Abstract
Gene function during mouse development is often studied through the production and analysis of transgenic and knock-out models. However, these techniques are time- and resource-consuming, and require specialized equipment and expertise. We have established a new protocol for functional studies that combines organ culture of explanted fetal tissues with micro-injection and magnetically-induced transfection (“magnetofection”) of gene expression constructs. As proof-of-principle, we magnetofected cDNA constructs into genital ridge tissue as a means of gain-of-function analysis, and shRNA constructs for loss-of-function analysis. Ectopic expression of Sry induced female-to-male sex-reversal, whereas knockdown of Sox9 expression caused male-to-female sex-reversal, consistent with the known functions of these genes. Further, ectopic expression of Tmem184a, a gene of unknown function, in female genital ridges, resulted in failure of gonocytes to enter meiosis. This technique will likely be applicable to the study of gene function in a broader range of developing organs and tissues.
Keywords: gene delivery, sex determination, germ cells, meiosis, Tmem184a, Sry, Sox9
Introduction
Functional genomic studies in various animal models have contributed greatly to our understanding of the genetic programs underpinning normal development and disease. In particular, functional studies in mouse have illuminated the genetic programs governing vertebrate embryo development and an array of various experimental strategies have been employed, ranging from transient gene function assays to targeted knock-out animals. The development of several different strategies have been necessary to obviate numerous technical difficulties inherent for each experimental procedure, and still today researchers are continuing to devise new methodologies allowing for gene-specific functional analyses. In particular, transient gene function assays are desirable as these technologies often allow for more rapid and cost-effective experiments compared to stable mutagenesis approaches.
Of the various transient gene function assays, virus-mediated gene transfer has the advantage of high efficiency, but is hampered by issues of virus production and handling, and generally poor tissue penetration (Matsuda and Cepko, 2004; Baker et al., 2005; Srivastava, 2008). The latter is also true for liposome-based gene transfection systems, which additionally has a lower transfection efficiency (Zabner et al., 1995; Ewert et al., 2004). Alternatively, electroporation as a means of gene delivery has been used in a variety of different systems such as chicken embryos (Muramatsu et al., 1997a; Yasuda et al., 2000; Yasugi and Nakamura, 2000), mouse testis (Muramatsu et al., 1997b; Yamazaki et al., 2000) and the developing nervous system (Yozu et al., 2005). However, in our experience, it is difficult to perform electroporation successfully with small embryonic mouse tissues (unpublished data). Despite these technical difficulties, such alternative approaches can allow researchers to determine gene function rapidly before pursuing a transgenic and/or knock-out model. Furthermore, these approaches can allow genes that are functional in several developing organ systems to be studied in isolation in the organ of interest, for example in explant culture of embryonic tissues.
Although transient mutagenesis is a powerful tool to study gene function, stable mutagenesis assays are regarded more desirable. Since the discovery that X-rays could induce random mutations in the mouse in the 1920s (Little and Bagg, 1923; Muller, 1927), and after a renaissance in the 1980s with the introduction of N-ethyl-N-nitrosourea (ENU) mutagenesis programs, forward genetics (from phenotype to gene) has been a powerful tool with which to study gene function in vivo, now involving numerous large-scale mutagenesis programs worldwide (reviewed by Gondo, 2008).
Whereas forward genetics involves creating random mutations in the genome, many experimental approaches call for targeting pre-determined genes by site-directed or gene-specific mutagenesis—a process known as reverse genetics (from gene to phenotype). For example, known disease-causing genes mapped by studies of human patients can be targeted in mice, producing useful models for in-depth mechanistic studies, and novel genes with unknown disease association can be functionally annotated by creating transgenic and/or knock-out mice (Palmiter et al., 1982; Capecchi, 1989). This strategy has served the genetics and developmental biology communities well since the 1980s, yielding thousands of transgenic and knock-out models now available for research.
Despite the success of these strategies however, many obstacles remain in the pursuit of functional genomics studies on a broad scale. For example, embryonic lethality caused by any introduced alteration in the genome can impede the study of many morphological processes. One strategy to obviate this hurdle is to establish conditionally mutant animals using the cre-LoxP system, but this strategy involves the creation of two mouse lines (a cre driver mouse line and a “floxed” targeted mouse line), and often results in incomplete inactivation of the targeted gene. Further, the production of targeted knock-out and transgenic mouse models have traditionally been very time- and resource-consuming, and have called for sophisticated equipment and a high level of technical training. As a result, alternative technologies are still very desirable for functional studies in vivo.
One organ system particularly well suited for explant organ culture and ex vivo functional analyses is the developing gonads. The morphologically and functionally distinct testes and ovaries arise from a common organ, the genital ridge, and have the potential to differentiate into either depending on genetic cues. During normal development, the initiator of gonadal sex differentiation is the Y-chromosomal gene Sry (Sex-determining region on the Y), which triggers a cascade of regulatory pathways driving the XY gonad towards testis differentiation (Berta et al., 1990; Jäger et al., 1990; Koopman et al., 1991). In the absence of Sry, or rather SRY function, the XX gonads differentiate into ovaries (reviewed by Wilhelm and Koopman, 2006). This binary process provides an unparalleled opportunity to investigate the molecular pathways that lead to the formation of distinctively different organs.
Since the discovery of Sry as the mammalian sex-determining gene, much has been learned regarding the molecular principles governing gonadal differentiation. One of the most important genes acting downstream of Sry is Sox9 (SRY-box containing gene 9). Like Sry, Sox9 has been shown to be necessary and sufficient for testis differentiation. Gain-of-function in humans and mice result in XX sex reversal (Huang et al., 1999; Bishop et al., 2000; Vidal et al., 2001), while loss-of-function leads to skeletal defects and XY sex reversal (Foster et al., 1994; Wagner et al., 1994). SOX9 is an HMG-domain transcription factor, expressed in the pre-Sertoli cells of male genital ridges shortly after the onset of Sry expression around 10.5 days post coitum (dpc) in the mouse (Kent et al., 1996; Morais da Silva et al., 1996). Sox9 is thought to be a direct target of SRY (Sekido and Lovell-Badge, 2008) and to be the main transcriptional regulator of effector genes necessary for proper testis morphogenesis. Shortly after Sox9 transcription begins at 11.5 dpc, the testis becomes compartmentalized into cords encapsulating the gonocytes, and the interstitium. In contrast, no apparent morphological differentiation occurs in the developing ovary until after 13.5 dpc. Rather than being enclosed in cords, the female gonocytes gather into smaller clusters dispersed throughout the developing ovary.
In the testis, target genes of SOX9 involved in sex differentiation have been described, of which the best characterised encode anti-Müllerian hormone (AMH) (de Santa Barbara et al., 1998; Arango et al., 1999) and prostaglandin D synthase (PGDS) (Wilhelm et al., 2007). In the ovary, less is known regarding molecular pathways, albeit female-specific gene expression is well characterized from as early as 12 dpc onwards, with genes such as Wnt4 (wingless-related MMTV integration site 4), FoxL2 (forkhead-box L2), and Cav1 (caveolin-1) (Bullejos et al., 2002; Loffler et al., 2003; Yao et al., 2004). In addition, microarray analysis comparing male versus female transcriptomes at early time points of gonadal development identified many more genes that are female-specifically expressed (Nef et al., 2005; Beverdam and Koopman, 2006). Despite this high number, it is not clear if a female counterpart to Sry and Sox9, i.e. a female-determining factor, exists.
In an effort to better understand the genetic principles underpinning sexual differentiation of XY and XX gonads into testes and ovaries, several large-scale expression screens have been undertaken (Bowles et al., 2000; Wertz and Herrmann, 2000; Boyer et al., 2004; Nef et al., 2005; Small et al., 2005; Beverdam and Koopman, 2006). These screens have all aimed at identifying genes that either show sexually dimorphic expression and/or are up- or down-regulated during critical time-points during gonadal differentiation. As a result, a plethora of candidate genes have been identified as potential regulators of gonad differentiation, all warranting further investigations. However, with such a large number of candidate genes, classic transgenic and knock-out protocols are not feasible approaches in the short term. Hence, an assay designed to more rapidly screen for loss- and gain-of-function phenotypes of the many candidate genes would be of great benefit.
To address this issue, we have developed an ex vivo functional assay that involves the use of gonad explant cultures (Martineau et al., 1997). In this assay, mouse genital ridges are explanted at 11.5 dpc, before any overt signs of differentiation, and cultured on agar blocks to provide an air/medium interface that allows these ridges to develop for several days in culture. Micro-injection of gene and shRNA expression constructs followed by magnetically-mediated gene transfection (“magnetofection”) was used to provide insight into the function of candidate genes. Using this technique, we show that delivery of an Sry- expression plasmid into XX gonads or a Sox9 shRNA-expression plasmid into XY gonads resulted in localized sex-reversal with the formation of ovotestes. We also show that ectopic expression of Tmem184a (transmembrane protein 184a), a gene whose function has not been established, in female genital ridges caused XX germ cells to commit to the male fate. Our results indicate that magnetofection may provide a rapid indicator of gene function during organ development, a system that is likely to be broadly applicable in developmental biology.
Results
Microarray analyses and other expression screening approaches have identified hundreds of candidate genes in a variety of developmental processes such as sex determination and gonad development. Our present aim was to develop and establish proof-of-principle for a rapid and general method to analyze gain- and loss-of-function gene effects of these candidates. We developed an assay in which plasmid DNA associated with magnetic nanoparticles is injected into explanted genital ridges. Upon placing the tissue in a strong magnetic field, the DNA is drawn by the magnetic particles into the tissue, a process called magnetofection (Fig. 1A).
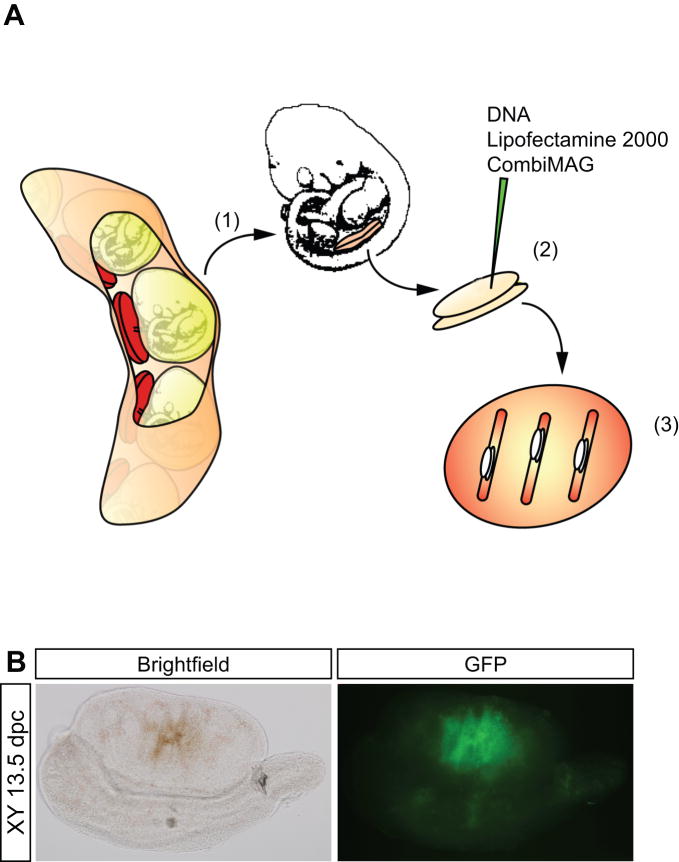
Figure 1. The magnetofection procedure.
(A) Schematic representation. Urogenital ridges are explanted from mouse embryos at 11.5 dpc (1), injected with DNA / Lipofectamine 2000 / CombiMag (DLC) mixture (2), placed on a magnetic plate, and cultured for 36 h on agar blocks (3). (B) Magnetofection results in efficient DNA delivery into gonad explants. An EGFP reporter construct was injected and magnetofected into 11.5 dpc XY gonad explants, which were subsequently cultured for three days. The injection site is clearly visible under brightfield by the distinct bronze-coloured magnetic beads (left panel). Expression of EGFP at this site was visualized by UV irradiation (right panel).
Using this method, we first tested whether a transgene could be successfully delivered and expressed. An expression plasmid for enhanced green fluorescence protein (EGFP) was injected into 11.5 dpc isolated gonads. After magnetofection, the gonads were cultured on agar blocks for up to five days. We detected EGFP expression at the site of injection within four hours after magnetofection, and expression was still detectable after five days of culture (Fig. 1B).
To optimize the procedure, various concentrations of expression constructs combined with Lipofectamine 2000 (Invitrogen) and CombiMAG (Chemicell) were tested, and the optimal cocktail for achieving expression in cultured genital ridges (see Methods) was used for all subsequent experiments. In our hands, excess CombiMAG or impure DNA caused the solution to coagulate, thus interfering with subsequent injection. Coagulation also occurred when the mixture was allowed to incubate beyond the optimum 20 min incubation time (see Methods), and so we adhered strictly to this timing for our experiments.
Microinjection and magnetofection were performed on genital ridges from several embryonic stages between 11.0 and 13.0 dpc. Although genital ridges from all stages were successfully magnetofected, we observed variations in transduction efficiencies after 72 hour culturing (data not shown). In general, later-staged genital ridges showed more abundant, and less regionally restricted, gene expression than early-stage samples (11.0-11.5 dpc). Also, with the early-staged gonads being very small organs, subsequent expression of the introduced constructs often ended up being restricted to the adjacent mesonephros, possibly a direct consequence of the mechanical difficulties of microinjection into a small and fragile tissue. Finally, although we subjected genital ridges to a constant magnetic field of 230 mT for 1 hour, extended periods of up to several hours did not affect the efficiency of the procedure.
Using these conditions, approximately 20% of genital ridges expressed the introduced construct. Of these, all showed regionally restricted expression confined to an area around the microinjection site. There was considerable variation of expression levels of the introduced expression constructs, likely reflecting the amount of plasmid injected into each sample. We were unable to accurately control or determine the amount of expression plasmids introduced with each injection. However, we successfully increased the area of transduction within individual samples by subjecting the genital ridges to more than one microinjection (data not shown). Finally, we did not observe any regional differences depending on the site of injection: transduction efficiencies were comparable independent of whether the expression constructs were introduced at the poles or in the centre of the gonad, or in the adjacent mesonephros.
Gain-of-function of Sry induces testicular development in cultured XX genital ridges
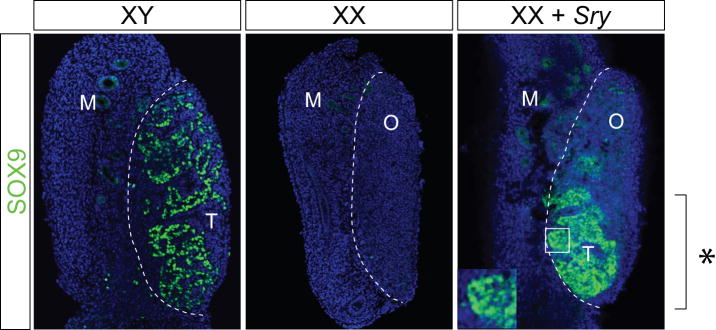
We next tested whether over-expression of genes known to be involved in sex determination and gonad development would direct downstream marker gene expression and alter the tissue architecture in the predicted way. XX genital ridges were injected and magnetofected with either an empty expression plasmid or an expression plasmid from which the testis-determining gene Sry is expressed under the control of the CMV promoter (pcHA-Sry). After 36 hours in culture, the gonads were subjected to paraffin section immunofluorescence for SOX9 to indicate differentiation along the male pathway, and FOXL2, a female-specific marker known to be expressed in granulosa cells (Cocquet et al., 2002; Schmidt et al., 2004). At this stage in development, Sox9 is normally expressed in XY but not XX genital ridges (Fig. 2). Ectopic expression of Sry in explanted XX genital ridges induced the expression of Sox9 (Fig. 2, right panel) and reduced expression of the female marker FOXL2 (data not shown) around the site of DNA injection. These effects are not due to the injection and magnetofection itself, because gonads treated with an empty expression vector did not up-regulate SOX9 (data not shown). Furthermore, ex vivo gene transfer of Sry induced gross alteration in tissue structure, with localized development of testis cords visible in the treated XX gonads at the site of expression (Fig. 2). These results demonstrate that micro-injection and magnetofection of Sry resulted in functional gene expression in sufficient cells to induce localized testis differentiation in intact XX gonads. Nuclear localisation of SOX9 was observed in the sex-reversed region (Fig. 2, right panel, inset), as expected since the upregulation of endogenous SOX9 is a result of ectopic Sry expression.
Figure 2. Delivery of an Sry expression construct induces localized testis differentiation in XX genital ridges.
11.5 dpc XY (left panel) and XX (middle panel) and XX genital ridges magnetofected with an Sry expression plasmid (right panel, XX + Sry) were cultured for three days. Subsequently, immunofluorescence was performed on sagittal paraffin sections for SOX9 (green). SOX9 is detectable in the Sertoli cells of the XY gonad but not in an XX gonad. The ectopic expression of Sry leads to the induction of nuclear SOX9 expression and the formation of testis cords around the area of injection (right panel and inset). M, mesonephros; O, ovarian tissue; T, testicular tissue.
Loss-of-function of Sox9 suppresses testis cord formation and induces female gene expression in XY genital ridges
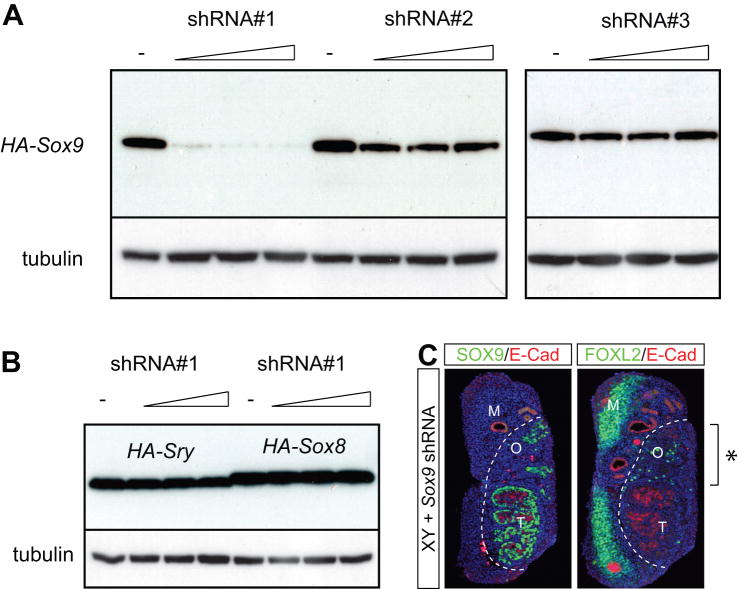
Having shown the ability to achieve gain-of-function of a gene of interest, we next investigated the use of this technique for loss-of-function studies. For this purpose, we designed three siRNAs to silence Sox9 expression, and cloned the corresponding DNA sequences into a vector that allowed them to be expressed as short hairpin RNAs (shRNAs). To test the ability of these shRNA constructs to suppress SOX9 expression, we transfected them in increasing amounts together with a HA-Sox9 expression plasmid into HEK293 cells. Western blotting demonstrated that shRNA-construct #1 was over 95% effective in silencing the expression of the co-transfected pcHA-Sox9 expression construct, while #2 was approximately 40% effective, and #3 showed no silencing effect compared to the level of expression observed without shRNA (Fig. 3A). To confirm the specificity of the most effective shRNA construct #1, we co-transfected this construct into HEK293 cells, together with expression constructs for Sry and Sox8 (SRY-box containing gene 8). We did not observe any knock-down of expression of these closely-related proteins by Western blotting (Fig. 3B).
Figure 3. Delivery of a Sox9 shRNA expression construct represses cord formation in XY genital ridges.
(A) Validation of knock-down efficiency and specificity of Sox9 shRNA constructs. Anti-HA Western blot analysis showed the effect of increasing amounts of three different Sox9 shRNA expression constructs (shRNA#1, 2 and 3) on HA-SOX9 protein levels. shRNA#1 is the most efficient in blocking SOX9 expression. (B) Anti-HA Western blot analysis demonstrated that the closely related genes Sry and Sox8 are not affected by Sox9 shRNA#1 at all concentrations tested.α–Tubulin Western blot served as a loading control in all experiments. (C) Magnetofection of Sox9-shRNA construct into XY genital ridges. Immunofluorescence of serial paraffin sections of the same sample for SOX9 or FOXL2 (green) and E-Cadherin (red). The expression of the Sox9-shRNA leads to the disruption of SOX9 expression and testis cord formation as well as up-regulation of the female marker FOXL2 in the magnetofected region. M, mesonephros; O, ovarian tissue; T, testicular tissue.
We injected and magnetofected Sox9 shRNA-construct #1 into 11.5 dpc XY genital ridges. After culturing the gonad explants, immunofluorescence analysis revealed localized inhibition of SOX9 expression and the induction of the female program as shown by the expression of FOXL2 (Fig. 3C). Strikingly, magnetofection of SOX9 shRNA construct resulted in a greatly reduced number of SOX9-positive cells, and disrupted cord structure in this region (Fig. 3C). Therefore, similar to the SRY gain-of-function experiments, inhibition of SOX9 expression resulted in the formation of ovotestes. These experiments indicate that both gain- and loss-of-function approaches successfully resulted not only in the expected alterations of gene expression, but also in profound morphological changes indicating localized gonadal sex reversal.
Mis-expression of Tmem184a results in altered germ cell fate in the developing ovary
Finally, we used this functional assay to study the effect of altering the expression of a male-specific gene of unknown function, Tmem184a, which encodes a putative transmembrane protein. We have previously shown that Tmem184a is specifically up-regulated within the Sertoli cells of the developing testes shortly after the onset of Sox9 expression (Svingen et al., 2007). We have also found that Tmem184a transcription is dependent on Sox9 (data not shown). Recently, the protein encoded by Tmem184a (also referred to as Sdmg1) was verified as being sex-specifically expressed, and was implicated in signalling by Sertoli cells to influence germ cell sex differentiation (Best et al., 2008). However, the biochemical function of TMEM184A is still to be characterized.
We designed and cloned a construct to express mouse TMEM184A fused to the HA-tag at the N-terminus, under the control of the CMV promoter. Transfection of TM3 and 12XG cells with the TMEM184A expression construct confirmed expression of the TMEM184A protein in these cells (data not shown). Since protein tags can interfere with normal protein trafficking, expression was also verified by a C-terminally tagged expression construct. The expression pattern corresponded with that observed by Best and co-workers (2008).
Using magnetofection, we ectopically expressed Tmem184a in 11.5 dpc XX genital ridges. We assessed germ cell progression after 72 hours of culture by immunofluorescence experiments using the germ cell markers OCT4 (Octamer binding transcription factor 4; POU domain, class 5, transcription factor 1, Pou5f1) and SYCP3 (Synaptonemal complex protein 3) relative to XY and XX controls (Fig. 4A-C). OCT4 is expressed by all germ cells in the bipotential gonads and is maintained in male germ cells as they enter mitotic arrest, but ceases to be expressed in female germ cells as they enter meiosis (Fig. 4D,F; Pesce et al., 1998). SYCP3 is female-specifically up-regulated at the onset of meiosis (Fig. 4G,I), however some SYCP3 expression is evident also in early and mitotically arrested spermatogonia, seen by spotted aggregates compared to the more uniform staining reflecting the open conformation of the synaptonemal complex of meiotic oocytes (Fig. 4M,O; Di Carlo et al., 2000; Chuma and Nakatsuji, 2001).
Figure 4. Ectopic expression of Tmem184a in XX genital ridge results in germ cell sex reversal.
Section ISH indicates injection sites (arrow) of the cultured genital ridges (panels A-C). Immunofluorescence analyses revealed altered germ cell fate in TMEM184A magnetofected genital ridges as assayed with the marker proteins OCT4 (green) and SYCP3 (red). The pluripotency marker OCT4 is expressed by all germ cells at early stages during gonadogenesis. From 13.5 dpc onwards, OCT4 is down-regulated in XX gonads as the germ cells enters meiosis (panel F), but continues to be expressed by germ cells in the XY gonads as the germ cells enter mitotic arrest (panel D). SYCP3, which is a component of the synaptonemal complex, is only detectable in mitotically arrested germ cells as nuclear aggregates (panels G, M), but is upregulated in XX germ cells as the chromatin forms extensive thread-like complexes (panels I, O). In Tmem184a magnetofected XX genital ridges (panel B), we observed a mixture of OCT- and SYCP3-positive germ cells (panels E, H, K, N), indicating the presence of mitotically arrested gonocytes in later stage ovaries. Hence, by ectopically expressing the testis-specific gene Tmem184a in differentiating ovaries, the gonocytes are driven towards mitotic arrest; a hallmark of early testis differentiation. Arrowheads denote mitotically arrested gonocytes; arrows denote meiotic oocytes. Scale bars: panel A = 200 μm; panel M = 20 μm.
Ectopic expression of Tmem184a in the XX genital ridges resulted in a large number of germ cells failing to enter meiosis after 72 hours in culture (Fig. 4K). In XX control gonads, the majority of the germ cells showed a marked up-regulation of SYCP3 and lack of OCT4 (Fig. 4L), indicating that the time of gonad culture was sufficient for most of the female germ cells to reach meiotic prophase I. High magnification of SYCP3 immunostaining revealed a large population of non-meiotic germ cells (arrowheads) in the Tmem184a-magnetofected ovaries compared to control testis and control ovary (Fig. 4M-O), the latter showing meiotic oocytes (arrows) characterized by strong up-regulation and redistribution of SYCP3 protein. Although the commitment of germ cells into meiosis is reported to occur sequentially, with the up-regulation of Sycp3 following an anterior-to-posterior wave in the ovary (Bullejos and Koopman, 2004), we note that the observed spermatogonial-like germ cells of Tmem184a-magnetofected ovaries generally located closer to the injection site and that the meiotic germ cells were dispersed along the entire gonadal axis, seemingly independent of an anterior-posterior wave. Therefore, our data suggest that expression of TMEM184A is sufficient to prevent germ cells from entering meiosis.
Discussion
In this study we sought to develop a system for rapid functional analysis of genes of unknown function, particularly those with roles in organ development in the vertebrate embryo. Using mouse fetal explant cultures combined with magnetofection, we were able to stably alter the developmental fate of an indifferent organ: XX genital ridges gave rise to testicular tissue in response to Sry action, and XY genital ridges generated ovarian tissue in response to Sox9 shRNA. These changes were induced only at the site of injection, providing an internal control for comparison with untreated parts of the gonad.
Typically, 20% of magnetofected genital ridges expressed the introduced construct. Although a transient technique, the success rate is comparatively similar to the rate of incorporation and expression of transgenes introduced by pronuclear injection or the range of electroporation efficiencies for gene knockout constructs electroporated into ES cells. This rate varied between experiments from zero to 30%, associated with variation between batches of CombiMAG and developmental stage of genital ridges. 11.5 dpc samples supported more robust gene expression, were more refractory to tissue damage caused by injection, and were easier to inject accurately, than 11.0 dpc samples. Therefore, conditions need to be optimized for each laboratory, reagent set and target tissue to achieve desired results.
For example, we achieved a magnetic field of 230 mT by placing the microinjected genital ridges directly on top of the commercially supplied magnet, separated only by the thickness of the Petri dish. This magnetic field was sufficient for successful magnetofection of genital ridges, and is likely to suffice for other similarly small, soft tissues, since it has been optimized by the manufacturer for use with cultured cells. With other tissues it is conceivable that the optimal strength of the magnetic field could differ. The magnetic field strength can be reduced by altering the distance between the magnetic plate and the microinjected tissues, with doubling of the distance approximately reducing the magnetic strength by a factor of four, or increased by using a customized magnet. Further, we expect the stability of the transfected constructs will depend on the type of construct, the type of reporter, and the type of tissue being studied.
In addition to proof-of-principle experiments involving the known sex-determining genes Sox9 and Sry, we showed that this technique is also useful for studying the functions of novel factors. Ectopic expression of the testis-specific gene Tmem184a by magnetofection in explant female genital ridges resulted in a large number of gonocytes failing to enter meiosis within the ovary. Recently, a similar phenotype was reported for Tmem184a by aggregating explant gonad cultures with cells of a Sertoli cell line (SK11) that shows endogenous Tmem184a expression (Best et al., 2008). These results indicate that the signal for mitotic arrest is of a paracrine nature. However, such aggregation protocols are only possible if the downstream effect of the specific protein function is mediated through secretory signalling pathways. With ex vivo magnetofection, protein function can be studied without reliance on paracrine signalling, as the gene is injected directly into the developing tissue.
Whereas both the Sry and Sox9-shRNA magnetofection experiments described give rise to phenotypes of the somatic compartments of the developing gonads, the Tmem184a magnetofected XX genital ridges showed a gonocyte-specific phenotype. Concomitant with the sex-specific somatic differentiation of XY and XX gonads, the germ cells follow different fates depending on the gonadal environment they inhabit. In testes the germ cell lineage becomes committed to the spermatogenic pathway around 12.5 dpc (McLaren and Southee, 1997). In the ovaries, germ cells commit to oogenesis, marked by initiation of meiosis. Since uncommitted PGCs have the innate ability to commit to either the spermatogenic or oogenic pathway depending on the surrounding environment, they must be responsive to sex-determining signals from either the testis or ovary, or both. It has been shown that meiosis is brought about in the ovary by retinoic acid diffusing into the XX gonad from the adjacent mesonephros (Bowles et al., 2006; Koubova et al., 2006), whereas degradation of retinoic acid by the testis-specific enzyme CYP26B1 (cytochrome P450, family 26, subfamily b, polypeptide 1) is thought to prevent gonocytes from entering meiosis in the developing testis (Bowles et al., 2006). In addition to a retinoic acid-driven signalling system inducing germ cells to enter meiosis in the ovary, another signalling mechanism has been proposed to induce mitotic arrest of germ cells within the testis (McLaren, 2003). Best and co-workers (2008) reported that TMEM184A is likely involved in this testis-specific signalling pathway. However, the phenotype was achieved by culturing female gonads aggregated to a Sertoli cell line rather than ectopic expression of Tmem184a in the gonad proper. Here we report a direct gain-of-function effect by ectopic Tmem184a expression in explanted female genital ridges, circumventing potential interfering mechanisms from an in vitro cell culture system. However, as both studies result in the same phenotype (altered germ cell fate), together they strengthen the hypothesis that TMEM184A is a Sertoli cell-specific factor necessary for gonocyte progression down the male pathway during embryonic testis differentiation and development. Finally, expression of Tmem184a in explanted female genital ridges resulted in a mixed population of OCT4 and SYCP3 positive germ cells within the same gonad. It is possible that this pathway acts antagonistically with the retinoic acid-induced meiotic pathway in the female gonad. We hypothesize that threshold values of either signalling pathway are required within a given time-frame for successful commitment to the male or female differentiation pathway.
In summary, our study shows that delivery of expression or knock-down constructs results in dramatic alteration of downstream gene regulation and morphogenesis, thus making this technique not only useful to introduce lineage tracers but also to gain functional insight into candidate genes. We expect this technique will provide a useful adjunct to existing methods of functional analysis of genes involved in sex determination and gonad development. With further tissue-specific optimization, we also predict that this methodology will be applicable to other mammalian organogenetic systems including kidney (Gupta et al., 2003) and lung (Blewett et al., 1995), in which organ explant cultures have been carried out successfully.
Experimental Procedures
Plasmids
pcHA-Sry, pcHA-Sox9 and pcHA-Sox8 were used to express mouse SRY, SOX9 and SOX8 protein, respectively, tagged with a hemagglutinin (HA) epitope at the amino-terminal end (Beverdam et al., 2003). pcHA-Tmem184a was cloned by inserting the open reading frame of mouse Tmem184a in frame with the HA-tag at the amino-terminus into pcDNA3.1 (Promega). pSil-Sox9 was used to express a short hairpin RNA targeting the mouse Sox9 gene. For this, pSil-Sox9 was constructed by inserting a small DNA insert (#1: Sox9 184 to 204; #2: Sox9 853 to 873; #3 Sox9-3′UTR 1682 to 1702 according to the number from the first base of the start codon, into the pSilencer vector (Ambion). Plasmids were purified with JETstar 2.0 maxi kit (GENOMED, Australia) and used in a 1 μg/μl concentration.
Animals and cell lines
Embryos were collected from timed matings of outbred CD1 strain mice, with noon of the day on which the mating plug was observed designated 0.5 dpc. For more accurate staging, the tail somite (ts) stage of the embryo was determined by counting the number of somites posterior to the hind limb (Hacker et al., 1995). Using this method, 10.5 dpc corresponds to approx. 8 ts, 11.5 dpc to 18 ts, and 12.5 dpc to 30 ts.
The 12XG cell line was established by preparing a single cell suspension of 12.5 dpc XX gonads from the transgenic mouse line expressing EGFP from the X-chromosome (Hadjantonakis et al., 1998). Cells were cultured at 37°C, 5% CO2 in Dulbecco's Modified Eagle Medium (DMEM, Gibco) supplemented with 10% serum (Serum Supreme, BioWhittaker) and 2 mM glutamine until spontaneously immortalized.
HEK293 and TM3 cells were cultured at 37°C, 5% CO2 in Dulbecco's Modified Eagle Medium (DMEM, Gibco) supplemented with 10% serum (Serum Supreme, BioWhittaker) and 2 mM glutamine.
Western blot analysis
HEK293 cells were seeded at 7.5×105 cells per well in 6-well plates. Eight hours later the cells were transfected with 1 μg of pcHA-Sox8, pcHA-Sox9 or pcHA-Sry with or without increasing amounts of pSil-Sox9 (total amount of 4 μg DNA was achieved by adding empty pSilencer plasmid) using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturers' recommendations. After 24 h, proteins were extracted in 2X sample buffer (125mM tris, pH 6.8; 4% SDS; 20% glycerol and 5% β-mercaptoethanol). Primary anti-HA (Sigma) and anti-tubulin (Sigma) were used at 1:2000 dilutions overnight at 4°C. HRP-conjugated secondary antibody followed by chemiluminescence detection with Super Signal West Pico Chemiluminescence reagent (Pierce, Rockford, IL, USA) to reveal protein expression.
Magnetofection
Genital ridges were dissected in L15 medium (Sigma). For each microgram of expression plasmid, 2.5 μl Lipofectamine 2000 (Invirogene) was added, incubated for 20 min at room temperature, mixed with 3.5 μl CombiMAG (Chemicell) and incubated for another 20 min at room temperature before injected into genital ridges using a glass capillary (KIMAX-51). Genital ridges were placed on a 230 mT NdFeB (5× 5mm) magnetic plate (Chemicell) in L15 medium for 1 h at room temperature before placed on a 1.5% agar (Difco)/DMEM block in DMEM supplemented with 10% fetal bovine serum (Cambrex) and 100 μg/ml ampicillin (Sigma). Gonads were cultured for 36-72 h under the same conditions before being subjected to further analysis. Mock injections were performed alongside experimental injections as a control.
Immunofluorescence
Immunofluorescence on cells was performed as described previously (Beverdam et al., 2003). Cultured gonads were fixed in 4% paraformaldehyde in PBS at 4°C for 2 h, rinsed three times with PBTx (PBS containing 0.1% Triton X-100), dehydrated and embedded in paraffin. Section immunofluorescence of 7 μm thick paraffin sections was performed as described previously (Wilhelm et al., 2005). The following antibodies were used: rabbit anti-SOX9 (1:300, (Wilhelm et al., 2005), rabbit anti-FOXL2 (1:500, kindly provided by M. Fellous), mouse anti-E-Cadherin (1:200, BD Biosciences), mouse-anti-WT1 (1:1000, DAKO), rabbit-anti-DAX1 (1:100, Santa Cruz), mouse-anti-OCT4 (1:200, Santa Cruz) and rabbit-anti-SCP3 (1:300, Abcam) All secondary antibodies, goat anti-rabbit Alexa Fluor 488, goat anti-rabbit Alexa Fluor 594, goat anti-mouse Alexa Fluor 488, and goat anti-mouse Alexa Fluor 594 were obtained from Molecular Probes and used at 1:200 dilution.
Acknowledgments
We thank Marc Fellous for the FOXL2 antibody, Fred Martinson for help with Fig. 1, Tara Davidson for technical assistance, and Josephine Bowles for helpful comments on the manuscript. DW is a CDA Fellow of the National Health and Medical Research Council of Australia; PK is a Federation Fellow of the Australian Research Council.
Grant sponsors: Australian Research Council, National Health and Medical Research Council of Australia, National Institutes of Health, USA (NIH HD049431).
References
- Arango NA, Lovell-Badge R, Behringer RR. Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell. 1999;99:409–419. doi: 10.1016/s0092-8674(00)81527-5. [DOI] [PubMed] [Google Scholar]
- Baker AH, Kritz A, Work LM, Nicklin SA. Cell-selective viral gene delivery vectors for the vasculature. Exp Physiol. 2005;90:27–31. doi: 10.1113/expphysiol.2004.028126. [DOI] [PubMed] [Google Scholar]
- Berta P, Hawkins JR, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, Fellous M. Genetic evidence equating SRY and the male sex determining gene. Nature. 1990;348:448–450. doi: 10.1038/348448A0. [DOI] [PubMed] [Google Scholar]
- Best D, Sahlender DA, Walther N, Peden AA, Adams IR. Sdmg1 is a conserved transmembrane protein associated with germ cell sex determination and germline-soma interactions in mice. Development. 2008;135:1415–1425. doi: 10.1242/dev.019497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverdam A, Koopman P. Expression profiling of purified mouse gonadal somatic cells during the critical time window of sex determination reveals novel candidate genes for human sexual dysgenesis syndromes. Hum Mol Genet. 2006;15:417–431. doi: 10.1093/hmg/ddi463. [DOI] [PubMed] [Google Scholar]
- Beverdam A, Wilhelm D, Koopman P. Molecular characterization of three gonad cell lines. Cytogenet Genome Res. 2003;101:242–249. doi: 10.1159/000074344. [DOI] [PubMed] [Google Scholar]
- Bishop CE, Whitworth DJ, Qin Y, Agoulnik AI, Agoulnik IU, Harrison WR, Behringer RR, Overbeek PA. A transgenic insertion upstream of Sox9 is associated with dominant XX sex reversal in the mouse. Nat Genet. 2000;26:490–494. doi: 10.1038/82652. [DOI] [PubMed] [Google Scholar]
- Blewett CJ, Cilley RE, Ehrlich HP, Dillon PW, Blackburn JH, Krummel TM. Regenerative healing of incisional wounds in murine fetal lungs maintained in organ culture. J Pediatr Surg. 1995;30:945–948. doi: 10.1016/0022-3468(95)90318-6. [DOI] [PubMed] [Google Scholar]
- Bowles J, Bullejos M, Koopman P. A subtractive gene expression screen suggests a role for vanin-1 in testis development in mice. Genesis. 2000;27:124–135. [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- Boyer A, Lussier JG, Sinclair AH, McClive PJ, Silversides DW. Pre-Sertoli specific gene expression profiling reveals differential expression of Ppt1 and Brd3 genes within the mouse genital ridge at the time of sex determination. Biol Reprod. 2004;71:820–827. doi: 10.1095/biolreprod.104.029371. [DOI] [PubMed] [Google Scholar]
- Bullejos M, Bowles J, Koopman P. Extensive vascularization of developing mouse ovaries revealed by caveolin-1 expression. Dev Dyn. 2002;225:95–99. doi: 10.1002/dvdy.10128. [DOI] [PubMed] [Google Scholar]
- Bullejos M, Koopman P. Germ cells enter meiosis in a rostro-caudal wave during development of the mouse ovary. Mol Reprod Dev. 2004;68:422–428. doi: 10.1002/mrd.20105. [DOI] [PubMed] [Google Scholar]
- Capecchi MR. Altering the genome by homologous recombination. Science. 1989;244:1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Chuma S, Nakatsuji N. Autonomous transition into meiosis of mouse fetal germ cells in vitro and its inhibition by gp130-mediated signaling. Dev Biol. 2001;229:468–479. doi: 10.1006/dbio.2000.9989. [DOI] [PubMed] [Google Scholar]
- Cocquet J, Pailhoux E, Jaubert F, Servel N, Xia X, Pannetier M, De Baere E, Messiaen L, Cotinot C, Fellous M, Veitia RA. Evolution and expression of FOXL2. J Med Genet. 2002;39:916–922. doi: 10.1136/jmg.39.12.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Sudbeck P, Scherer G, Poulat F, Berta P. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Mullerian hormone gene. Mol Cell Biol. 1998;18:6653–6665. doi: 10.1128/mcb.18.11.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo A, Travia G, De Felici M. The meiotic specific synaptonemal complex protein SCP3 is expressed by female and male primordial germ cells of the mouse embryo. Int J Dev Biol. 2000;44:241–244. [PubMed] [Google Scholar]
- Ewert K, Slack NL, Ahmad A, Evans HM, Lin AJ, Samuel CE, Safinya CR. Cationic lipid-DNA complexes for gene therapy: understanding the relationship between complex structure and gene delivery. Curr Med Chem. 2004;11:133–149. doi: 10.2174/0929867043456160. [DOI] [PubMed] [Google Scholar]
- Foster JW, Domingues-Steglich MA, Guioli S, Kowk G, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, Brook JD, Schafer AJ. Campomelic dysplasia and autosomal sex reversal caused my mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- Gondo Y. Trends in large-scale mouse mutagenesis: from genetics to functional genomics. Nat Rev Genet. 2008;9:803–810. doi: 10.1038/nrg2431. [DOI] [PubMed] [Google Scholar]
- Gupta IR, Lapointe M, Yu OH. Morphogenesis during mouse embryonic kidney explant culture. Kidney Int. 2003;63:365–376. doi: 10.1046/j.1523-1755.2003.00715.x. [DOI] [PubMed] [Google Scholar]
- Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121:1603–1614. doi: 10.1242/dev.121.6.1603. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A. Non-invasive sexing of preimplantation stage mammalian embryos. Nat Genet. 1998;19:220–222. doi: 10.1038/893. [DOI] [PubMed] [Google Scholar]
- Huang B, Wang S, Ning Y, Lamb AN, Bartley J. Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet. 1999;87:349–353. doi: 10.1002/(sici)1096-8628(19991203)87:4<349::aid-ajmg13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Jäger RJ, Anvret M, Hall K, Scherer G. A human XY female with a frame shift mutation in the candidate sex-determining gene SRY. Nature. 1990;348:452–454. doi: 10.1038/348452a0. [DOI] [PubMed] [Google Scholar]
- Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little CC, Bagg HJ. The occurrence of two heritable types of abnormality among the descendants of X-rayed mice. Amer J Roentgenol Radiat Therap. 1923;10:975–989. [Google Scholar]
- Loffler KA, Zarkower D, Koopman P. Etiology of ovarian failure in blepharophimosis ptosis epicanthus inversus syndrome: FOXL2 is a conserved, early-acting gene in vertebrate ovarian development. Endocrinology. 2003;144:3237–3243. doi: 10.1210/en.2002-0095. [DOI] [PubMed] [Google Scholar]
- Martineau J, Nordquist K, Tilmann C, Lovell-Badge R, Capel B. Male specific cell migration into the developing gonad. Curr Biol. 1997;7:958–968. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci USA. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- McLaren A, Southee D. Entry of mouse embryonic germ cells into meiosis. Dev Biol. 1997;187:107–113. doi: 10.1006/dbio.1997.8584. [DOI] [PubMed] [Google Scholar]
- Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;14:62–68. doi: 10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- Muller HJ. Artificial transmutation of the gene. Science. 1927;66:84–87. doi: 10.1126/science.66.1699.84. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Mizutani Y, Ohmori Y, Okumura J. Comparison of three nonviral transfection methods for foreign gene expression in early chicken embryos in ovo. Biochem Biophys Res Commun. 1997a;230:376–380. doi: 10.1006/bbrc.1996.5882. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Shibata O, Ryoki S, Ohmori Y, Okumura J. Foreign gene expression in the mouse testis by localized in vivo gene transfer. Biochem Biophys Res Commun. 1997b;233:45–49. doi: 10.1006/bbrc.1997.6361. [DOI] [PubMed] [Google Scholar]
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, Parker KL, Vassalli JD. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 2005;287:361–377. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Brinster RL, Hammer RE, Trumbauer ME, Rosenfeld MG, Birnberg NC, Evans RM. Dramatic growth of mice that develop from eggs microinjected with metallothionein-growth hormone fusion genes. Nature. 1982;300:611–615. doi: 10.1038/300611a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce M, Wang X, Wolgemuth DJ, Scholer H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev. 1998;71:89–98. doi: 10.1016/s0925-4773(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–942. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- Small CL, Shima JE, Uzumcu M, Skinner MK, Griswold MD. Profiling gene expression during the differentiation and development of the murine embryonic gonad. Biol Reprod. 2005;72:492–501. doi: 10.1095/biolreprod.104.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A. Adeno-associated virus-mediated gene transfer. J Cell Biochem. 2008;105:17–24. doi: 10.1002/jcb.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingen T, Beverdam A, Bernard P, McClive P, Harley VR, Sinclair AH, Koopman P. Sex-specific expression of a novel gene Tmem184a during mouse testis differentiation. Reproduction. 2007;133:983–989. doi: 10.1530/REP-06-0379. [DOI] [PubMed] [Google Scholar]
- Vidal VPI, Chaboissier MC, de Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Wertz K, Herrmann BG. Large-scale screen for genes involved in gonad development. Mech Dev. 2000;98:51–70. doi: 10.1016/s0925-4773(00)00452-4. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Hiramatsu R, Mizusaki H, Widjaja L, Combes AN, Kanai Y, Koopman P. SOX9 regulates Prostaglandin D Synthase gene transcription in vivo to ensure testis development. J Biol Chem. 2007;282:10553–10560. doi: 10.1074/jbc.M609578200. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Koopman P. The makings of maleness: Towards an integrated view of male sexual development. Nat Rev Genet. 2006;7:620–631. doi: 10.1038/nrg1903. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Martinson F, Bradford S, Wilson MJ, Combes AN, Beverdam A, Bowles J, Mizusaki H, Koopman P. Sertoli cell differentiation is induced both cell-autonomously and through prostaglandin signaling during mammalian sex determination. Dev Biol. 2005;287:111–124. doi: 10.1016/j.ydbio.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Yagi T, Ozaki T, Imoto K. In vivo gene transfer to mouse spermatogenic cells using green fluorescent protein as a marker. J Exp Zool. 2000;286:212–218. doi: 10.1002/(sici)1097-010x(20000201)286:2<212::aid-jez13>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Yao HHC, Matzuk MM, Jorgez CJ, Menke DB, Page DC, Swain A, Capel B. Follastatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn. 2004;230:210–215. doi: 10.1002/dvdy.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K, Momose T, Takahashi Y. Applications of microelectroporation for studies of chick embryogenesis. Dev Growth Differ. 2000;42:203–206. doi: 10.1046/j.1440-169x.2000.00502.x. [DOI] [PubMed] [Google Scholar]
- Yasugi S, Nakamura H. Gene transfer into chicken embryos as an effective system of analysis in developmental biology. Dev Growth Differ. 2000;42:195–197. doi: 10.1046/j.1440-169x.2000.00500.x. [DOI] [PubMed] [Google Scholar]
- Yozu M, Tabata H, Nakajima K. The caudal migratory stream: a novel migratory stream of interneurons derived from the caudal ganglionic eminence in the developing mouse forebrain. J Neurosci. 2005;25:7268–7277. doi: 10.1523/JNEUROSCI.2072-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabner J, Fasbender AJ, Moninger T, Poellinger KA, Welsh MJ. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem. 1995;270:18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]