role for the AHR in attenuating the macrophage inflammatory response to lipopolysaccharide challenge has been established in mice (Sekine et al., 2009). Such data have important physiological implications, indicating a pivotal role for AHR in immune homeostasis. Indeed, evidence exists that deregulation of AHR signaling, resulting from diminished or sustained AHR activity, can result in or exacerbate autoimmune pathologies, such as diabetes and cancer (Ishimaru et al., 2009; Poland et al., 1982; Remillard and Bunce, 2002).
In mammals, it is widely held that the overwhelming majority of AHR-mediated effects occur in response to ligand binding rather than in a ligand-independent manner. However, no physiologically relevant endogenous ligand for the AHR has been unequivocally identified. Consequently, the physiological activity associated with AHR has been inferred from studies utilizing potentially toxic xenobiotic AHR ligands. The use of these ligands may reflect biological adaptations to a toxic compound rather than highlight a physiological function of AHR. Unless endogenous AHR ligands can be identified, the true physiological activity of AHR will remain a matter for debate. The search for endogenous AHR ligands has typically relied on the utilization of murine AHR. AHR expressed by the Ahrb allele exhibits a 10-fold higher affinity for classical ligands relative to its human counterpart, the Ahrd allele. Additionally, murine AHR is inherently more stable than human AHR due to a stronger association with its obligate chaperone complex (Petrulis and Perdew, 2002). These characteristics allow the use of [3H]TCDD in direct ligand competition assays. The use of [3H]TCDD competition binding studies with the human AHR are difficult to perform and usually require the use of sucrose density gradients due to the 10-fold lower affinity for TCDD (Roberts et al., 1990). The photoaffinity ligand, 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin, overcomes such issues and allows the binding of putative human AHR ligands to be assessed at equilibrium (Ramadoss and Perdew, 2004). Utilizing this approach, we have examined a number of tryptophan derivatives in an effort to identify physiologically relevant endogenous human AHR ligands.
The data presented here indicate that two products formed through the IDO pathway have AHR ligand–binding capacity. The observation that KA, XA, and other tryptophan derivatives in general exhibit AHR-binding potential is somewhat surprising. Indeed, such findings challenge established dogma associated with the physical and chemical nature of AHR ligands. Despite the absence of a crystal structure profiling the ligand-binding domain of the AHR and any large-scale coordinated structure-activity relationships, a number of general characteristics have been assigned to compounds exhibiting high-affinity AHR binding. Based on past extensive empirical evidence, high-affinity AHR ligands have been shown to be neutrally charged, nonpolar, planar polycyclic aromatic hydrocarbons with molecular weights in the range of 270–325 (e.g., benzo(a)pyrene, TCDD). In contrast, KA has a molecular weight of 189 and is a carboxylic acid. Clearly, tryptophan derivatives such as, KA, XA, indirubin, or FICZ do not comfortably fit within this established profile, yet interestingly, they demonstrate relatively potent human AHR activity. These observations suggest that bona fide endogenous AHR ligands may be structurally and chemically atypical when compared to high-affinity xenobiotics, such as TCDD. The relative differences in binding affinity exhibited by xenobiotic and endogenous tryptophan ligands suggest that high-affinity xenobiotics likely excessively compete with their endogenous counterparts to mediate toxicity.
While performing a human versus mouse comparison with regard to the binding characteristics of these newly identified ligands, we observed an atypical binding profile. Mouse AHR generally exhibits a higher affinity for ligands than does the human AHR (Ramadoss and Perdew, 2004); however, in the case of KA, this situation is reversed. Furthermore, this reversal is reflected at the level of receptor transformation and transactivation potential, as assessed by gel shift and AHR-dependent reporter assays. The atypical binding/activity profile displayed by KA is interesting and not without precedent since indirubin exhibits a similar profile with higher affinity and activity in the human context (Flaveny et al., 2009). The nature of this reversal implies that a divergence has occurred across species, with humans adapting to alternate endogenous AHR ligands. Such observations may indicate that endogenous ligands may elicit species-dependent effects. Additionally, the species-dependent toxic effects of xenobiotic AHR ligands, usually attributed to differences in affinity, may actually be more profound and fundamental. Despite a relatively high degree of sequence homology, a species comparison between mouse and human AHR has revealed that their differences in agonist-mediated activity are likely at least in part due to either differential coactivator recruitment or a more generalized difference in coactivator/AHR affinity (Flaveny et al., 2008).
Having established that KA and XA represent endogenous AHR ligands, what are the physiological consequences of AHR activation by these ligands? The IDO pathway plays a central role in immune tolerance through localized depletion of tryptophan and the production of metabolites, with the capacity to inhibit T-cell responses (Bauer et al., 2005). Inflammatory cytokines generated in response to infection have been demonstrated to induce IDO activity in dendritic cells, resulting in the polarization of naïve CD4+ T cells into anti-inflammatory TReg cells and immune tolerance (Cherayil, 2009). Interestingly, TCDD has been shown to induce IDO expression in dendritic cells, providing a link between AHR and IDO (Vogel et al., 2008). Similarly, the differentiation of TReg cells has been revealed to have an AHR-dependent component, although it has not been established whether AHR ligands generated by the IDO pathway are involved. Additional evidence for the involvement of AHR in immune tolerance has been demonstrated with VAF347, a compound with anti-inflammatory properties (Lawrence et al., 2008). Acting as an AHR ligand, VAF347 has the capacity to influence dendritic and T-cell function, resulting in an elevated level of immune tolerance and subsequently leading to diminished allograft rejection. Interestingly, the survival and propagation of tumor cells is believed to rely, in part, on the induction of immune tolerance. How tumor cells escape immune surveillance is likely to involve multiple mechanisms, but we may speculate that the interplay between AHR and IDO could be a contributing factor. Tumor aggressiveness correlates well with excessive IL6 secretion by cancerous cells, what is even more intriguing, IL6 has been revealed to synergistically induce dendritic IDO expression in concert with other cytokines (Fujigaki et al., 2006). Consequently, levels of IDO-derived products, including KA, may become elevated above the normal low micromolar concentrations found in humans. It is noteworthy that, based on our observations, such concentrations would be expected to activate human AHR but not mouse AHR. Interestingly, we have previously demonstrated that AHR activated by xenobiotic ligands can synergize with proinflammatory cytokines to mediate enhanced IL6 expression and secretion by tumor cells (Hollingshead et al., 2008). The data presented here reveal that this synergy can also be evoked through activation of human AHR with KA. Thus, we can envision the formation of a positive feedback loop in which tumor-derived IL6 elevates dendritic IDO to promote a higher state of immune tolerance. Elevated IDO expression would generate higher localized levels of KA, which in turn could activate tumor cell AHR to facilitate IL6 expression and thus complete the feedback loop, allowing tumor cells to escape immune surveillance and propagate.
Here, we provide evidence that tryptophan derivatives generated by the IDO pathway represent endogenous activators of the human AHR. Furthermore, these products, KA and XA, are direct ligands of the AHR, with the capacity to stimulate AHR-dependent gene expression at physiologically attainable concentrations, as evidenced by cell reporter– and competitive ligand–binding assays. KA has been detected in the colon of pigs and rats at 1.5–16μM, while in humans, KA has been detected at 0.8μM in bile (Kuc et al., 2008; Paluszkiewicz et al., 2009). The level of KA in normal human serum is 31nM, while in patients with late stage kidney disease, serum levels are as high as 5μM (Schefold et al., 2009). These observations, coupled with data presented here, suggest that the human AHR would be activated by KA in certain tissues, such as the lining of the colon, in dendritic cells, and perhaps systemically in kidney disease patients. Moreover, the agonist potential of KA and XA appears to be species specific, demonstrating a greater degree of efficacy with the human AHR when compared to its murine homologue. Such observations challenge established dogma associated with the physical and chemical nature of AHR ligands. Furthermore, we propose that activation of human AHR by KA and XA may facilitate selective pressure, allowing tumor cells secreting high levels of IL6 in response to AHR activation to escape immune surveillance mechanisms.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
The National Institutes of Health (ES04869 to G.H.P, GM066411 to C.J.O.); Dow Chemical Company.
Supplementary Material
Acknowledgments
The authors thank Steve Safe and Michael Denison for providing TCDD and H1L1.1c2 cells, respectively. We thank Marcia Perdew for editorial assistance.
References
- Adachi J, Mori Y, Matsui S, Takigami H, Fujino J, Kitagawa H, Miller CA, III, Kato T, Saeki K, Matsuda T. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J. Biol. Chem. 2001;276:31475–31478. doi: 10.1074/jbc.C100238200. [DOI] [PubMed] [Google Scholar]
- Bauer TM, Jiga LP, Chuang JJ, Randazzo M, Opelz G, Terness P. Studying the immunosuppressive role of indoleamine 2,3-dioxygenase: tryptophan metabolites suppress rat allogeneic T-cell responses in vitro and in vivo. Transpl. Int. 2005;18:95–100. doi: 10.1111/j.1432-2277.2004.00031.x. [DOI] [PubMed] [Google Scholar]
- Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 2008;18:207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger MK, Glover E, Moran SM, Walisser JA, Lahvis GP, Hsu EL, Bradfield CA. Abnormal liver development and resistance to 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in mice carrying a mutation in the DNA-binding domain of the aryl hydrocarbon receptor. Toxicol. Sci. 2008;106:83–92. doi: 10.1093/toxsci/kfn149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherayil BJ. Indoleamine 2,3-dioxygenase in intestinal immunity and inflammation. Inflamm. Bowel Dis. 2009;15:1391–1396. doi: 10.1002/ibd.20910. [DOI] [PubMed] [Google Scholar]
- Chiaro CR, Morales JL, Prabhu KS, Perdew GH. Leukotriene A4 metabolites are endogenous ligands for the Ah receptor. Biochemistry. 2008a;47:8445–8455. doi: 10.1021/bi800712f. [DOI] [PubMed] [Google Scholar]
- Chiaro CR, Patel RD, Perdew GH. 12(R)-Hydroxy-5(Z),8(Z),10(E),14(Z)-eicosatetraenoic acid [12(R)-HETE], an arachidonic acid derivative, is an activator of the aryl hydrocarbon receptor. Mol. Pharmacol. 2008b;74:1649–1656. doi: 10.1124/mol.108.049379. [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Flaveny C, Reen RK, Kusnadi A, Perdew GH. The mouse and human Ah receptor differ in recognition of LXXLL motifs. Arch. Biochem. Biophys. 2008;471:215–223. doi: 10.1016/j.abb.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaveny CA, Murray IA, Chiaro CR, Perdew GH. Ligand selectivity and gene regulation by the human aryl hydrocarbon receptor in transgenic mice. Mol. Pharmacol. 2009;75:1412–1420. doi: 10.1124/mol.109.054825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, Seishima M. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J. Biochem. 2006;139:655–662. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- Garrison PM, Tullis K, Aarts JM, Brouwer A, Giesy JP, Denison MS. Species-specific recombinant cell lines as bioassay systems for the detection of 2,3,7,8-tetrachlorodibenzo-p-dioxin-like chemicals. Fundam. Appl. Toxicol. 1996;30:194–203. doi: 10.1006/faat.1996.0056. [DOI] [PubMed] [Google Scholar]
- Ho PP, Steinman L. The aryl hydrocarbon receptor: a regulator of Th17 and Treg cell development in disease. Cell Res. 2008;18:605–608. doi: 10.1038/cr.2008.63. [DOI] [PubMed] [Google Scholar]
- Hollingshead BD, Beischlag TV, Dinatale BC, Ramadoss P, Perdew GH. Inflammatory signaling and aryl hydrocarbon receptor mediate synergistic induction of interleukin-6 in MCF-7 cells. Cancer Res. 2008;68:3609–3617. doi: 10.1158/0008-5472.CAN-07-6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead BD, Petrulis JR, Perdew GH. The aryl hydrocarbon (Ah) receptor transcriptional regulator hepatitis B virus X-associated protein 2 antagonizes p23 binding to Ah receptor-Hsp90 complexes and is dispensable for receptor function. J. Biol. Chem. 2004;279:45652–45661. doi: 10.1074/jbc.M407840200. [DOI] [PubMed] [Google Scholar]
- Ishimaru N, Takagi A, Kohashi M, Yamada A, Arakaki R, Kanno J, Hayashi Y. Neonatal exposure to low-dose 2,3,7,8-tetrachlorodibenzo-p-dioxin causes autoimmunity due to the disruption of T cell tolerance. J. Immunol. 2009;182:6576–6586. doi: 10.4049/jimmunol.0802289. [DOI] [PubMed] [Google Scholar]
- King NJ, Thomas SR. Molecules in focus: indoleamine 2,3-dioxygenase. Int. J. Biochem. Cell Biol. 2007;39:2167–2172. doi: 10.1016/j.biocel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Kuc D, Zgrajka W, Parada-Turska J, Urbanik-Sypniewska T, Turski WA. Micromolar concentration of kynurenic acid in rat small intestine. Amino Acids. 2008;35:503–505. doi: 10.1007/s00726-007-0631-z. [DOI] [PubMed] [Google Scholar]
- Lawrence BP, Denison MS, Novak H, Vorderstrasse BA, Harrer N, Neruda W, Reichel C, Woisetschlager M. Activation of the aryl hydrocarbon receptor is essential for mediating the anti-inflammatory effects of a novel low-molecular-weight compound. Blood. 2008;112:1158–1165. doi: 10.1182/blood-2007-08-109645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lob S, Konigsrainer A. Is IDO a key enzyme bridging the gap between tumor escape and tolerance induction? Langenbecks Arch. Surg. 2008;393:995–1003. doi: 10.1007/s00423-007-0245-7. [DOI] [PubMed] [Google Scholar]
- Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH. Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol. Cell. Biol. 1998;18:978–988. doi: 10.1128/mcb.18.2.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AJ, Sharma MD, Chandler PR, Duhadaway JB, Everhart ME, Johnson BA, III, Kahler DJ, Pihkala J, Soler AP, Munn DH, et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc. Natl. Acad. Sci. U.S.A. 2008;105:17073–17078. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsavsky KM, Page JL, Johnson MC, Zarbl H, Strom SC, Omiecinski CJ. Gene expression profiling and differentiation assessment in primary human hepatocyte cultures, established hepatoma cell lines, and human liver tissues. Toxicol. Appl. Pharmacol. 2007;222:42–56. doi: 10.1016/j.taap.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluszkiewicz P, Zgrajka W, Saran T, Schabowski J, Piedra JL, Fedkiv O, Rengman S, Pierzynowski SG, Turski WA. High concentration of kynurenic acid in bile and pancreatic juice. Amino Acids. 2009;37:637–641. doi: 10.1007/s00726-008-0183-x. [DOI] [PubMed] [Google Scholar]
- Patel RD, Murray IA, Flaveny CA, Kusnadi A, Perdew GH. Ah receptor represses acute-phase response gene expression without binding to its cognate response element. Lab. Invest. 2009;89:695–707. doi: 10.1038/labinvest.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew GH. Association of the Ah receptor with the 90-kDa heat shock protein. J. Biol. Chem. 1988;263:13802–13805. [PubMed] [Google Scholar]
- Petrulis JR, Perdew GH. The role of chaperone proteins in the aryl hydrocarbon receptor core complex. Chem. Biol. Interact. 2002;141:25–40. doi: 10.1016/s0009-2797(02)00064-9. [DOI] [PubMed] [Google Scholar]
- Phelan D, Winter GM, Rogers WJ, Lam JC, Denison MS. Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch. Biochem. Biophys. 1998;357:155–163. doi: 10.1006/abbi.1998.0814. [DOI] [PubMed] [Google Scholar]
- Poland A, Palen D, Glover E. Tumour promotion by TCDD in skin of HRS/J hairless mice. Nature. 1982;300:271–273. doi: 10.1038/300271a0. [DOI] [PubMed] [Google Scholar]
- Ramadoss P, Perdew GH. Use of 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin as a probe to determine the relative ligand affinity of human versus mouse aryl hydrocarbon receptor in cultured cells. Mol. Pharmacol. 2004;66:129–136. doi: 10.1124/mol.66.1.129. [DOI] [PubMed] [Google Scholar]
- Remillard RB, Bunce NJ. Linking dioxins to diabetes: epidemiology and biologic plausibility. Environ. Health Perspect. 2002;110:853–858. doi: 10.1289/ehp.02110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EA, Johnson KC, Harper PA, Okey AB. Characterization of the Ah receptor mediating aryl hydrocarbon hydroxylase induction in the human liver cell line HepG2. Arch. Biochem. Biophys. 1990;276:442–450. doi: 10.1016/0003-9861(90)90743-i. [DOI] [PubMed] [Google Scholar]
- Sato S, Shirakawa H, Tomita S, Ohsaki Y, Haketa K, Tooi O, Santo N, Tohkin M, Furukawa Y, Gonzalez FJ, et al. Low-dose dioxins alter gene expression related to cholesterol biosynthesis, lipogenesis, and glucose metabolism through the aryl hydrocarbon receptor-mediated pathway in mouse liver. Toxicol. Appl. Pharmacol. 2008;229:10–19. doi: 10.1016/j.taap.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Schefold JC, Zeden JP, Fotopoulou C, von Haehling S, Pschowski R, Hasper D, Volk HD, Schuett C, Reinke P. Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: a possible link between chronic inflammation and uraemic symptoms. Nephrol. Dial. Transplant. 2009;24:1901–1908. doi: 10.1093/ndt/gfn739. [DOI] [PubMed] [Google Scholar]
- Sekine H, Mimura J, Oshima M, Okawa H, Kanno J, Igarashi K, Gonzalez FJ, Ikuta T, Kawajiri K, Fujii-Kuriyama Y. Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol. Cell. Biol. 2009;29:6391–6400. doi: 10.1128/MCB.00337-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KP, Wyman A, Casado FL, Garrett RW, Gasiewicz TA. Treatment of mice with the Ah receptor agonist and human carcinogen dioxin results in altered numbers and function of hematopoietic stem cells. Carcinogenesis. 2009;30:11–19. doi: 10.1093/carcin/bgn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slinker BK. The statistics of synergism. J. Mol. Cell. Cardiol. 1998;30:723–731. doi: 10.1006/jmcc.1998.0655. [DOI] [PubMed] [Google Scholar]
- Togawa H, Shinkai S, Mizutani T. Induction of human UGT1A1 by bilirubin through AhR dependent pathway. Drug Metab. Lett. 2008;2:231–237. doi: 10.2174/187231208786734120. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J. Exp. Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 2008;375:331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.