Abstract
Alcohol-induced liver injury (ALI) has been associated with, among other molecular changes, abnormal hepatic methionine metabolism, resulting in decreased levels of S-adenosylmethionine (SAM). Dietary methyl donor supplements such as SAM and betaine mitigate ALI in animal models; however, the mechanisms of protection remain elusive. It has been suggested that methyl donors may act via attenuation of alcohol-induced oxidative stress. We hypothesized that the protective action of methyl donors is mediated by an effect on the oxidative metabolism of alcohol in the liver. Male C57BL/6J mice were administered a control high-fat diet or diet enriched in methyl donors with or without alcohol for 4 weeks using the enteral alcohol feeding model. As expected, attenuation of ALI and an increase in reduced glutathione:oxidized glutathione ratio were achieved with methyl donor supplementation. Interestingly, methyl donors led to a 35% increase in blood alcohol elimination rate, and while there was no effect on alcohol metabolism in the stomach, a profound effect on liver alcohol metabolism was observed. The catalase-dependent pathway of alcohol metabolism was induced, yet the increase in CYP2E1 activity by alcohol was blunted, which may be mitigating production of oxidants. Additional factors contributing to the protective effects of methyl donors in ALI were increased activity of low- and high-Km aldehyde dehydrogenases leading to lower hepatic acetaldehyde, maintenance of the efficient mitochondrial energy metabolism, and promotion of peroxisomal β-oxidation. Profound changes in alcohol metabolism represent additional important mechanism of the protective effect of methyl donors in ALI.
Keywords: acetaldehyde, catalase, cytochrome P4502E1, S-adenosylmethionine, peroxisome proliferator–activated receptor alpha
Hepatic methionine metabolism, also referred to as one-carbon metabolism, is critical for a number of vital liver functions. One of the most important roles is the production of S-adenosylmethionine (SAM), the principal methyl donor required for the methylation of DNA, RNA, proteins, and phospholipids and as a precursor for polyamine and reduced glutathione (GSH) synthesis. Increasing evidence has shown that deficiencies or impairment of this pathway can contribute to the pathogenesis and progression of liver disease (Mato and Lu, 2007). Experimental animal models using diets deficient in methyl donors, such as methionine or choline, result in the spontaneous development of nonalcoholic steatohepatitis (Oz et al., 2008) and hepatocellular carcinoma (HCC) (Shivapurkar and Poirier, 1983). Interestingly, the methionine adenosyltransferase (Mat1a) knockout mouse model that results in the chronic depletion of SAM and the glycine N-methyltransferase (Gnmt) knockout mouse model that results in the overproduction of SAM (Luka et al., 2006) both promote steatohepatitis (Lu et al., 2001; Mato and Lu, 2007) and HCC (Martinez-Chantar et al., 2002; Tseng et al., 2003). These observations suggest that SAM is tightly regulated and should be well balanced in order to maintain liver physiological function.
Aberrant hepatic methionine metabolism is commonly observed in patients with alcoholic liver disease. Both decreases in SAM and folate (Cravo et al., 1996) and elevations in homocysteine (de la Vega et al., 2001) in liver or serum samples have been reported. This condition is multifactorial in origin and results not only from poor nutrition but also due to the deleterious effects of alcohol on intestinal absorption and hepatic uptake of essential amino acids and B vitamins necessary to support one-carbon metabolism (Halsted et al., 1973). Moreover, the oxidative metabolism of alcohol resulting in changes in the hepatic redox state shifts the cycle away from recycling methionine for SAM production to synthesizing the antioxidant glutathione.
The use of dietary methyl donor supplementation in patients with alcoholic cirrhosis has produced mixed results (Mato et al., 1999; Vendemiale et al., 1989). Only one clinical study reported improved survival with long-term dietary supplementation with SAM alone but only among those with less advanced liver disease (Mato et al., 1999). Thus, better understanding of the mechanisms underlying the linkages between one-carbon metabolism and alcohol-induced liver injury (ALI), especially the protective effects of dietary supplementation with methyl donors, is needed. In this study, unlike previous works to date that have employed single nutritional supplements, we have used a combination of methionine and its metabolites (choline, betaine, and folate), as well as cofactors (vitamins B6, B12, and zinc), to fortify one-carbon metabolism in the liver. We report that the protective effects are mediated via profound changes in alcohol metabolism and describe the mechanistic underpinnings of this effect.
MATERIALS AND METHODS
Animals, diets, and treatment.
Male C57BL/6J mice (8 weeks old; Jackson Laboratory, Bar Harbor, ME) were randomized into two groups and provided either a standard (Isopro RMH 3000; Lab Diet, Richmond, IN) or methyl-enriched (TD.01310; Harlan-Teklad, Madison, WI) rodent chow for 1 week prior to and immediately following surgical intragastric intubation until the intragastric feeding was initiated (Kono et al., 2000). Following surgery, mice were housed in individual metabolic cages and allowed a week to recover with ad libitum access to food and water. Following the recovery period, mice were administered via gastric cannula high-fat diets (HFDs) prepared as detailed in Thompson and Reitz (1978), which contained either maltose-dextrin (isocaloric control, referred to as “control”) or ethyl alcohol (referred to as “alcohol”) with or without methyl donor enrichment (added per kilogram diet: 15 g betaine, 13.2 g choline chloride, 13.6 mg folic acid, 7.5 g L-methionine, 0.13 mg vitamin B12, and 648 mg zinc sulfate). The high-fat (corn oil–based) diet is necessary to facilitate the development of steatohepatitis in this animal model of ALI (Nanji et al., 1989). Alcohol was delivered initially at 17.3 g/kg/day and was gradually increased to 1.3 g/kg every 2 days until day 8. The dose was then incrementally adjusted 1 g/kg every 4 days until the dose reached 27 g/kg/day. 5-bromo-2′-deoxyuridine (BrdU) was administered in the diet (0.02 ml/g) the last 3 days of the experiment. Mice were monitored four times daily throughout the study and sacrificed after 28 days of treatment. There were five to seven mice per experimental group at the end of these experiments. In parallel experiments, which assessed alcohol elimination rates, mice were either provided a standard or methyl-enriched rodent chow (see above) for 2 weeks and administered a single bolus (1.5 g/kg) of alcohol by gavage in saline vehicle (20% dilution) or treated with alcohol in a HFD (with or without methyl donor supplementation) via gastric cannulation exactly as described above for 8 days. In both experiments, tail vein blood samples were collected at 30-min intervals for 2.5 h after alcohol dosing. All animals were given humane care in compliance with National Institutes of Health guidelines, and this work was approved by the institutional animal care and use committee.
Histopathology.
Formalin-fixed paraffin-embedded liver or kidney sections (6 μm) were stained with hematoxylin/eosin (H&E) or periodic acid Schiff (PAS) stain, respectively, for light microscopy evaluation of liver or kidney pathology. H&E-stained slides were scored as described by Nanji et al. (1989) as follows: steatosis (the percentage of hepatocytes containing fat): < 25% = 1+, < 50% = 2+, <75% = 3+, and > 75% = 4+; inflammation and necrosis: one focus per low-power field = 1+ and two or more foci = 2+. PAS-stained slides were scored as follows: +, slight and focal lesions (less than 30% of area effected); 2+, moderate and diffuse lesions (30–70% effected); and 3+, severe and massive lesions (more than 70% effected).
Immunohistochemical detection of BrdU.
Formalin-fixed paraffin-embedded liver sections (6 μm) were mounted on glass slides. Sections were deparaffinized and rehydrated, and immunodetection was performed using DAKO EnVision System HRP (DakoCytomation, Carpinteria, CA) with primary anti-BrdU monoclonal antibody (clone Bu20a; DAKO) diluted in PBS containing 1% bovine serum albumin and incubated overnight at 4°C. Quantitative analysis was performed by averaging percent positively stained nuclei to total nuclei within 10 random fields at ×200.
Clinical chemistry.
Alcohol and acetaldehyde concentrations in serum, urine, or liver tissue homogenates were determined as described elsewhere (Bergmeyer, 1988). Serum alanine aminotransferase (ALT) levels were determined spectrophotometrically with the Thermo Scientific Infinity ALT Liquid stable reagent (Thermo Electron, Melbourne, Australia).
Determination of aminothiols.
The content of one-carbon metabolites in liver tissue extracts was determined using high-performance liquid chromatography with coulometric electrochemical detection as previously described (Trasler et al., 2003).
Protein level and activity measurements.
Cytosol, membrane, mitochondrial, and nuclear proteins were obtained by subcellular fractionation of liver tissue using the Qproteome Cell Compartment Kit (Qiagen, Valencia, CA). Proteins were resolved using 4–12% Bis-Tris gradient gel electrophoresis (Invitrogen, Carlsbad, CA) and transferred to 0.45-μm polyvinylidene difluoride membranes (Invitrogen) by electroblotting. Membranes were blocked in 5% nonfat dry milk in PBS-T (0.1% Tween 20 in PBS); probed with 1 μg/ml anti-alcohol dehydrogenase (ADH) (Abcam, Cambridge, MA), 1:1000 anti-Cyp2e1 (Biomol, Plymouth Meeting, PA), 0.25 μg/ml anti-catalase (Calbiochem, San Diego, CA), 0.5 μg/ml anti-aldehyde dehydrogenase (ALDH)1 (Calbiochem), 1 μg/ml anti-ALDH2 (Santa Cruz Biotechnology, Santa Cruz, CA), or 1 μg/ml anti-peroxisome proliferator–activated receptor (PPAR)α (Abcam); and incubated overnight at 4°C. Blots were then washed in Tris-Buffered Saline Tween-20, incubated with the appropriate secondary antibody, and detected using chemiluminescence Western blotting analysis kit (Amersham, Pittsburgh, PA). Experiments were repeated two to three times, and representative blots are shown. The “ADH activity” assay was modified from a method described elsewhere (Crow et al., 1977) for 96-well plate format. Enzyme activity was determined based on the linear decline in absorbance at 340 nm resulting from the reduction of nicotinamide adenine dinucleotide (NAD+) by ADH per milligram protein. “Cyp2e1 activity” was measured by the rate of hydroxylation of p-nitrophenol to p-nitrocatechol by isolated hepatic microsomes (Koop, 1986). The “peroxidatic function of catalase” was measured using the catalase kit (Cayman Chemical Company, Ann Arbor, MI) according to manufacturer’s protocol. The activity of the peroxisomal enzyme, “acyl-CoA oxidase,” was measured as previously described (Inestrosa et al., 1979) and modified for 96-well format. To measure “ALDH activity,” liver or kidney S9 fractions were diluted in buffer containing 50mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 8.4) and 0.33 mM dithiothreitol. For high-Km ALDH activity (ALDH1), fractions were added to a reaction mixture containing 0.1mM pyrazole, 5 mM acetaldehyde, and 0.5 mM NAD+ in 50 mM sodium pyrophosphate buffer (pH 8.8). Samples for low-Km ALDH (ALDH2) were sonicated for 10 s prior to analysis. ALDH2 activity was measured as described for ALDH1 except 0.1 mM acetaldehyde, and the addition of 2 μM rotenone was used. Activity was determined by monitoring the reduction of NAD+ by ALDH at 340 nm. Chymotrypsin-like activity or trypsin-like “activity of the proteasome” was determined with fluorogenic synthetic peptides, Suc-LLVY-AMC, or LSTR-AMC, respectively (Donohue et al., 2004).
Hepatic acetaldehyde content.
Levels of acetaldehyde were determined in liver supernatants as described in Bergmeyer (1988) and modified for use in a 96-well plate format. Samples of liver supernatant were incubated for 1 h in buffer containing 1M KCl, 0.5M Tris (pH 7.0), 47mM 2-mercaptoethanol, 1.5mM NAD+, and 0.13 mg/ml ALDH (Sigma-Aldrich) in a ratio of 10:1 buffer:sample or standard. The reduction of NAD+ by ALDH was measured at 340 nm. Acetaldehyde concentrations were determined from a standard curve of known amounts of acetaldehyde (0–89μM) and were normalized to protein content.
Nuclear magnetic resonance–based serum metabolite analysis.
To each 40 μl aliquot of serum, 60 μl of 200mM formate and 500 μl H2O with 0.02% NaN3 was added and transferred to 5-mm nuclear magnetic resonance (NMR) tubes. The formate was added as a chemical shift reagent as well as internal standard with a final concentration of 20mM. NMR spectra were acquired on a Varian Inova 400 MHz (Varian, Inc., Palo Alto, CA) as detailed previously (Bradford et al., 2008). The bins containing alcohol were removed from all spectra, and bins were renormalized. Profiling of the metabolites was carried out using the Chenomx NMR metabolite database (Chenomx Inc., Edmonton, Canada). Confirmation of assignments was aided by two-dimensional (1H-13C) experiments on selected samples. Statistical analysis was performed using SimcaP+ v.11.5 (Umetrics, Umea, Sweden).
RESULTS
Methyl Donor–Enriched Diet Attenuates Alcohol-Induced Steatohepatitis
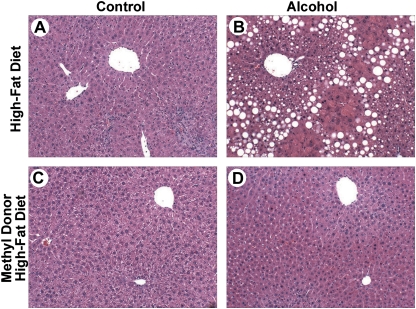
All mice gained weight throughout the study; however, mice on methyl donor–enriched diets gained ∼10% less weight than those in high-fat control group (Table 1, Supplementary fig. 1). Increased liver-to-body weight ratios were also observed in HFD + alcohol group, of which a fatty liver and increased hepatocyte proliferation (Table 1) are likely contributing factors. Liver-to-body weight ratios and proliferation rates in the methyl donor high-fat diet (MDHFD) + alcohol group were not different from controls. Mild inflammation was observed in livers of mice of all groups (Fig. 1, Table 1). Pronounced steatohepatitis, as evidenced by macrovesicular and microvesicular steatosis, necrosis, and inflammation (Fig. 1B, Table 1), was observed in liver sections from mice in the HFD + alcohol group. In sharp contrast, mice in the MDHFD + alcohol group showed remarkably reduced appearance of fatty liver and no signs of necrosis (Fig. 1D, Table 1). No appreciable kidney toxicity was observed in this study (Supplementary table 3).
TABLE 1.
Effect of Methyl Donor–Enriched Diet on Routine Clinical Parameters
| HFD |
MDHFD |
|||
| Control | Alcohol | Control | Alcohol | |
| Steatosis score | 0 | 2.8 ± 0.3a,d | 0 | 0.9 ± 0.4 |
| Inflammation score | 1.7 ± 0.3 | 1.1 ± 0.1 | 1.5 ± 0.3 | 1.2 ± 0.3 |
| Necrosis score | 0.7 ± 0.4 | 1.7 ± 0.3d | 0 | 0 |
| Total pathology score | 2.3 ± 0.6 | 5.6 ± 0.5a,d | 1.5 ± 0.3 | 2.1 ± 0.4 |
| ALT (U/l) | 16 ± 4 | 76 ± 26 | 14 ± 2 | 28 ± 7 |
| BAC (mg/dl) | 24 ± 5 | 194 ± 61a,c | 30 ± 5 | 239 ± 36a,c |
| LAC (μg EtOH/μg protein) | 11 ± 1 | 22 ± 5a,c | 8 ± 1 | 17 ± 2a,c |
| UAC (mg/dl) | 23 ± 9 | 338 ± 102a,c | 20 ± 8 | 175 ± 44a,c |
| Mean daily UAC (mg/dl) | 23 ± 2 | 225 ± 34a,c,d | 16 ± 1 | 123 ± 17a,c |
| BAE rate (mg/kg/h) | ND | 395 ± 89 | ND | 535 ± 111b |
| Terminal body weight (g) | 28 ± 1 | 26 ± 1 | 25 ± 0a | 23 ± 1a,b |
| Terminal liver weight (g) | 1.9 ± 0.1 | 2.3 ± 0.1 | 1.5 ± 0.1a | 1.4 ± 0.0a,b |
| % Liver:body weight | 6.8 ± 0.5 | 8.6 ± 0.4a,c,d | 6.1 ± 0.1 | 6.0 ± 0.2 |
| Proliferation (% BrdU) | 0.1 ± 0.2 | 10.9 ± 5.4a | 3.6 ± 2.4 | 3.8 ± 0.7 |
Notes. Values given are mean ± SEM (n = 5–7). Statistical significance of means was determined using Mann-Whitney rank sum test for histopathology scores, while one-way ANOVA followed by Tukey’ post hoc test were used for all other measurements. Significance (p < 0.05) is indicated in bold and by an asterisk when compared to (a) HFD control, (b) HFD + alcohol, (c) MDHFD, or (d) MDHFD + alcohol. ALT, alanine aminotransferase; BAC, blood alcohol concentration; LAC, liver alcohol concentration; ND, not determined.
FIG. 1.
Attenuation of ALI in mice administered methyl donor–enriched HFD. Male C57BL/6J mice were administered a control HFD (A and B) or methyl donor–enriched HFD (C and D) with (B and D) or without (A and C) alcohol for 4 weeks using an intragastric feeding protocol. Representative photomicrographs of H&E-stained liver sections from mice administered various types of treatments as indicated; original magnification ×100.
Rate of Alcohol Metabolism Is Increased by a Methyl Donor–Enriched Diet
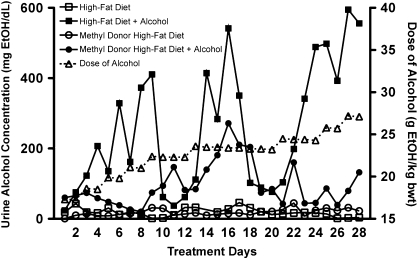
Rodents fed alcohol continuously exhibit a cyclic fluctuation in urine alcohol concentrations (UAC) due to diurnal variation in basal metabolism and hormones (Li et al., 2000). In animals receiving HFD + alcohol, cyclic oscillations in UAC occurred approximately every 8 days, with concentrations ranging between 50 and 600 mg/dl, despite constant steady increase in the dose of alcohol (Fig. 2). However, in animals receiving MDHFD + alcohol, the cyclic oscillation in UAC was profoundly blunted with peak and trough concentrations fluctuating only between 250 and 50 mg/dl. The net result of this was an approximately twofold reduction in the mean daily UAC even though blood and liver alcohol concentrations at sacrifice were high (Table 1). Since these data infer that the rate of alcohol metabolism is increased in methyl donor–supplemented animals, blood alcohol elimination (BAE) rates were determined by measuring blood alcohol concentration every hour over 6-h time period on day 8 of treatment as the first peak in UAC begins to fall. A 35% increase in BAE rate was observed in mice given MDHFD + alcohol over those receiving HFD + alcohol (Table 1).
FIG. 2.
Representative plots of daily UAC. Typical urine alcohol levels in mice administered different diets as indicated. The dose of alcohol administered over the 28 days of treatment is plotted on the right y-axis.
Methyl Donor–Enriched Diet Has No Effect on First-Pass Metabolism of Alcohol
Although many tissues can metabolize alcohol, considerable metabolism occurs in the liver and, to a lesser extent, in the stomach. The lack of liver injury along with reduced UAC and increase in BAE rate in mice given MDHFD + alcohol could lead one to speculate that increased metabolism of alcohol in the stomach prevented significant quantities of alcohol reaching the liver. Stomach catalase/D-amino acid oxidase pathway has been shown to be partially responsible for the protective action of glycine on ALI (Iimuro et al., 1996). To address the role of first-pass metabolism in the protection afforded by the methyl donor–enriched diet, we assessed the activity of stomach ADH and catalase in mice fed the diets for 28 days and observed no differences between control and methyl donor–enriched diet groups (Supplementary table 1). In addition, disposition of alcohol after a single acute dose was unaffected by methyl donors when mice were fed ad libitum with a pelleted control or methyl donor–enriched diet for 2 weeks followed by an oral bolus (1.5 g/kg) of alcohol (data not shown).
Methyl Donor–Enriched Diet Leads to Stimulation of the Catalase-Dependent Alcohol Metabolism in the Liver
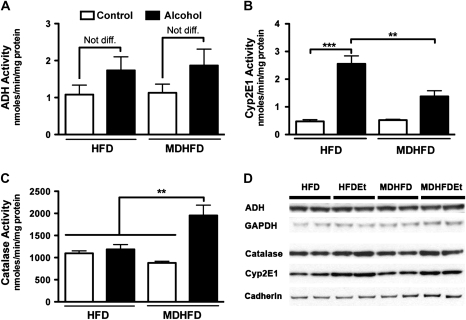
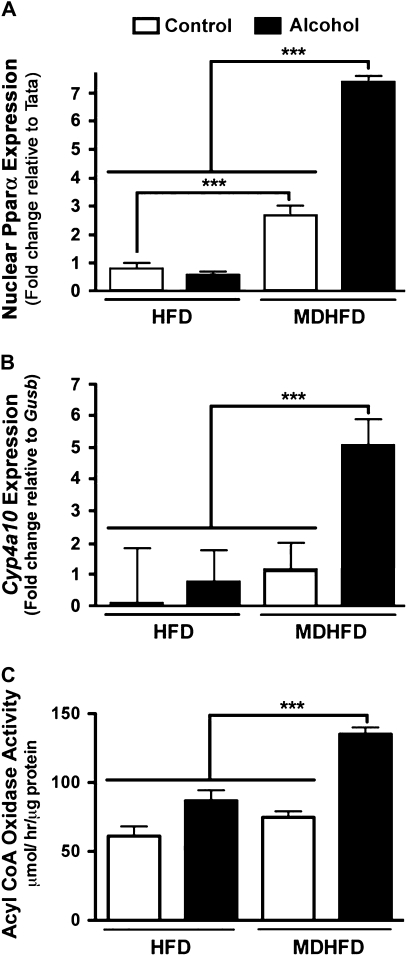
During moderate alcohol consumption, the majority of alcohol is metabolized to acetaldehyde by ADH in the liver, but under conditions of chronic high-dose exposures, a significant portion is metabolized by the catalase-peroxide complex and, to a lesser degree, Cyp2e1. Thus, hepatic activity of all three enzymes was measured in this study. ADH activity was found to be marginally, but not significantly, increased in both alcohol-treated groups, and no effect of the methyl donors was observed (Fig. 3A). While Cyp2e1 activity (Fig. 3B) and protein levels (Fig. 3D) were significantly elevated in both alcohol-fed groups, the induction in MDHFD + alcohol group was significantly lower than that in HFD + alcohol group. Interestingly, activity of catalase was increased significantly in mice given a MDHFD + alcohol compared to all other groups (Fig. 3C) even though protein levels did not change significantly (Fig. 3D). The activity of acyl-CoA oxidase, enzyme that provides hydrogen peroxide needed for catalase-dependent alcohol metabolism, was also significantly increased only in the MDHFD + alcohol group (Fig. 4C). Since acyl-CoA oxidase is controlled by PPARα, we also assessed liver (nuclear) protein levels of PPARα (Fig. 4A) and expression of CYP4A10 (Fig. 4B), a PPARα-controlled gene. All were induced significantly in the MDHFD + alcohol group.
FIG. 3.
Liver alcohol metabolism. Liver activity of (A) ADH, (B) Cyp2e1, and (C) catalase was determined in mice administered a control HFD or MDHFD with or without alcohol for 4 weeks. Asterisks indicate statistically significant (**p < 0.01 and ***p < 0.001) differences using one-way ANOVA followed by Tukey’s post hoc test. N = three to five animals per group. (D) Representative Western blots for ADH, catalase, and Cyp2e1. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and cadherin were used for cytosol and membrane protein loading controls, respectively.
FIG. 4.
Activation of PPARα pathway in mice administered methyl donor–enriched HFD and alcohol. (A) Fold change in liver levels (nuclear extracts) of PPARα in mice administered a control HFD or MDHFD with or without alcohol for 4 weeks. TATA expression was used as control. (B) Fold change in expression of Cyp4a10 was derived using 2ΔΔCt values and normalized to expression of the housekeeping gene Gusb. (C) Liver activity of acyl-CoA oxidase. Asterisks indicate statistically significant (***p < 0.001) differences using one-way ANOVA followed by Tukey’s post hoc test. N = three to five animals per group.
Methyl Donor–Enriched Diet Reduces Hepatic Acetaldehyde Content
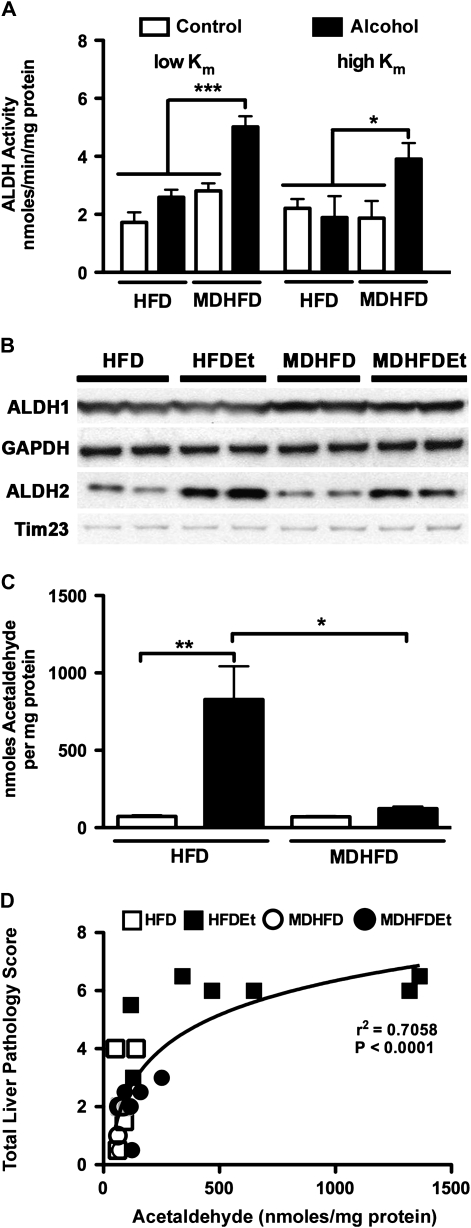
Alcohol metabolism results in the production of acetaldehyde, a reactive metabolite, which in turn is rapidly metabolized to acetate by ALDH. The activity of liver ALDH1 and 2 in the MDHFD + alcohol group was statistically different from all other groups (Fig. 5A), and protein levels for ALDH1 and ALDH2 were increased (Fig. 5B). Although liver ALDH2 protein content was also increased in mice given HFD + alcohol (Fig. 5B), it did not result in an increase in enzyme activity (Fig. 5A). Kidney activity of ALDH1 and 2 was much lower than that in the liver, and little effect of the methyl donor supplementation was observed (Supplementary fig. 2). Striking differences in hepatic acetaldehyde content were also observed between two alcohol-fed groups. Levels in mice given HFD + alcohol were eightfold higher than those receiving MDHFD + alcohol (Fig. 5C). Hepatic acetaldehyde content was determined to also strongly correlate with liver injury (Fig. 5D).
FIG. 5.
Liver acetaldehyde content is reduced by methyl donor–enriched diet following subchronic alcohol exposure. Activity (A) and protein content (B) for ALDH1 and 2 were assessed in mice administered a control HFD or MDHFD with or without alcohol for 4 weeks. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and Tim23 were used as cytosol and mitochondrial loading controls, respectively. (C) Acetaldehyde content in liver. (D) Semi-log correlation plot of acetaldehyde content with total pathology score (Spearman r2 = 0.71). Asterisks indicate statistically significant (*p < 0.05 and **p < 0.01) differences using one-way ANOVA followed by Tukey’s post hoc test.
Effect of MDHFD on Hepatic One-Carbon Metabolism
Methyl donor–enriched diets were formulated to provide substantially increased amounts of cofactors (Cooney, 1993). Indeed, in both MDHFD and MDHFD + alcohol–treated mice, hepatic levels of SAM were significantly increased by 10- to 20-fold, respectively (Table 2). SAM:S-adenosylhomocysteine (SAH) ratios, an indicator of transmethylation potential, were also considerably improved fivefold to sevenfold. Homocysteine, a metabolic by-product of SAM methylation reactions, is typically methylated to regenerate methionine but during conditions of oxidative stress is diverted for GSH production. HFD + alcohol treatment reduced the levels of GSH and raised the levels of oxidized glutathione (GSSG) while reducing the GSH:GSSG ratio, an index of overall oxidant stress. MDHFD + alcohol treatment did not increase GSH but reduced the levels of GSSG.
TABLE 2.
Effect of Methyl Donor–Enriched Diet on Hepatic One-Carbon Metabolism
| HFD |
MDHFD |
||||
| Control | Alcohol | Control | Alcohol | ||
| Methionine metabolism | |||||
| Methionine | 1.0 ± 0.12 | 0.8 ± 0.05 | 5.0 ± 0.94 | 12.1 ± 2.95a,b,c | |
| SAM | 0.3 ± 0.05 | 0.1 ± 0.02 | 2.2 ± 0.41a,b | 4.3 ± 0.27a,b,c | |
| SAH | 0.2 ± 0.03 | 0.2 ± 0.02 | 0.5 ± 0.07a,b | 0.6 ± 0.03a,b | |
| SAM:SAH | 1.4 ± 0.19 | 0.8 ± 0.14 | 5.3 ± 1.51a,b | 7.1 ± 0.52a,b | |
| Adenosine | 0.7 ± 0.04 | 0.6 ± 0.07 | 0.5 ± 0.06 | 0.5 ± 0.02 | |
| Homocysteine | 0.2 ± 0.02 | 0.2 ± 0.01 | 0.1 ± 0.01 | 0.2 ± 0.01 | |
| Glutathione biosynthesis | |||||
| Cysteine | 11 ± 1.1 | 9 ± 1.1 | 8 ± 0.5 | 9 ± 0.2 | |
| Glu-Cys | 0.12 ± 0.02 | 0.23 ± 0.03 | 0.12 ± 0.02 | 0.13 ± 0.02 | |
| GSH | 84 ± 3 | 42 ± 4a,c | 73 ± 8 | 60 ± 2 | |
| GSSG | 0.49 ± 0.12 | 0.82 ± 0.10a,c,d | 0.28 ± 0.09 | 0.22 ± 0.02 | |
| GSH:GSSG | 191 ± 43 | 55 ± 12c,d | 321 ± 92 | 277 ± 36 | |
| Cys-Gly | 1.2 ± 0.09c,d | 1.2 ± 0.11c,d | 0.23 ± 0.03 | 0.41 ± 0.05 | |
Notes. Metabolite concentrations (nanomoles per milligram protein) were measured in liver tissue homogenates using coulometric electrochemical high-performance liquid chromatography detection as described in Materials and Methods section. Values given are the mean ± SEM from (n = 5–7 mice). Significance of the difference in means (p < 0.05, one-way ANOVA followed by Tukey’s post hoc test) is indicated by bold font and by an asterisk when compared to (a) HFD control, (b) HFD + alcohol, (c) MDHFD, or (d) MDHFD + alcohol. Cys-Gly, cysteinylglycine; Glu-Cys, glutaminecysteine.
Methyl Donors Affect Cellular Energy Metabolism
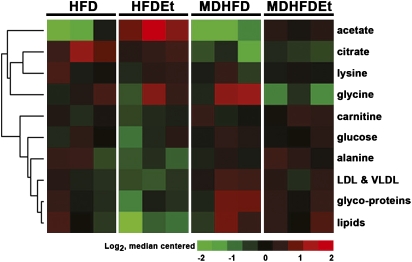
Analysis of the serum metabolome determined that feeding a methyl donor–enriched diet results in changes in carbohydrate and lipid metabolism following alcohol treatment (Fig. 6). Levels of acetate and citrate were reduced in mice given MDHFD + alcohol compared to those receiving HFD + alcohol, a condition that would preferentially stimulate carbohydrate metabolism over fatty acid oxidation. Lipid metabolism, however, appeared to be stimulated in mice given MDHFD + alcohol as serum lipids and, in particular very low–density lipoprotein, were found to be elevated.
FIG. 6.
Alterations in serum metabolites by methyl donors and alcohol. Metabolites in serum from mice (three per group) given a control HFD or MDHFD with or without alcohol for 4 weeks. Heatmap of metabolites significantly different (one-way ANOVA, p < 0.05) between two alcohol-treated groups is shown. Values are log2 transformed and median centered and metabolites clustered using average linkage–centered correlation. A complete list of metabolite data is available in Supplementary table 2.
DISCUSSION
The association between decreased hepatic SAM content and the severity of alcoholic hepatitis in humans, a well-established phenomenon (Herbert et al., 1963), has prompted studies of methyl donors in a variety of animal models of ALI. Administration of diets deficient in methionine, choline, or folate or combinations thereof to rats, mice, or mini-pigs have been shown to exacerbate ALI (Gyamfi et al., 2008). To the contrary, dietary supplementation with SAM or betaine to alcohol-fed rats (Garcia-Ruiz et al., 1995) or baboons (Lieber et al., 1990) attenuated liver injury, restored GSH, and improved mitochondrial function. Several hypotheses have been proposed to explain the mechanism of protection offered by methyl donors, including attenuation of oxidative stress by restoring levels of GSH (Garcia-Ruiz et al., 1995), an increase in SAM:SAH ratio (Barak et al., 1993), and an attenuated inflammatory response (Song et al., 2007). It is likely that these mechanisms are interdependent, yet the causality and temporality are not well understood.
In this study, the expected attenuation of ALI (e.g., restoration of the liver glutathione balance and attenuation of liver injury phenotypes) by dietary methyl donor supplementation was achieved in a subchronic mouse model and known hallmarks of methyl donor’s protective effects (Purohit et al., 2007) were observed. While it is without a doubt that upregulation of the transsulfuration pathway, which leads to a decrease in oxidative stress via increased synthesis of glutathione, is a major contributor to protection, our data suggest a novel mechanism via the effect on alcohol metabolism in the liver.
Through observations of the daily changes in the urinary alcohol content, we detected an effect of the methyl donor–enriched diet on the oxidative metabolism of alcohol. Importantly, it has been reported that SAM accelerates the clearance of alcohol and acetaldehyde in humans (di Padova et al., 1984), but no mechanism for this effect was proposed. Thus, the phenotype studied here in the mouse model of ALI is relevant to clinical practice.
We found that a 35% increase in BAE rate in methyl donor–supplemented animals was due to changes in liver metabolism but not due to first-pass metabolism in the gut. Interestingly, the most pronounced effect of methyl donor supplementation was the induction of the catalase-dependent pathway since increased activity of both catalase and acyl-CoA oxidase, source of hydrogen peroxide necessary for catalase function, was observed.
Not only was alcohol metabolism enhanced through this route but also the activity of low- and high-Km ALDH was also increased, which created favorable conditions for fast and efficient catabolism of alcohol and lower liver levels of acetaldehyde, a highly cytotoxic molecule and a key mediator of liver injury by alcohol (Seitz and Stickel, 2007). The effect of methyl donor supplementation on alleviating the net levels of acetaldehyde produced during feeding with alcohol is a potentially important part of the protection against ALI. Indeed, in humans, SAM was shown to favor the elimination of alcohol without increasing blood levels of acetaldehyde (di Padova et al., 1984).
It is not known, however, how SAM and/or other methyl donors can activate catalase and acyl-CoA oxidase, a potential means through which the methyl donor–supplemented diet appears to exhibit its protective action against ALI in the mouse. One possible mechanism could be through epigenetic regulation as it was suggested that the degree of methylation of the CpG-rich promoter of the catalase gene may be important for regulation of its expression (Reimer et al., 1994). In addition, it is plausible that a higher order regulatory mechanism that may affect both catalase and acyl-CoA oxidase is involved. A potential key pathway may be through PPARα, which controls expression of both proteins of interest, since induction of Cyp4a10 was also observed in the same group. Indeed, we observed that methyl donor supplementation led to an increase in nuclear protein levels for PPARα. It was suggested that SAM may act through a putative RNA methyltransferase PIMT (PPAR-interacting protein with methyltransferase domain) to enhance the transcriptional activity of both PPARs and retinoid-X-receptors (Zhu et al., 2001). Since in our study the marked elevations in liver methionine and SAM were detected in livers of methyl donor–supplemented mice, in both control and alcohol-treated groups, it is plausible that this mechanism may be responsible for an increase in nuclear levels of PPARα.
Alcohol may impair PPARα-dependent signaling in the liver, which is an important factor in deregulation of lipid and overall metabolism, thus contributing to the development of ALI. Specifically, evidence exists that ALI is exacerbated in Pparα-null mice (Nakajima et al., 2004) and that PPARα agonists are protective against ALI (Fischer et al., 2003). Alcohol is also known to lead to a decrease in liver Pparα messenger RNA and protein as well as reduced expression of PPARα-controlled genes, such as acyl-CoA oxidase (Fischer et al., 2003; Lu et al., 2008). Our data suggest that methyl donors are able to block this deleterious effect of alcohol by activating PPARα and/or supporting peroxisomal and mitochondrial metabolism (see below), resulting in activation of catalase-dependent alcohol metabolism. It should be noted that the modest but significant activation of PPARα in methyl donor–enriched HFD group had no observable effect on alcohol metabolism, activity of acyl-CoA oxidase, or Cyp4a10 expression, which suggests that additional factors, such as increased stability of the enzymes (Horie and Suga, 1990), may be responsible for the overall protective effect.
We also observed that alcohol-induced activation of Cyp2e1, a protein that is known to contribute to liver oxidative stress (Lu and Cederbaum, 2008), was blunted by methyl donors. While SAM is known to be a weak inhibitor of Cyp2e1 (Caro and Cederbaum, 2005), the Ki for inhibition is relatively high, and it is not likely that a direct effect of SAM on Cyp2e1 was responsible for the effect observed here. Interestingly, a similar protective effect on Cyp2e1 activation by alcohol was reported recently with betaine (1% in drinking water for 2 weeks) supplementation (Kim et al., 2008). The increase in Cyp2e1 protein levels and activity in the liver following treatment with alcohol is due to reduced protein turnover by the ubiquitin-dependent proteasomal pathway (Morishima et al., 2005) and not due to increased transcription. While blunted activation of Cyp2e1 in mice given MDHFD + alcohol was associated with remarkably higher proteasome activity as compared to HFD + alcohol group, we did not observe an inhibition of proteasome with alcohol in this mouse model (data not shown). Nonetheless, a blunted increase in Cyp2e1 activity in methyl donor diet + alcohol group may be an important factor for alleviation of liver injury by way of prevention of the formation of cytotoxic reactive oxygen species and lipid peroxides. Pathogenesis of alcohol-induced hepatic steatosis and steatohepatitis is thought to be dependent on oxidative stress, whereby lipid peroxidation products facilitate the inflammatory response (Day and James, 1998).
The metabolomic analysis suggested that additional factors contributing to the protective effects of methyl donors in ALI may be the improved maintenance of the efficient mitochondrial energy metabolism and promotion of peroxisomal β-oxidation of fatty acids. It is known that folate is responsible for stimulating methyl transfer pathways in the cytosol and mitochondria (Depeint et al., 2006). Indeed, serum acetate, a key transmitochondrial membrane shuttle, was lower in the mice fed methyl donors and alcohol compared to alcohol group without supplementation, suggesting that mitochondrial function may be improved. In addition, methionine was found to be markedly elevated in serum of mice fed methyl donors and alcohol. Methionine and its metabolite carnitine play an important role in shuttling long-chain fatty acids into the mitochondrial matrix. Even though the elevation of serum carnitine in this group was not significant, it is evident that adequate levels were present in the liver to facilitate β-oxidation of fatty acids and dampen the untoward effects of alcohol on fatty acid metabolism. In view of our suggestion that a PPARα-related alcohol metabolism pathway may be induced by methyl donor supplementation, it is noteworthy that Makowski et al. (2009) demonstrated that PPARα is a regulator of carnitine levels and that Pparα-null mice have lower levels of carnitine and fasted ketone levels suggestive of lowered rates of β-oxidation.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (F32 AA016860, R01 AA016258, and P30 ES010126).
Supplementary Material
Acknowledgments
C.L.P. was recipient of Ruth L. Kirschstein National Service Award and the Leon and Bertha Goldberg Postdoctoral Fellowship in Toxicology (University of North Carolina).
References
- Barak AJ, Beckenhauer HC, Junnila M, Tuma DJ. Dietary betaine promotes generation of hepatic S-adenosylmethionine and protects the liver from ethanol-induced fatty infiltration. Alcohol Clin. Exp. Res. 1993;17:552–555. doi: 10.1111/j.1530-0277.1993.tb00798.x. [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU. Methods of Enzymatic Analysis. New York: Academic Press; 1988. [Google Scholar]
- Bradford BU, O'Connell TM, Han J, Kosyk O, Shymonyak S, Ross PK, Winnike J, Kono H, Rusyn I. Metabolomic profiling of a modified alcohol liquid diet model for liver injury in the mouse uncovers new markers of disease. Toxicol. Appl. Pharmacol. 2008;232:236–243. doi: 10.1016/j.taap.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. Inhibition of CYP2E1 catalytic activity in vitro by S-adenosyl-L-methionine. Biochem. Pharmacol. 2005;69:1081–1093. doi: 10.1016/j.bcp.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Cooney CA. Are somatic cells inherently deficient in methylation metabolism? A proposed mechanism for DNA methylation loss, senescence and aging. Growth Dev. Aging. 1993;57:261–273. [PubMed] [Google Scholar]
- Cravo ML, Gloria LM, Selhub J, Nadeau MR, Camilo ME, Resende MP, Cardoso JN, Leitao CN, Mira FC. Hyperhomocysteinemia in chronic alcoholism: correlation with folate, vitamin B-12, and vitamin B-6 status. Am. J. Clin. Nutr. 1996;63:220–224. doi: 10.1093/ajcn/63.2.220. [DOI] [PubMed] [Google Scholar]
- Crow KE, Cornell NW, Veech RL. The rate of ethanol metabolism in isolated rat hepatocytes. Alcohol Clin. Exp. Res. 1977;1:43–50. doi: 10.1111/j.1530-0277.1977.tb05765.x. [DOI] [PubMed] [Google Scholar]
- Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- de la Vega MJ, Santolaria F, Gonzalez-Reimers E, Aleman MR, Milena A, Martinez-Riera A, Gonzalez-Garcia C. High prevalence of hyperhomocysteinemia in chronic alcoholism: the importance of the thermolabile form of the enzyme methylenetetrahydrofolate reductase (MTHFR) Alcohol. 2001;25:59–67. doi: 10.1016/s0741-8329(01)00167-7. [DOI] [PubMed] [Google Scholar]
- Depeint F, Bruce WR, Shangari N, Mehta R, O'Brien PJ. Mitochondrial function and toxicity: role of B vitamins on the one-carbon transfer pathways. Chem. Biol. Interact. 2006;163:113–132. doi: 10.1016/j.cbi.2006.05.010. [DOI] [PubMed] [Google Scholar]
- di Padova C, Tritapepe R, Rovagnati P, Pozzoli M, Stramentinoli G. Decreased blood levels of ethanol and acetaldehyde by S-adenosyl-L-methionine in humans. Arch. Toxicol. Suppl. 1984;7:240–242. doi: 10.1007/978-3-642-69132-4_34. [DOI] [PubMed] [Google Scholar]
- Donohue TM, Jr, Kharbanda KK, Casey CA, Nanji AA. Decreased proteasome activity is associated with increased severity of liver pathology and oxidative stress in experimental alcoholic liver disease. Alcohol Clin. Exp. Res. 2004;28:1257–1263. doi: 10.1097/01.alc.0000134233.89896.19. [DOI] [PubMed] [Google Scholar]
- Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J. Biol. Chem. 2003;278:27997–28004. doi: 10.1074/jbc.M302140200. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Morales A, Colell A, Ballesta A, Rodes J, Kaplowitz N, Fernandez-Checa JC. Feeding S-adenosyl-L-methionine attenuates both ethanol-induced depletion of mitochondrial glutathione and mitochondrial dysfunction in periportal and perivenous rat hepatocytes. Hepatology. 1995;21:207–214. doi: 10.1002/hep.1840210133. [DOI] [PubMed] [Google Scholar]
- Gyamfi MA, Damjanov I, French S, Wan YJ. The pathogenesis of ethanol versus methionine and choline deficient diet-induced liver injury. Biochem. Pharmacol. 2008;75:981–995. doi: 10.1016/j.bcp.2007.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsted CH, Robles EA, Mezey E. Intestinal malabsorption in folate-deficient alcoholics. Gastroenterology. 1973;64:526–532. [PubMed] [Google Scholar]
- Herbert V, Zalusky R, Davidson CS. Correlation of folate deficiency with alcoholism and associated macrocytosis, anemia, and liver disease. Ann. Intern. Med. 1963;58:977–988. doi: 10.7326/0003-4819-58-6-977. [DOI] [PubMed] [Google Scholar]
- Horie S, Suga T. Different regulation of hepatic peroxisomal beta-oxidation activity in rats treated with clofibrate and partially hydrogenated marine oil. Biochem. Biophys. Res. Commun. 1990;166:780–786. doi: 10.1016/0006-291x(90)90877-p. [DOI] [PubMed] [Google Scholar]
- Iimuro Y, Bradford BU, Forman DT, Thurman RG. Glycine prevents alcohol-induced liver injury by decreasing alcohol in the rat stomach. Gastroenterology. 1996;110:1536–1542. doi: 10.1053/gast.1996.v110.pm8613061. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Bronfman M, Leighton F. Detection of peroxisomal fatty acyl-coenzyme A oxidase activity. Biochem. J. 1979;182:779–788. doi: 10.1042/bj1820779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Jung YS, Kwon d. Y, Kim YC. Alleviation of acute ethanol-induced liver injury and impaired metabolomics of S-containing substances by betaine supplementation. Biochem. Biophys. Res. Commun. 2008;368:893–898. doi: 10.1016/j.bbrc.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Kono H, Bradford BU, Rusyn I, Fujii H, Matsumoto Y, Yin M, Thurman RG. Development of an intragastric enteral model in the mouse: studies of alcohol-induced liver disease using knockout technology. J. Hepatobiliary Pancreat. Surg. 2000;7:395–400. doi: 10.1007/s005340070034. [DOI] [PubMed] [Google Scholar]
- Koop DR. Hydroxylation of p-nitrophenol by rabbit ethanol-inducible cytochrome P-450 isozyme 3a. Mol. Pharmacol. 1986;29:399–404. [PubMed] [Google Scholar]
- Li J, Nguyen V, French BA, Parlow AF, Su GL, Fu P, Yuan QX, French SW. Mechanism of the alcohol cyclic pattern: role of the hypothalamic-pituitary-thyroid axis. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G118–G125. doi: 10.1152/ajpgi.2000.279.1.G118. [DOI] [PubMed] [Google Scholar]
- Lieber CS, Casini A, DeCarli LM, Kim CI, Lowe N, Sasaki R, Leo MA. S-adenosyl-L-methionine attenuates alcohol-induced liver injury in the baboon. Hepatology. 1990;11:165–172. doi: 10.1002/hep.1840110203. [DOI] [PubMed] [Google Scholar]
- Lu SC, Alvarez L, Huang ZZ, Chen L, An W, Corrales FJ, Avila MA, Kanel G, Mato JM. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5560–5565. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- Luka Z, Capdevila A, Mato JM, Wagner C. A glycine N-methyltransferase knockout mouse model for humans with deficiency of this enzyme. Transgenic Res. 2006;15:393–397. doi: 10.1007/s11248-006-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski L, Noland RC, Koves TR, Xing W, Ilkayeva OR, Muehlbauer MJ, Stevens RD, Muoio DM. Metabolic profiling of PPARalpha-/- mice reveals defects in carnitine and amino acid homeostasis that are partially reversed by oral carnitine supplementation. FASEB J. 2009;23:586–604. doi: 10.1096/fj.08-119420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Chantar ML, Corrales FJ, Martinez-Cruz LA, Garcia-Trevijano ER, Huang ZZ, Chen L, Kanel G, Avila MA, Mato JM, Lu SC. Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1A. FASEB J. 2002;16:1292–1294. doi: 10.1096/fj.02-0078fje. [DOI] [PubMed] [Google Scholar]
- Mato JM, Camara J, Fernandez d. P, Caballeria L, Coll S, Caballero A, Garcia-Buey L, Beltran J, Benita V, Caballeria J, et al. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial. J. Hepatol. 1999;30:1081–1089. doi: 10.1016/s0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]
- Mato JM, Lu SC. Role of S-adenosyl-L-methionine in liver health and injury. Hepatology. 2007;45:1306–1312. doi: 10.1002/hep.21650. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Peng HM, Lin HL, Hollenberg PF, Sunahara RK, Osawa Y, Pratt WB. Regulation of cytochrome P450 2E1 by heat shock protein 90-dependent stabilization and CHIP-dependent proteasomal degradation. Biochemistry. 2005;44:16333–16340. doi: 10.1021/bi0515570. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Kamijo Y, Tanaka N, Sugiyama E, Tanaka E, Kiyosawa K, Fukushima Y, Peters JM, Gonzalez FJ, Aoyama T. Peroxisome proliferator-activated receptor alpha protects against alcohol-induced liver damage. Hepatology. 2004;40:972–980. doi: 10.1002/hep.20399. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Mendenhall CL, French SW. Beef fat prevents alcoholic liver disease in the rat. Alcohol Clin. Exp. Res. 1989;13:15–19. doi: 10.1111/j.1530-0277.1989.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Oz HS, Chen TS, Neuman M. Methionine deficiency and hepatic injury in a dietary steatohepatitis model. Dig. Dis. Sci. 2008;53:767–776. doi: 10.1007/s10620-007-9900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit V, Abdelmalek MF, Barve S, Benevenga NJ, Halsted CH, Kaplowitz N, Kharbanda KK, Liu QY, Lu SC, McClain CJ, et al. Role of S-adenosylmethionine, folate, and betaine in the treatment of alcoholic liver disease: summary of a symposium. Am. J. Clin. Nutr. 2007;86:14–24. doi: 10.1093/ajcn/86.1.14. [DOI] [PubMed] [Google Scholar]
- Reimer DL, Bailley J, Singh SM. Complete cDNA and 5′ genomic sequences and multilevel regulation of the mouse catalase gene. Genomics. 1994;21:325–336. doi: 10.1006/geno.1994.1273. [DOI] [PubMed] [Google Scholar]
- Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- Shivapurkar N, Poirier LA. Tissue levels of S-adenosylmethionine and S-adenosylhomocysteine in rats fed methyl-deficient, amino acid-defined diets for one to five weeks. Carcinogenesis. 1983;4:1051–1057. doi: 10.1093/carcin/4.8.1051. [DOI] [PubMed] [Google Scholar]
- Song Z, Zhou Z, Song M, Uriarte S, Chen T, Deaciuc I, McClain CJ. Alcohol-induced S-adenosylhomocysteine accumulation in the liver sensitizes to TNF hepatotoxicity: possible involvement of mitochondrial S-adenosylmethionine transport. Biochem. Pharmacol. 2007;74:521–531. doi: 10.1016/j.bcp.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JA, Reitz RC. Effects of ethanol ingestion and dietary fat levels on mitochondrial lipids in male and female rats. Lipids. 1978;13:540–550. doi: 10.1007/BF02533593. [DOI] [PubMed] [Google Scholar]
- Trasler J, Deng L, Melnyk S, Pogribny I, Hiou-Tim F, Sibani S, Oakes C, Li E, James SJ, Rozen R. Impact of Dnmt1 deficiency, with and without low folate diets, on tumor numbers and DNA methylation in Min mice. Carcinogenesis. 2003;24:39–45. doi: 10.1093/carcin/24.1.39. [DOI] [PubMed] [Google Scholar]
- Tseng TL, Shih YP, Huang YC, Wang CK, Chen PH, Chang JG, Yeh KT, Chen YM, Buetow KH. Genotypic and phenotypic characterization of a putative tumor susceptibility gene, GNMT, in liver cancer. Cancer Res. 2003;63:647–654. [PubMed] [Google Scholar]
- Vendemiale G, Altomare E, Trizio T, Le Grazie C, di Padova C, Salerno MT, Carrieri V, Albano O. Effects of oral S-adenosyl-L-methionine on hepatic glutathione in patients with liver disease. Scand. J. Gastroenterol. 1989;24:407–415. doi: 10.3109/00365528909093067. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Qi C, Cao WQ, Yeldandi AV, Rao MS, Reddy JK. Cloning and characterization of PIMT, a protein with a methyltransferase domain, which interacts with and enhances nuclear receptor coactivator PRIP function. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10380–10385. doi: 10.1073/pnas.181347498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.