Abstract
Spontaneous brain activity has recently received increasing interest in the neuroimaging community. However, the value of resting-state studies to a better understanding of brain–behavior relationships has been challenged. That altered states of consciousness are a privileged way to study the relationships between spontaneous brain activity and behavior is proposed, and common resting-state brain activity features observed in various states of altered consciousness are reviewed. Early positron emission tomography studies showed that states of extremely low or high brain activity are often associated with unconsciousness. However, this relationship is not absolute, and the precise link between global brain metabolism and awareness remains yet difficult to assert. In contrast, voxel-based analyses identified a systematic impairment of associative frontoparieto–cingulate areas in altered states of consciousness, such as sleep, anesthesia, coma, vegetative state, epileptic loss of consciousness, and somnambulism. In parallel, recent functional magnetic resonance imaging studies have identified structured patterns of slow neuronal oscillations in the resting human brain. Similar coherent blood oxygen level–dependent (BOLD) systemwide patterns can also be found, in particular in the default-mode network, in several states of unconsciousness, such as coma, anesthesia, and slow-wave sleep. The latter results suggest that slow coherent spontaneous BOLD fluctuations cannot be exclusively a reflection of conscious mental activity, but may reflect default brain connectivity shaping brain areas of most likely interactions in a way that transcends levels of consciousness, and whose functional significance remains largely in the dark.
Keywords: functional neuroimaging, resting state, disorders of consciousness, vegetative state
Introduction
In recent years, there has been a growing interest from the neuroscientific community concerning spontaneous brain activity and its relation to cognition and behavior. The concept of a “default mode of brain function” arose from the need to explain consistent brain-activity decreases in a set of areas during cognitive processing as compared to a passive resting baseline.1 These areas, encompassing the posterior cingulate cortex/precuneus, the medial prefrontal cortex, and bilateral temporoparietal junctions, began to be known as the “default network.” Furthermore, Raichle et al.2 showed that most brain areas at rest manifest a high level of “default” functional activity. This work has called attention to the importance of intrinsic functional activity in assessing brain behavior relationships, and has now been extended in several functional magnetic resonance imaging (fMRI) studies.
An ongoing controversy concerns the value and interpretability of resting-state studies and their contribution to a better understanding of brain–behavior relationships.3,4 It has been suggested that intrinsic brain activity would have a limited role for behavioral outcomes. In this view, observations made under resting conditions have no privileged status as a fundamental metric of brain functioning, and the link between the processing taking place at rest and its physiology would be one without direct relevance to neuroscience. In contrast, the aims of cognitive neuro-science would be best served by the study of specific task manipulations, rather than of rest.3
In response to this criticism, Raichle and Snyder5 argued that “there is likely much more to brain function than that revealed by experiments manipulating momentary demands of the environment.” In their view, a first argument in this direction is the cost of intrinsic brain activity, which far exceeds that of evoked activity.6 Indeed, relative to the high rate of ongoing or “basal” brain metabolism,6 the amount dedicated to task-evoked regional imaging signals is remarkably small (estimated to be less than 5%). The brain continuously expends a considerable amount of energy, even in the absence of a particular task (i.e., when a subject is awake and at rest). A significant fraction of the energy consumed by the brain (quite possibly the majority) has been shown to be a result of functionally significant spontaneous neuronal activity.7 From this cost-based analysis of brain functional activity, it seems reasonable to conclude that intrinsic activity may be as significant, if not more so, than evoked activity in terms of overall brain function.6 Another argument for the interest of studying spontaneous brain activity is the striking degree of functional organization exhibited by this intrinsic activity.5 The first clue of this organization is the consistent activity decreases in default network during cognitive tasks.1 Even more striking data recently arose from fMRI blood oxygen level–dependent (BOLD) connectivity studies in awake resting subjects, which will be discussed later in this chapter. Maps of spontaneous network correlations have also been proposed to provide tools for functional localization, for the understanding of clinical conditions such as Alzheimer's disease8,9 and autism,10 or for the study of comparative anatomy between primate species.11 However, the functional significance of the observed patterns of intrinsic brain activity remains actually poorly understood.
We here propose that disorders of consciousness are a privileged way to investigate the links between spontaneous brain activity and behavior. These states are indeed mainly characterized by the alteration of intrinsic brain activity, which induces dramatic changes in the contents of awareness and responses to environmental stimuli and demands. We will illustrate our view, reviewing common features of spontaneous brain-activity patterns in altered states of consciousness, as shown by metabolic positron emission tomography (PET) data as well as recent BOLD fMRI studies. We will also discuss methodological issues of resting-state neuroimaging experiments.
Consciousness as a Multidimensional Concept
Consciousness has two major components: awareness (i.e., the content of consciousness) and arousal (i.e., the level of consciousness).12 Arousal and awareness are usually positively correlated: when your arousal decreases, so does your awareness [rapid eye movement (REM) sleep being a notable exception]. Awareness can also be divided into two components: self-awareness and external awareness. Self- and external awareness usually behave in an anti-correlated manner. When you are engaged in self-related processes, you are less receptive to environmental demands, and vice versa.13,14 A number of studies have compared brain activation in circumstances that do or do not give rise to consciousness in either of its two main senses of awareness and arousal. Very few groups, however, have studied situations in which wakefulness and arousal are dissociated.
The vegetative state (VS) is a classic example of a dissociated state of unconsciousness. VS patients are fully aroused, but are unaware of themselves and their environment. They can show automatic reactions like moving their eyes, head, and limbs in a meaningless manner, and may even grimace, cry, or smile (albeit never contingently upon specific external stimuli). Some patients might evolve toward full recovery or remain in the minimally conscious state,15 where some nonreflexive or nonmeaningful behaviors are shown, but patients are still unable to communicate. In addition to their clinical and ethical importance, the study of vegetative and minimally conscious states offers a still widely unexploited means of studying human consciousness.12 In contrast to other unconscious states, such as general anesthesia and deep sleep, where impairment in arousal cannot be disentangled from impairment in awareness, these states represent a unique lesional approach enabling us to identify the neural correlates of (un)awareness.
PET Studies of Brain Metabolism in Altered States of Consciousness
PET studies modulating arousal, and hence awareness, by means of anaesthetic drugs such as halothane16 or propofol17 have shown a drop in global brain metabolism to around half of normal values. Similar global decreases in metabolic activity are observed in deep slow-wave sleep,18 although in rapid eye movement (REM) sleep brain metabolism returns to normal waking values. On average, grey-matter metabolism is 50–70% of the normal range in comatose patients of traumatic or hypoxic origin.19 In VS, that is, in “arousal without awareness,” global brain metabolic activity also decreases to about 50% of normal levels.20,21 As vegetative patients are fully aroused, global brain metabolism seems to correlate with awareness rather than with arousal in altered consciousness states.
However, contradictory data exist concerning the positive correlation between global brain metabolism and levels of consciousness. First, not all anesthetics suppress global cerebral metabolism. Some studies have also reported that ketamine, a so-called dissociative anesthetic agent, increases global cerebral metabolism and fast EEG rhythms at doses associated with a loss of consciousness.22,23 In the same line, in comatose patients with traumatic diffuse axonal injury, hyperglycolysis, leading to increased brain metabolism, has sometimes been reported.19 Another counterexample is the loss of consciousness induced by generalized epilepsia24 and some absence seizures,25 where global brain metabolism is diffusely increased. Finally, a similar return from abnormally high global brain metabolism to a normal balance of activation and inhibition could be evoked in rare cases of zolpidem-evoked (a GABAergic agent) clinical improvement in VS patients.26
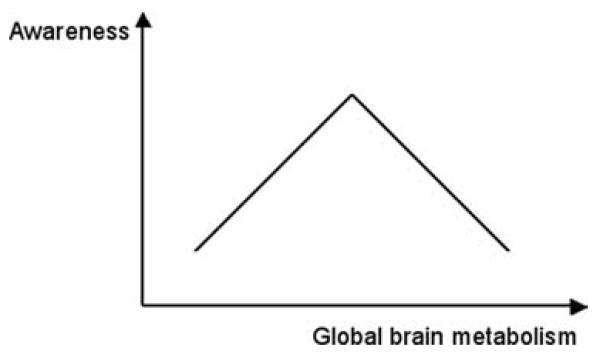
Looking at these data as a whole (summarized in FIG. 1), the complex relationships between global brain metabolism and awareness could be linked to Tononi's theory of information integration.27,28 This theory claims that consciousness is reflected in a system's capacity to integrate information, and proposes a way—the value φ—to measure such a capacity.28 Computer simulation shows that φ is very low in the case of an hyperpolarized state of the system, is maximized in conditions of “intermediate” neural activity, and decreases in states where the neural activity is extremely high and near-synchronous.27 It has been proposed that a proper balance between excitatory and inhibitory activity would be necessary to allow neurons to respond appropriately to correlational changes in their input, and to establish the functional connectivity as required for a particular cognitive task or behavior.29 In the same line, Raichle and Gusnard suggested that a large part of the brain's default activity could be devoted to ongoing synaptic processes associated with the maintenance of this balance.30 In this view, global brain metabolism would have a potentially decisive role by allowing the presence of conscious perception or behavior.
FIGURE 1.
Relationships between global brain metabolism and awareness. The link between global brain energy consumption and awareness is complex. Evidence exists that both states of extremely low and extremely high global brain metabolism are associated with small amounts of awareness. An intermediate level of brain metabolism, corresponding to a proper balance between inhibitory and excitatory neural activity, seems to be necessary to allow the genesis of awareness.
The equivocal link between global brain activity and consciousness is, however, further challenged by the fact that in some patients who subsequently recovered from a VS to normal consciousness, global metabolic rates for glucose metabolism did not show substantial changes.31 Moreover, some awake healthy volunteers have global brain metabolism values comparable to those observed in some patients in a VS.12 Inversely, some well-documented vegetative patients have shown close to normal global cortical metabolism.32 These data led us to focus rather on regional metabolism in our quest for a better understanding of the links between consciousness and resting-brain activity.
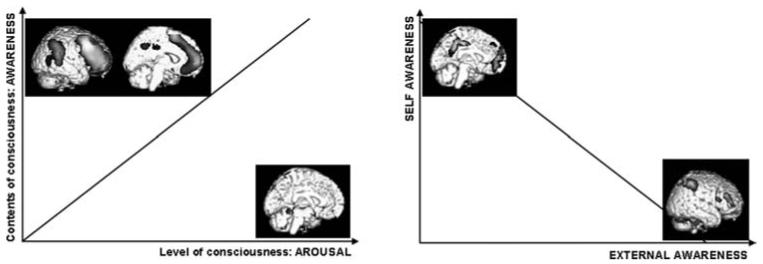
Voxel-based statistical analyses have sought to identify regions showing metabolic dysfunction in VS patients as compared with the conscious resting state in healthy controls. These studies have identified a systematic metabolic dysfunction, not in one brain region but in a wide frontoparietal network encompassing the polymodal associative cortices in VS: lateral and medial frontal regions bilaterally, parietotemporal and posterior parietal areas bilaterally, posterior cingulated, and precuneal cortices,12,21 known to be the most active “by default” in resting nonstimulated conditions.33 In contrast, arousal structures (encompassing the pedunculopontine reticular formation, the hypothalamus, and the basal forebrain) are relatively preserved in these patients.19 The same frontoparietal functional impairment is found in various other states of unconsciousness, that is, in sleep,34 coma,35 general anesthesia,36 generalized seizures,37 or in other dissociated unconscious states like absence seizures,38,39 complex partial seizures,40 or somnambulism.41 Figure 2 illustrates the involvement of the frontoparietal cortical network in awareness, while arousal rather relies on subcortical structures.
These findings emphasize the importance of frontoparietal association areas in consciousness, and are in line with the global workspace theory as introduced by Baars.42,43 This theory views the brain as a massive parallel set of specialized processors. Consciousness might be a gateway to brain integration, enabling access between otherwise separate neuronal functions. In such a system, coordination and control may take place by way of a central information exchange, allowing some processors—such as sensory systems in the brain—to distribute information to the system as a whole. According to Baars,35 frontoparietal association areas would be an ideal candidate for being the global workspace processor.
As reported below, awareness can in turn be divided in two main components: self- and external awareness. In the same line, FIGURE 2 illustrates that frontoparietal network can be subdivided in areas involved in external awareness, and in self-awareness. External awareness network activity is crucial for conscious external stimuli perception, as documented in healthy awake volunteers.13,44 Self-awareness network encompasses the so-called “default network” and has been involved in various aspects of self-related processes.45 In awake healthy volunteers, self- and external awareness networks usually show an anticorrelated pattern of activity. Anticorrelations between self- and external awareness networks have indeed been observed during cognitive tasks,46 sensory perception,13 as well as in studies of resting-state brain activity.45 In contrast, in most states of altered consciousness, both the activity of subnetworks is similarly impaired.
FIGURE 2.
(Left) Consciousness has two main components: arousal, or the level of consciousness, and awareness, corresponding to the contents of consciousness per se. Arousal and awareness are usually positively correlated. However, they involve different brain structures. Arousal involves the activity of subcortical structures encompassing brain-stem reticular formation, hypothalamus, and basal forebrain. Awareness is related to the activity of a widespread set of frontoparietal associative areas, both on the convexity and on the midline. (Right) Awareness can in turn be divided into two main components: self and external awareness. In healthy volunteers, self- and external awareness are usually negatively correlated. Similarly, the frontoparietal awareness network can in turn be divided into two sub-systems, involved in self-and external awareness. Self-awareness networks encompass the posterior cingulate/precuneal cortices, medial frontal cortex, and bilateral temporoparietal junctions. The external awareness network encompasses lateral frontal and parietal cortices. In healthy volunteers, self- and external awareness networks usually show an anticorrelated pattern of activity.
In addition to activity in frontoparietal network, awareness seems also to relate to the functional connectivity within this network, and with the thalami. Functional disconnections in long-range cortico–cortical (between laterofrontal and midline-posterior areas) and cortico–thalamo–cortical (between nonspecific thalamic nuclei and lateral and medial frontal cortices) pathways have been identified in the vegetative state.21,47 In the same line, disruptions of thalamo–cortical48 and cortico–cortical49 connectivity have been reported during other unconscious states like sleep or anesthesia. Moreover, recovery from VS is accompanied by a functional restoration of the frontoparietal network31 and some of its cortico–thalamo–cortical connections.47
These results are in line with Dehaene and Changeux's recent computational model of the relationships between spontaneous brain activity and external stimuli awareness.50 This model emphasizes the importance of both thalamo–cortical and cortico–cortical cerebral connections to create patterns of spontaneous brain activity hypothesized to allow conscious perception.
Methodological Considerations in the Study of Spontaneous Brain Activity Using Functional Magnetic Rersonance
Up to now, a large majority of functional neuroimaging focused on brain activity elicited by external stimuli or “evoked responses.” Likewise, current mathematical tools have been mainly devised for the analysis of data acquired during external stimulation and not for spontaneous activity. Since the late 1990s, brain activity fluctuations in the default resting state have received increasing interest. Analytical tools have therefore been developed for the processing of spontaneous fMRI (and electroencephalographic) data.
Spontaneous brain activity is by definition not triggered by external stimuli. We therefore have no control and, importantly, no a priori knowledge about when a “spontaneous event” occurs. The problem of analyzing spontaneous data is thus twofold, as we must simultaneously determine the “where” and “when” of brain activity. Knowing one or the other, that is, location or timing, allows a more complete characterization of the spontaneous activity. Most methods rely on this idea, that is, make assumptions about the timing or location of some activity pattern to determine the location or timing, respectively, of the activated brain system.
The BOLD signal recorded with fMRI allows the mapping of brain activity with good spatial resolution (submillimetric) but poor timing (around 1 s, at best), due to the physiological origin of the hemodynamic signal. Unfortunately, the signal recorded also contains some noise on top of the brain signal. This noise component has typically two origins: the scanner itself (such as scanner instability)51 and nonneural physiological fluctuations due to, for example, cardiac or respiratory artefacts.52,53 Before investigating spontaneous brain activity, it is necessary to correct the fMRI data for these artefacts. One option to account for part of the noise is to use a high sampling rate. With a very short repetition time, the higher-frequency spurious (mainly cardiac and respiratory) signal will not be aliased and can be directly filtered out.54-56 There are technical limitations, though, as a compromise must be found between speed of acquisition, field of view, and spatial resolution. High-pass filtering is the method of choice to remove the slowly varying “scanner drift” signal but, of course, as for any filtering method, this removes possible information carrying signals. Alternatively, linear regression can be employed to remove nonneural signal from the data.52,57 For example, if physiological parameters are measured along side the fMRI acquisition, any BOLD signal correlated with (functions of) these measurements can be regressed out. Other regressors generated directly from the fMRI data are also possible, such as the mean BOLD signal over all voxels (usually called “global”), or the BOLD signal from areas where there should be little or no neural activity (e.g., the ventricles or white matter). As hinted by its name, “linear regression” assumes a linear relationship between the “noise regressor” and the noise part of the signal in all the voxels. If, by chance, some neural signal was also correlated with the “noise regressor,” that part of the signal would be lost for further analysis.
Finally, “independent component analysis” (ICA)—a relatively new approach—is capable of directly separating the signals of interest (due to brain activity) from the noise.58,59 This approach is discussed later on in more detail. The goal of the described procedures is to ensure that the further analyzed signal is neurobiologically meaningful and corresponds to the spontaneous brain activity of interest. We now focus on ways to identify and characterize patterns of spontaneous activity.
The most straightforward approach is correlation or “functional connectivity” analyses. After choosing a “seed region,” that is, a region of interest, the time course of the BOLD signal is extracted (averaged over the region or the first principal component) and a correlation coefficient is calculated for all the other voxels, providing a correlation map. This method has the advantage of being simple, sensitive, and easily interpretable, but is limited to one seed region at a time.45,60 Results rely heavily on the a priori choice of seed region and provide no information about the causality of the observed correlated activation. Indeed the activity in two disconnected areas can be correlated because they are driven by a third independent area. To study the interaction between two seed regions, “physiophysiological interaction” models are useful.61 These models provide evidence for the interaction between distributed brain systems: voxels whose correlation with one seed region is modulated by the other seed region are highlighted.
A more mathematically sophisticated approach to analyze spontaneous fMRI data is the previously mentioned ICA. ICA is a data-driven “blind source separation” algorithm that tries to decompose the entire data set into components, spatial and temporal, that are statistically independent.62-64 The way statistical independence is defined and reached leads to different flavors of ICA decompositions. The main advantage of ICA is the direct extractions of spatial maps, with their associated time course: the sources of interest, that is, spontaneous brain activity, can be automatically separated from the noise components. However, there remain two major difficulties with ICA. First, the number of components to be extracted has to be defined a priori, and results are highly dependent on that chosen number. Second, components are not ranked during the decomposition. It is the investigator's duty to manually and subjectively decide, based on his or her experience, knowledge, or priors, which components correspond to noise or neural systems. Solutions to these practical problems have been proposed, for example, the “probabilistic ICA.”59
So far, we have only considered fMRI recordings, but the electroencephalogram (EEG) is more and more routinely recorded alongside fMRI to study spontaneous brain activity.65 Importantly, EEG data provide access to very useful information regarding the timing of spontaneous brain activity. Features can be detected in the EEG signal and used to build an “activation” regressor for fMRI. For example, during sleep studies, typical waves (e.g., slow waves or spindles; or epileptic spikes) are easily detected on the EEG trace and a “spontaneous hemodynamic event” is associated with each occurrence of such wave. The analysis of the fMRI data can then proceed as usual in stimulus induced tasks.66-68 EEG data can also be processed to yield a continuous regressor associated with spontaneous brain dynamics. For example, correlation between the BOLD signal of each voxel and the EEG power in a specific frequency band, convoluted with the standard hemodynamic “response function,” provides a correlation map, similar to what is done with the seed-region activity. Typically, the spectrogram, that is, the power spectrum evolving over time, of some or all EEG channels is calculated and resampled at the fMRI acquisition frequency. Then the time course of power within frequency bands of interest is used to build a correlations map.69,70
Functional Magnetic Resonance Imaging Resting-State Studies in Awake Healthy Subjects
Even in the absence of sensory inputs, structured patterns of ongoing spontaneous activity can be observed in cortical and thalamic neurons.71-73 In parallel, recent fMRI studies have identified spontaneous fluctuations in neural activity in the resting human brain. These slow BOLD fluctuations (in the range of 0.1 Hz) are not random but coherent within specific neuroanatomical systems. Biswal and colleagues60 were the first to describe correlations between the activity of bilateral somatomotor cortices in the awake resting human brain. The finding that spontaneous fluctuations in the fMRI BOLD signal at rest in one area of the cerebral cortex exhibited system-relevant correlations with signal fluctuations in other areas has then been replicated several times for motor cortices54,74-76 and extended to other neuroanatomical systems, including visual,54,77 auditory,77 default-mode network,45,78-80 memory,57,81 language,77,82 and attention systems.80,83 Similar results were derived from other methods like hierarchical clustering84,85 and ICA.56,58,63,64,86 The joint finding of these studies is that regions similarly modulated by tasks or stimuli tend to exhibit correlated spontaneous fluctuations even in the absence of these tasks or stimuli.7 Resting-state fMRI patterns have also been shown to be spatially very consistent across subjects.87
In parallel, other resting-state fMRI studies showed anticorrelated patterns of spontaneous fluctuations, in regions with apparent opposing functionality. In particular, two independent studies45,79 recently showed that even in the absence of any task or behavior, in the so-called “conscious resting state” of the human brain, two networks very similar to self- and external awareness networks show a pattern of anticorrelated activity (illustrated in FIG. 3). It has been suggested that these anticorrelations could be a reflection of periodical shifts from introspective or self-oriented processes into a state-of-mind of extrospectively oriented attention, and an engagement of networks that support sensorimotor planning.79 Another recent fMRI study88 investigated the positive and negative correlations of three regions of interest (ROIs) located in the auditory, visual, and somatosensory systems by using resting-state fMRI. They found that all three sensory systems exhibited significant negative correlation with the default network (self-awareness or “intrinsic” system). This study extends former findings by indicating that multiple subsystems rather than a single subsystem of the “extrinsic system” are inherently negatively correlated with the self-awareness network. These negative correlations may explain the phenomenon that externally and internally oriented processes can always disturb or even interrupt each other. These data resemble our recent findings of a competitive effect between self-awareness network activity and conscious somatosensory stimuli perception.13
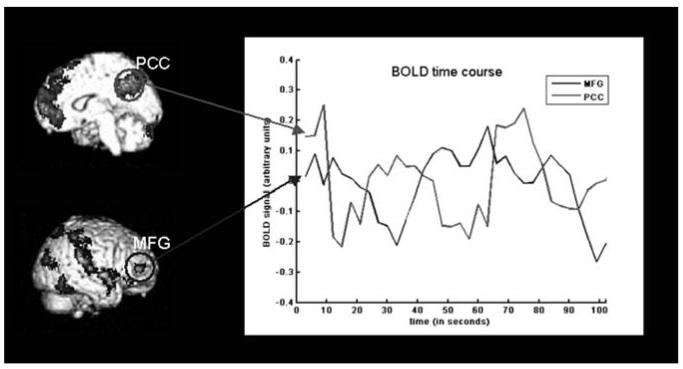
FIGURE 3.
Spontaneous anticorrelations between self- and external awareness networks in the conscious resting state, as observed in an individual volunteer. (Left) Areas correlated (above) and anticor-related (below) with the blood oxygen level–dependent (BOLD) time course of a seed voxel located in the posterior cingulate/precuneus. (Right) Plot of the BOLD time courses of posterior cingulate/precuneus (PCC, red/gray line) and of middle frontal gyrus (MFG, blue/dark line) in the same volunteer. As previously reported, anticorrelations between these area time courses occur in slow frequencies with a period below 0.1 Hz. (In color in Annals online.)
To date, only a few studies combined EEG and fMRI data to better characterize spontaneous brain activity fluctuations in the awake resting state. A simultaneous EEG/fMRI study showed a strong negative correlation of parietal and frontal cortical activity with spontaneous fluctuations in EEG alpha power (8–12 Hz).69 Beta activity was shown to be positively correlated with activity in retrosplenial, temporoparietal, and dorsomedial prefrontal cortices, in the default network.80 These data were interpreted as alpha oscillations signaling a neural baseline with “inattention,” whereas beta rhythms index spontaneous cognitive operations during conscious rest. Conversely, a recent EEG/fMRI study showed that each fMRI resting-state network as identified by ICA was associated not to only one electrophysiological frequency, but to a coalescence of several brain rhythms in the delta, theta, alpha, beta, and gamma ranges.89 Furthermore, each functional network was shown to be characterized by a specific electrophysiological signature that involved the combination of these different brain rhythms. This neurophysiological signature was suggested to constitute a baseline for evaluating changes in oscillatory signals during active behavior.
Functional Magnetic Resonance Imaging Resting-state Studies in Altered States of Consciousness
It has been suggested that coherent spontaneous BOLD fluctuations observed in the resting state reflect unconstrained but consciously directed mental activity.3 One could argue that intrinsic activity simply represents unconstrained, spontaneous cognition, mind-wandering, or stimulus-independent thoughts.5,90 Alternatively, coherent BOLD fluctuations may persist in the absence of normal perception and behavior, reflecting a more fundamental or intrinsic property of functional brain organization.91 Importantly, the former view predicts that coherent BOLD fluctuations should be absent in coma, sleep, or deep anesthesia, in which conscious mental activity is thought to be absent.
Peltier et al.92 assessed the effect of sevoflurane anesthesia on the temporal BOLD correlations in activity in the motor cortices of healthy humans. Across all volunteers, they found that the number of significant voxels in the functional connectivity maps was reduced by 78% for light anesthesia and by 98% for deep anesthesia, compared with the awake state. Additionally, significant correlations in the connectivity maps were bilateral in the awake state, but unilateral in the light anesthesia state. Interestingly, this loss of interhemispheric connectivity was also found in an independent resting-state fMRI study on a minimally conscious patient compared to healthy volunteers.85
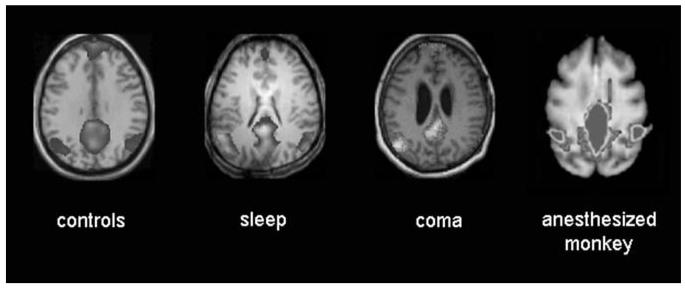
In contrast to these findings, recent data from several independent BOLD fMRIs suggest that low-frequency systemwide BOLD coherent spontaneous activity can be preserved in various states of unconsciousness. First, low-frequency BOLD fluctuations have recently been investigated using ICA during light sleep in humans.93,94 In this work (collapsing non-REM sleep stages 1 and 2), significant increases in the fluctuation level of the BOLD signal were observed in several cortical areas, among which visual cortex was the most significant.93 Furthermore, correlations among brain regions involved in the default network (encompassing posterior cingulate/precuneus, medial frontal cortex, and bilateral temporoparietal junctions) persisted during light non-REM sleep.94 Vincent et al.91 demonstrated in deeply isoflurane-anesthetised monkeys preserved and coherent resting-state spontaneous fluctuations within three well-known neuroanatomical systems (oculomotor, somatomotor, and visual) and within a network very close to the human “default” system (see FIG. 4), a set of brain regions thought by some to support uniquely human capabilities. These results demonstrate that cortical systems previously associated with performance in sensory, motor, and/or cognitive tasks are manifest in the correlation structure of spontaneous BOLD fluctuations observed in the absence of normal perception or behavior. Finally, using a method similar to that used in Vincent et al.,91 we could identify persisting coherent BOLD oscillations within the default-mode network in coma, and during stage 2 slow-wave sleep (FIG.4, unpublished results).
FIGURE 4.
Preserved coherent blood oxygen level–dependent (BOLD) oscillations in the default network persist in three documented states of unawareness. Brain areas showing correlations with a seed voxel in the posterior cingulate cortex, after correction for spurious variance as described in Reference 91. From the left to the right, results of 12 volunteers' random-effect analysis, from an individual sleeping volunteer scanned during sleep stage 2 (from Ref. 68), from a patient in coma due to a nontraumatic origin, and anaesthetized monkey data (reproduced by permission from Vincent et al.91). Sleep and coma patients were masked inclusively with healthy volunteers' results to check for spatial consistency of the resting-state connectivity patterns.
All these results indicate that coherent systemwide fluctuations probably reflect an aspect of brain functional organization that transcends levels of consciousness.91 Thus, coherent spontaneous BOLD fluctuations cannot be exclusively a reflection of conscious mental activity,3 but may reflect a more fundamental or intrinsic property of functional brain organization. They should be considered as certainly necessary, but not sufficient to support consciousness. One could argue that the temporal dynamics of our ongoing “stream of consciousness” (classically considered around 500 ms95) is much faster than the slow fMRI BOLD oscillations occurring at around the 10-s time period (0.1 Hz) observed here.
The physiological origin and functional significance of low-frequency spontaneous brain activity fluctuations remain to be assessed. Even if at least part of these systemwide default interactions correspond to unconscious processes, these fluctuations are likely to shape brain responses to environmental demands and to ongoingly modulate perception and behavior. Systemwide correlations in the absence of consciousness could also be seen as reflecting preserved anatomical connections dissociated from higher cognitive functions. According to the hypothesis of a tight correlate between low-frequency BOLD fluctuations and neuroanatomical connectivity,7 resting-state fMRI data would also be likely to bring prognostic information in acute brain-damaged patients. Further studies correlating diffusion tensor imaging measures to slow BOLD correlations are ongoing to test this hypothesis.
Conclusion
Even if states of extremely low or high brain activity are often associated with unconsciousness, the precise link between global brain metabolism and awareness remains difficult to assert. On the contrary, regional brain activity in a widespread frontoparietal associative network has been shown to be systematically altered in all documented states of unconsciousness. In line with studies in awake volunteers, these data emphasize the potential role of frontoparietal association cortices in the genesis of awareness.
Recent functional MRI studies have identified coherent low-frequency fluctuations among well-documented neuroanatomical networks. We, however, showed that these correlations can be similarly found in three documented states of unawareness, namely, sleep, coma, and deep anesthesia. We conclude that the presence of slow BOLD fluctuations is unlikely to merely reflect ongoing changes in the contents of consciousness and may be related to a more basic principle of brain function.
Acknowledgments
This work was supported by grants from the Belgian Fonds National de la Recherche Scientifique (FNRS) and from the Centre Hospitalier Universitaire Sart Tilman, the University of Liège, the Mind Science Foundation, the European Comission, the French Speaking Community Concerted Research Action, and the Fondation Médicale Reine Elisabeth. M.B. and T.D.V., C.P., S.L., and P.M. are, respectively, Research Fellows, Research Associate, Senior Research Associate, and Research Director at FNRS. M.S. was supported by an Austrian Science Fund Erwin-Schrödinger Fellowship J2470-B02 (to M.S.). We thank Dimitri Haye, Jacques Trantsieaux, and Charlemagne Noukoua for their assistance in acquiring the fMRI data.
Footnotes
Competing Interest
The authors declare no competing interest.
References
- 1.Shulman GL, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J. Cogn. Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 2.Raichle ME, et al. A default mode of brain function. Proc. Natl. Acad. Sci. USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morcom AM, Fletcher PC. Does the brain have a baseline? Why we should be resisting a rest. NeuroImage. 2007;37:1073–1082. doi: 10.1016/j.neuroimage.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Morcom AM, Fletcher PC. Cognitive neuro-science: the case for design rather than default. NeuroImage. 2007;37:1097–1099. [Google Scholar]
- 5.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 6.Raichle ME, Mintun MA. Brain work and brain imaging. Annu. Rev. Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 7.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 8.Greicius MD, et al. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Y, et al. Regional coherence changes in the early stages of Alzheimer's disease: a combined structural and resting-state functional MRI study. NeuroImage. 2007;35:488–500. doi: 10.1016/j.neuroimage.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 10.Cherkassky VL, et al. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- 11.Buckner RL, Vincent JL. Unrest at rest: Default activity and spontaneous network correlations. NeuroImage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Laureys S. The neural correlate of (un)awareness: lessons from the vegetative state. Trends Cogn. Sci. 2005;9:556–559. doi: 10.1016/j.tics.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Boly M, et al. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc. Natl. Acad. Sci. USA. 2007;104:12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duval S, Wicklund R. A Theory of Objective Self-awareness. Academy Press; New York: 1972. [Google Scholar]
- 15.Giacino JT, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- 16.Alkire MT, et al. Functional brain imaging during anesthesia in humans: effects of halothane on global and regional cerebral glucose metabolism. Anesthesiology. 1999;90:701–709. doi: 10.1097/00000542-199903000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Alkire MT, et al. Cerebral metabolism during propofol anesthesia in humans studied with positron emission tomography. Anesthesiology. 1995;82:393–403. doi: 10.1097/00000542-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Maquet P, et al. Functional neuroanatomy of human slow wave sleep. J. Neurosci. 1997;17:2807–2812. doi: 10.1523/JNEUROSCI.17-08-02807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537–546. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- 20.Schiff ND, et al. Residual cerebral activity and behavioural fragments can remain in the persistently vegetative brain. Brain. 2002;125:1210–1234. doi: 10.1093/brain/awf131. [DOI] [PubMed] [Google Scholar]
- 21.Laureys S, et al. Impaired effective cortical connectivity in vegetative state: preliminary investigation using PET. NeuroImage. 1999;9:377–382. doi: 10.1006/nimg.1998.0414. [DOI] [PubMed] [Google Scholar]
- 22.Itoh T, et al. Effects of anesthesia upon 18F-FDG uptake in rhesus monkey brains. Ann. Nucl. Med. 2005;19:373–377. doi: 10.1007/BF03027401. [DOI] [PubMed] [Google Scholar]
- 23.Maksimow A, et al. Increase in high frequency EEG activity explains the poor performance of EEG spectral entropy monitor during S-ketamine anesthesia. Clin. Neurophysiol. 2006;117:1660–1668. doi: 10.1016/j.clinph.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Blumenfeld H. Consciousness and epilepsy: Why are patients with absence seizures absent? Prog. Brain Res. 2005;150:271–286. doi: 10.1016/S0079-6123(05)50020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engel J, JR., et al. Local cerebral metabolic rate for glucose during petit mal absences. Ann. Neurol. 1985;17:121–128. doi: 10.1002/ana.410170204. [DOI] [PubMed] [Google Scholar]
- 26.Clauss R, Nel W. Drug induced arousal from the permanent vegetative state. NeuroRehabilitation. 2006;21:23–28. [PubMed] [Google Scholar]
- 27.Tononi G. Consciousness, information integration, and the brain. Prog. Brain Res. 2005;150:109–126. doi: 10.1016/S0079-6123(05)50009-8. [DOI] [PubMed] [Google Scholar]
- 28.Tononi G. An information integration theory of consciousness. BMC Neurosci. 2004;5:42. doi: 10.1186/1471-2202-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat. Rev. Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raichle ME, Gusnard DA. Appraising the brain's energy budget. Proc. Natl. Acad. Sci. USA. 2002;99:10237–10239. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laureys S, et al. Cerebral metabolism during vegetative state and after recovery to consciousness. J. Neurol. Neurosurg. Psychiatry. 1999;67:121. doi: 10.1136/jnnp.67.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiff ND, et al. Residual cerebral activity and behavioural fragments can remain in the persistently vegetative brain. Brain. 2002;125:1210–1234. doi: 10.1093/brain/awf131. [DOI] [PubMed] [Google Scholar]
- 33.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 34.Maquet P. Functional neuroimaging of normal human sleep by positron emission tomography. J. Sleep Res. 2000;9:207–231. doi: 10.1046/j.1365-2869.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 35.Baars BJ, Ramsoy TZ, Laureys S. Brain, conscious experience and the observing self. Trends Neurosci. 2003;26:671–675. doi: 10.1016/j.tins.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Kaisti KK, et al. Effects of surgical levels of propofol and sevoflurane anesthesia on cerebral blood flow in healthy subjects studied with positron emission tomography. Anesthesiology. 2002;96:1358–1370. doi: 10.1097/00000542-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Blumenfeld H, et al. Selective frontal, parietal, and temporal networks in generalized seizures. NeuroImage. 2003;19:1556–1566. doi: 10.1016/s1053-8119(03)00204-0. [DOI] [PubMed] [Google Scholar]
- 38.Salek-Haddadi A, et al. Functional magnetic resonance imaging of human absence seizures. Ann. Neurol. 2003;53:663–667. doi: 10.1002/ana.10586. [DOI] [PubMed] [Google Scholar]
- 39.Laufs H, et al. Linking generalized spike-and-wave discharges and resting state brain activity by using EEG/fMRI in a patient with absence seizures. Epilepsia. 2006;47:444–448. doi: 10.1111/j.1528-1167.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 40.Blumenfeld H, et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb. Cortex. 2004;14:892–902. doi: 10.1093/cercor/bhh048. [DOI] [PubMed] [Google Scholar]
- 41.Bassetti C, et al. SPECT during sleepwalking. Lancet. 2000;356:484–485. doi: 10.1016/S0140-6736(00)02561-7. [DOI] [PubMed] [Google Scholar]
- 42.Baars BJ. A Cognitive Theory of Consciousness. Cambridge University Press; Cambridge, UK: 1988. [Google Scholar]
- 43.Baars BJ. The conscious access hypothesis: origins and recent evidence. Trends Cogn. Sci. 2002;6:47–52. doi: 10.1016/s1364-6613(00)01819-2. [DOI] [PubMed] [Google Scholar]
- 44.Dehaene S, et al. Cerebral mechanisms of word masking and unconscious repetition priming. Nat. Neurosci. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- 45.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 47.Laureys S, et al. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet. 2000;355:1790–1791. doi: 10.1016/s0140-6736(00)02271-6. [DOI] [PubMed] [Google Scholar]
- 48.White NS, Alkire MT. Impaired thalamocortical connectivity in humans during general-anesthetic-induced unconsciousness. NeuroImage. 2003;19:402–411. doi: 10.1016/s1053-8119(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 49.Massimini M, et al. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 50.Dehaene S, Changeux JP. Ongoing spontaneous activity controls access to consciousness: a neuronal model for inattentional blindness. PLoS Biol. 2005;3:e141. doi: 10.1371/journal.pbio.0030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zarahn E, Aguirre GK, D'Esposito M. Empirical analyses of BOLD fMRI statistics. I. Spatially un-smoothed data collected under null-hypothesis conditions. NeuroImage. 1997;5:179–197. doi: 10.1006/nimg.1997.0263. [DOI] [PubMed] [Google Scholar]
- 52.Birn RM, et al. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 53.Lund TE, et al. Non-white noise in fMRI: Does modelling have an impact? NeuroImage. 2006;29:54–66. doi: 10.1016/j.neuroimage.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 55.Kiviniemi V, Ruohonen J, Tervonen O. Separation of physiological very low frequency fluctuation from aliasing by switched sampling interval fMRI scans. Magn. Reson. Imaging. 2005;23:41–46. doi: 10.1016/j.mri.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 56.De Luca M, et al. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 57.Rombouts SA, et al. Identifying confounds to increase specificity during a “no task condition”. Evidence for hippocampal connectivity using fMRI. NeuroImage. 2003;20:1236–1245. doi: 10.1016/S1053-8119(03)00386-0. [DOI] [PubMed] [Google Scholar]
- 58.Kiviniemi V, et al. Independent component analysis of nondeterministic fMRI signal sources. NeuroImage. 2003;19:253–260. doi: 10.1016/s1053-8119(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 59.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 60.Biswal B, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 61.Friston KJ, et al. Psychophysiological and modula-tory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 62.McKeown MJ, et al. Analysis of fMRI data by blind separation into independent spatial components. Hum. Brain Mapp. 1998;6:160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van de Ven VG, et al. Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Hum. Brain Mapp. 2004;22:165–178. doi: 10.1002/hbm.20022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beckmann CF, et al. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salek-Haddadi A, et al. Studying spontaneous EEG activity with fMRI. Brain Res. Brain Res. Rev. 2003;43:110–133. doi: 10.1016/s0165-0173(03)00193-0. [DOI] [PubMed] [Google Scholar]
- 66.Lemieux L, et al. Event-related fMRI with simultaneous and continuous EEG: description of the method and initial case report. NeuroImage. 2001;14:780–787. doi: 10.1006/nimg.2001.0853. [DOI] [PubMed] [Google Scholar]
- 67.Bagshaw AP, et al. EEG-fMRI of focal epileptic spikes: analysis with multiple haemodynamic functions and comparison with gadolinium-enhanced MR angiograms. Hum. Brain Mapp. 2004;22:179–192. doi: 10.1002/hbm.20024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schabus M, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc. Natl. Acad. Sci. USA. 2007;104:13164–13169. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laufs H, et al. EEG-correlated fMRI of human alpha activity. NeuroImage. 2003;19:1463–1476. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- 70.Czisch M, et al. Functional MRI during sleep: BOLD signal decreases and their electrophysiological correlates. Eur. J. Neurosci. 2004;20:566–574. doi: 10.1111/j.1460-9568.2004.03518.x. [DOI] [PubMed] [Google Scholar]
- 71.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 72.Tsodyks M, et al. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science. 1999;286:1943–1946. doi: 10.1126/science.286.5446.1943. [DOI] [PubMed] [Google Scholar]
- 73.Kenet T, et al. Spontaneously emerging cortical representations of visual attributes. Nature. 2003;425:954–956. doi: 10.1038/nature02078. [DOI] [PubMed] [Google Scholar]
- 74.Xiong J, et al. Interregional connectivity to primary motor cortex revealed using MRI resting state images. Hum. Brain Mapp. 1999;8:151–156. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<151::AID-HBM13>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fox MD, et al. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat. Neurosci. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- 76.De Luca M, et al. Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Exp. Brain Res. 2005;167:587–594. doi: 10.1007/s00221-005-0059-1. [DOI] [PubMed] [Google Scholar]
- 77.Cordes D, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am. J. Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- 78.Greicius MD, et al. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum. Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laufs H, et al. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc. Natl. Acad. Sci. USA. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vincent JL, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J. Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 82.Hampson M, et al. Detection of functional connectivity using temporal correlations in MR images. Hum. Brain Mapp. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fox MD, et al. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. USA. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cordes D, et al. Hierarchical clustering to measure connectivity in fMRI resting-state data. Magn. Reson. Imaging. 2002;20:305–317. doi: 10.1016/s0730-725x(02)00503-9. [DOI] [PubMed] [Google Scholar]
- 85.Salvador R, et al. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb. Cortex. 2005;15:1332–1342. doi: 10.1093/cercor/bhi016. [DOI] [PubMed] [Google Scholar]
- 86.Bartels A, Zeki S. The chronoarchitecture of the cerebral cortex. Philos. Trans. R Soc. Lond. B Biol. Sci. 2005;360:733–750. doi: 10.1098/rstb.2005.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Damoiseaux JS, et al. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tian L, et al. The relationship within and between the extrinsic and intrinsic systems indicated by resting state correlational patterns of sensory cortices. NeuroImage. 2007;36:684–690. doi: 10.1016/j.neuroimage.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 89.Mantini D, et al. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. USA. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mason MF, et al. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 92.Peltier SJ, et al. Functional connectivity changes with concentration of sevoflurane anesthesia. Neuroreport. 2005;16:285–288. doi: 10.1097/00001756-200502280-00017. [DOI] [PubMed] [Google Scholar]
- 93.Fukunaga M, et al. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn. Reson. Imaging. 2006;24:979–992. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 94.Horovitz SG, et al. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum. Brain Mapp. 2007 doi: 10.1002/hbm.20428. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Libet B. Reflections on the interaction of the mind and brain. Prog. Neurobiol. 2006;78:322–326. doi: 10.1016/j.pneurobio.2006.02.003. [DOI] [PubMed] [Google Scholar]