Summary
Background
Altered expression of apicobasal polarity factors is associated with cancer in vertebrates and tissue overgrowth in invertebrates, yet mechanisms by which these factors affect growth regulatory pathways are not well defined. We have tested the basis of an overgrowth phenotype driven by the Drosophila protein Crumbs (Crb), which nucleates an apical membrane complex that functionally interacts with the Par6/Par3/aPKC and Scrib/Dlg/Lgl apicobasal polarity complexes.
Results
We find that Crb-driven growth is dependent upon the Salvador-Warts-Hippo (SWH) pathway and its transcriptional effector Yorkie (Yki). Expression of the Crb intracellular domain elevates Yki activity and this correlates in tissues and cultured cells with loss of Expanded (Ex), an apically localized SWH component that inhibits Yki. Reciprocally, loss of crb elevates Ex levels, although this excess Ex does not concentrate to its normal location at apical junctions. The Ex-regulatory domain of Crb maps to the juxtamembrane FERM-domain binding motif (JM), a cytoskeletal interaction domain distinct from the PDZ-binding motif (PBM) through which Crb binds polarity factors. Expression of Crb-JM drives Yki activity and organ growth with little effect on tissue architecture, while reciprocally Crb-PBM produces tissue architectural defects without significant effect on Yki activity.
Conclusions
These studies identify Crb as a novel SWH regulator via JM-dependent effects on Ex levels and localization, and show that discrete domains within Crb may allow it to integrate junctional polarity signals with a conserved growth pathway.
Introduction
Mutations in any of three Drosophila melanogaster genes required for the maintenance of apicobasal polarity - Discs large (Dlg), lethal giant larvae (lgl), and scribble (scrib) - result in disorganized overgrowth of epithelial tissues [1]. The discoveries that homologs of these genes are downregulated in cancer and targeted for inactivation by mammalian tumor viruses [2] have further advanced the hypothesis that defective epithelial polarization can drive ectopic cell proliferation and tissue overgrowth in metazoans. The mechanisms by which Drosophila Dlg, Lgl and Scrib restrict cell proliferation are not well understood, although it is assumed that they are an extension of their more primary roles in cell polarization.
The polarization of Drosophila epithelial cells is controlled by functional interactions between membrane-associated protein complexes [1]. In the embryonic ectoderm, the Dlg/Scrib complex localizes to the basolateral cell membrane and functionally antagonizes the apical Par6/Par3/aPKC (Par) complex. The Par complex recruits a second apically localized complex, composed of the Crumbs (Crb), Patj and Pals1/Stardust (Sdt) proteins, which represses activity of the Scrib complex. Mutations in Dlg, lgl or scrib lead to membrane ‘apicalization’ in which the Crb complex spreads ectopically into the basolateral membrane. Overexpression of Crb in discs leads to a similar spreading phenotype and produces overgrowth in a manner overtly similar to that seen in Dlg, scrib, or lgl mutants [3, 4]. Certain endocytic mutants that block Crb turnover also cause disc overgrowth [4, 5], although the role of Crb in this phenotype is not clear. Intriguingly, a crb transgene encoding the transmembrane region and the small 37 amino acid intracellular tail is oncogenic when expressed in discs [3, 4], arguing that the Crb intracellular tail contains a growth regulatory domain. The Crb cytoplasmic tail has two recognized motifs, one that links to the spectrin and actin cytoskeleton and another that interacts with polarity regulatory factors such as Sdt, Patj, and Par6 [6]. The ability of Crb to drive tissue growth is thus either due to a previously described role for one of these motifs, or to a previously unrecognized growth regulatory domain embedded within the Crb cytoplasmic tail.
Here we show that the Crb cytoplasmic tail drives organ growth via the Salvador-Warts-Hippo (SWH) pathway, a conserved signaling network that restricts cell proliferation and promotes apoptosis by regulating the Yorkie (Yki) transcriptional co-factor [7]. The activities of the core SWH proteins are controlled by inputs from a variety of ‘non-core’ peripheral regulators that are required for growth inhibition by the SWH pathway, and in some cases render SWH responsive to different upstream inputs, including those from planar cell polarity pathways, morphogen gradients, and adhesion molecules [8]. We show that Crb restricts accumulation of Expanded [9], a FERM-domain protein that localizes to the apical-membrane and acts as a ‘peripheral’ regulator of the SWH pathway [10]. Moreover, this Ex-regulatory function maps to a small motif within the Crb cytoplasmic tail that coincides with juxtamembrane FERM-binding motif, which is distinct from the domain through which Crb binds polarity factors. These studies identify the Crb protein as a novel regulator of the SWH pathway via its effects on Ex, and suggest that discrete domains within Crb may allow it to simultaneously regulate apical polarity and tissue growth.
Results
The intracellular domain of crb requires yki to drive tissue growth
Previous studies have shown that engrailed-Gal4 (en-Gal4) driven expression of either a full-length UAS-crb transgene or one encoding only intact transmembrane and intracellular domains (UAS-crbi) [3] produces wing overgrowth [4]. Because the en>crb genotype led to significant embryonic lethality (data not shown), the en>crbi genotype was used to model the cell-autonomous effect of the Crb intracellular tail on wing development.
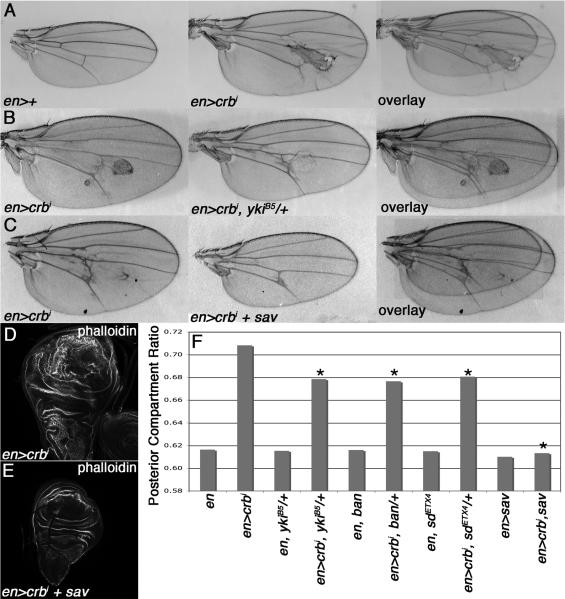
en>crbi larval wing discs show posterior domain overgrowth and tissue disorganization (Fig. 1D) [see also 4]. en>crbi adult wings also show an enlarged posterior compartment and cuticular defects (Fig. 1A). Quantification of posterior compartment size relative to the entire wing produces a value we termed the posterior compartment ratio (PCR) that is elevated in en>crbi animals (Fig. 1F). To understand the basis for this PCR phenotype, chromosomal deficiencies and selected alleles of pro-growth genes were screened for their ability to dominantly reduce en>crbi wing phenotypes. Multiple alleles of pro-growth members of the SWH growth regulatory pathway, including the transcriptional co-factor yorkie (yki), the microRNA bantam (ban), and the transcription factor scalloped (sd), dominantly suppressed the en>crbi PCR phenotype (Figs. 1B,F). These alleles did not modify PCR of control wings, indicating their effects on en>crbi wings do not reflect dosage-sensitive roles in wing growth (Fig. 1F). Co-expression of the Sav protein, which antagonizes Yki [7], also suppressed growth of larval and adult en>crbi wings (Fig. 1C,E-F). Alleles of Tor, rheb, rolled/ERK, stat92E, and Akt1 did not modify the en>crbi PCR phenotype (data not shown), indicating that crbi-driven growth is specifically sensitive to the dose of SWH pathway components.
Figure 1. Overgrowth driven by the crbi transgene is sensitive to the dose of SWH pathway genes.
Images and overlays of (A) transgenic en>crbi and control en>+, (B) en>crbi and en>crbi,ykiB5/+, (C) en>crbi and en>crbi,sav wings. (D-E) Phalloidin-FITC staining of en>crbi and en>crbi,sav larval wings. (F) PCR in the indicated genotypes (minimum 10 wings per genotype; * p<0.05 compared to en>crbi wings).
crbi expression elevates Yki activity in developing epithelia
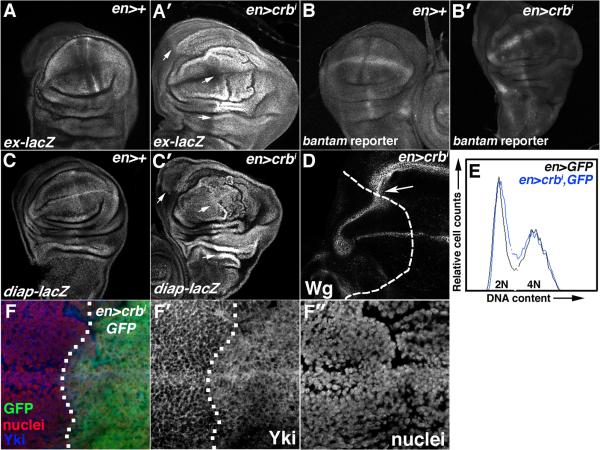
Consistent with the pattern of genetic interactions between crbi and SWH alleles, the expression of multiple Yki targets [7] is elevated in en>crbi larval wing cells (Fig 2A-D). The ex-lacZ and diap1-lacZ enhancer traps, which respectively report Yki-dependent transcription of the expanded (ex) and Drosophila Inhibitor of Apoptosis-1 (diap1) genes, are elevated in the posterior domain of en>crbi wing discs (Fig. 2A-A′,C-C′). The bantam-GFP reporter, whose expression inversely correlates with activity of the Yki-target and pro-growth miRNA bantam (ban), is also reduced in the posterior domain of en>crbi wing discs (Fig. 2B-B′). Wg protein levels, which are normally repressed by the SWH pathway in the proximal wing hinge, are elevated in the corresponding area of en>crbi wing discs (Fig. 2D). In parallel, the en>crbi genotype increased the amount of Yki that co-localizes with a DNA marker relative to the cortical pattern of Yki among cells in the anterior wing pouch (Fig. 2F-F″). Finally, crbi-driven disc growth is also not associated with a dramatic shift in cell cycle phasing (Fig. 2E) or cell size (data not shown), suggesting that crbi may promote balanced increases in the rates of cell growth and division as observed in core SWH mutants [7]. Thus the ability of multiple SWH pathway alleles to modify the en>crbi phenotype correlates with elevated Yki activity and with SWH-like phenotypes in en>crbi larval wing discs.
Figure 2. crbi elevates Yki-activity.
α-β-gal staining or GFP fluorescence in wing discs carrying (A) ex-lacZ, (B) ban-GFP, or (C) diap1-lacZ in the background of (A,B,C) en>+ or (A′,B′,C′) en>crbi. Arrows in A′ and C′ highlight elevated ex-lacZ and diap1-lacZ expression. (D) α-Wg stain in en>crbi wings discs (posterior = right of dashed line). (E) FACS-analysis of en>GFP (black) and transgenic en>crbi,GFP (blue) wing discs. (F) Co-staining for Yki (blue) and HP1 (nuclei; red) in en>crbi,GFP discs (posterior = right of dotted line).
The Notch receptor interacts with the SWH pathway in certain tissues [11-13] and is regulated by crb in certain developmental contexts [14]. The effect of Notch heterozygosity on en>crbi PCR could not be reliably measured due to significant wing-notching (data not shown). However expression of crbi had no significant effect on the Notch reporter E(spl)mβ-CD2 reporter [15] (Fig. S1,B-C) or expression of the Wg and Cut proteins at the dorsal/ventral margin of the wing (data not shown). Notch protein localization and levels in wing cells were also unaffected by expression of crbi (Fig. S1,G). Clonal loss of crb in the eye disc also did not affect E(spl)mβ-CD2 expression (Fig. S1,D). Thus, the crbi-induced changes in Yki activity do not coincide with detectable changes in Notch abundance, localization, or transcriptional induction of a Notch pathway reporter.
crbi downregulates Ex protein levels in wing disc cells
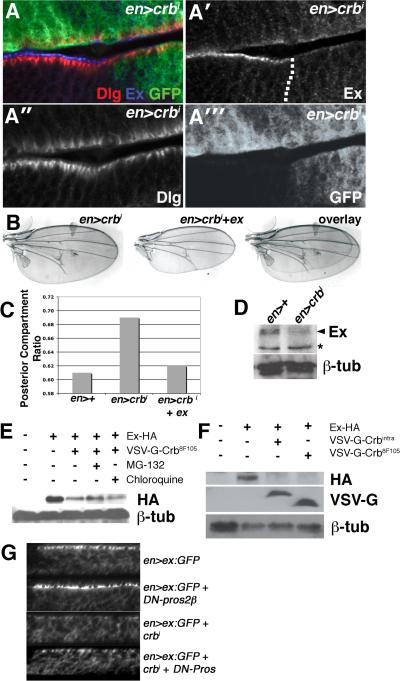
The localization of Crb to the apical membrane and apicolateral junctions of disc epithelial cells [16] suggests that expression of crbi might affect the activity of SWH proteins that also localize to these same domains. No significant alterations were noted in the levels and localization of either Fat or Merlin (Mer), two apically localized SWH regulators, in en>crbi wing discs (Fig. S1,E-F). Moreover, the Fat reporters dachs-V5 (data not shown) and four jointed-lacZ [17] are unaltered in en>crbi wing discs (Fig. S1,H-I), and RNAi-knockdown of the palmitoyltransferase approximated (app), which is required for the overgrowth of ft mutant cells [18], did not suppress the crbi-driven enlarged wing phenotype (Fig. S1,J). Thus crbi does not appear to control wing growth via a ft-dependent pathway. By contrast, the apical SWH regulator Ex is depleted from the apical membrane of crbi-expressing cells (Fig. 3A-A″′). In parallel, expression of en>crbi leads to a drop in overall levels of Ex detected by immunoblot analysis (Fig. 3D). The adherens junction (AJ) protein Armadillo was unaffected by crbi expression (Fig. S1,A-A′), indicating that the effect on Ex is not due to a general loss of AJ complexes in en>crbi cells.
Figure 3. crbi downregulates Ex levels.
(A-A′″) Lateral section of en>crbi,GFP wing disc co-stained for Dlg (red) and Ex (blue). Dotted line denotes A:P boundary. (B) Images and overlays of en>crbi and en>crbi,ex wings. (C) PCR in the indicated genotypes. (D) Immunoblot of Ex in en>+ and en>crbi wing discs. Arrowhead denotes Ex based on comigration with overexpressed Ex (not shown) (* = non-specific band). Lower panel is α-β-tub loading control. (E) Immunoblot of HA-Ex in Crb8F105-expressing cells treated with the MG132 (lane 4) or chloroquine (lane 5). (F) Corresponding α-HA, α-VSV-G, and α-β-tub immunoblots of S2 cells expressing HA-Ex from the pAct-HA-Ex plasmid (lanes 2-4), and VSV-G-tagged forms of either crbi (lane 3) or crb8F105 (lane 4) from the pMT plasmid. (G) Lateral images of Ex:GFP in the posterior region of the wing pouch in the indicated genotypes.
As transcription of ex-lacZ is elevated in en>crbi discs, crbi thus appears to promote post-transcriptional down-regulation of Ex. A similar effect occurs in S2 cells expressing Ex from the constitutively expressed pAct plasmid [10]: Ex levels are reduced following induction of a crb transgene expression from the inducible pMt plasmid [19] (Fig. 3E-F). Re-expression of Ex from a UAS-ex transgene is also sufficient to revert the en>crbi PCR phenotype (Fig. 3B,C). In view of this link between Crb overexpression and Ex loss, it is notable that the array of phenotypes produced by crbi overexpression are quite similar and those associated with the ex alleles in the intact organism (Fig. S2), including large wings lacking posterior cross veins, small eyes with reduced numbers of ommatidia [9], a delay in morphogenetic furrow (MF) progression in the ventral portion of the eye disc and an ectopic MF produced dorsally [20], and an increase in the number of interommatidial cells in the pupal eye disc [10]. Thus, while it is likely that the intracellular tail of Crb has effects on additional cellular pathways, these phenotypic similarities indicate that a significant subset of the effects of Crbi activity on developing tissues may be mediated via Ex.
The Crb juxtamembrane domain regulates Ex
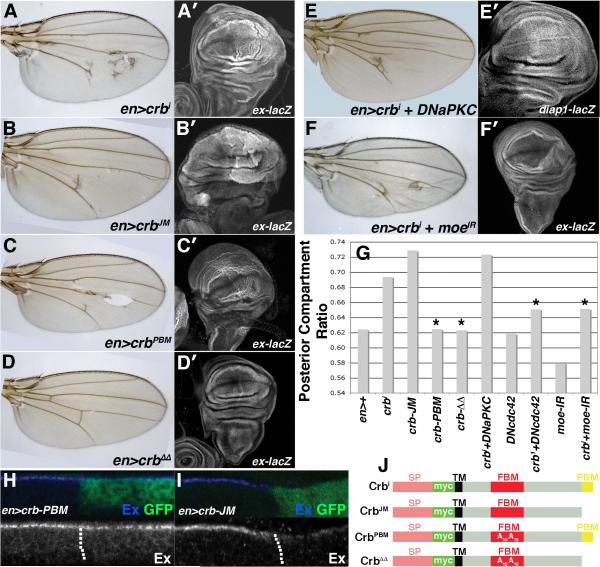
The 37 amino acid intracellular tail of Crb contains two functional motifs that are conserved across Crb proteins in multiple species: (i) the 15-amino acid juxtamembrane FERM-binding motif (JM), which mediates a direct interaction with the FERM-domain protein Yurt, and indirectly interacts with DMoesin (DMoe) and βH-spectrin to link Crb to the underlying actin/spectrin cytoskeleton, and (ii) the C-terminal PDZ-binding motif (PBM), which is composed of the last 4 residues of the Crb tail (ERLI) and directs interactions with Sdt and Patj to form a polarity regulatory module commonly referred to as the Crb complex [6]. In order to better understand the mechanism whereby crbi downregulates Ex and activates Yki, several previously utilized crb transgenes (Fig. 4J) that inactivate either the JM domain or the PBM within the Crbi protein [21] were assessed their ability to (i) increase wing size, (ii) activate Yki signaling, and (iii) eliminate apical Ex. Overexpression of a construct lacking the PBM, but maintaining the JM, increases PCR among adult wings to a similar degree as the intact crbi transgene (Fig. 4B,G). Expression of crb-JM also depletes apical Ex (Fig. 4I) and increases Yki activity as detected by the ex-lacZ reporter, particularly in the pouch region of the wing disc (Fig. 4B′). crb-JM thus phenocopies crbi in its effects on Ex and on SWH pathway activity. By contrast, a construct containing the PBM and inactivating mutations within the JM (crb-PBM) does not increase PCR or significantly elevate ex-lacZ expression (Fig. 4C-C′,G), and has no effect on apical Ex (Fig. 4H). Expression of crb-PBM did disrupt organization of the disc epithelium (Fig. S4,C-D) and wing morphology (Fig. 4C-C′) in a way not observed with crb-JM, indicating that the failure of the crb-PBM transgene to affect Ex is not due to a general lack of biological activity. A construct lacking intact JM and PBM domains (crbΔΔ) had no effect on wing size, structure or ex-lacZ (Fig. 4D-D′).
Figure 4. The Crb-JM controls Ex levels and Yki activity.
Paired light microscopic (A-F) and confocal images of α-β-gal staining to detect activity of the ex-lacZ transgene (A′-D′,F′) or diap1-lacZ (E′) in the indicated genotypes. (G) PCR values in the indicated genotypes. (*p<0.05 compared to en>crbi). α-Ex (blue) staining in (H) en>crb-PBM,GFP and (I) en>crb-JM,GFP wing discs. Dotted line marks the A:P boundary. (posterior = right). Cartoon of crb transgenes; signal peptide (SP), myc tag (Myc), transmembrane domain (TM), juxtamembrane FERM-binding motif (JM), PDZ-binding motif (PBM), and amino acid substitutions are indicated [adapted from 40].
A similar link between the Crb JM domain and Ex was observed in S2 cells. Expression of either VSV-G-tagged Crbi or VSV-G-tagged Crb8F105, which contains a stop codon that prevents the translation of the last 23 amino acids (including the PBM) while preserving much of the JM [19], is sufficient to downregulate co-expressed Ex (Fig. 3E-F). Treatment with the proteasome inhibitor MG132 was able to partially reverse this effect, whereas treatment with the lysosomal inhibitor chloroquine did not (Fig. 3E). In parallel experiments in intact wing discs, crbi is able to deplete levels of an Ex:GFP fusion protein [22] (Fig. 3G). Genetic reduction of proteasome activity with a dominant-negative allele of the proteasomal subunit Pros2β [23] also elevates levels of Ex:GFP levels in normal wing disc cells and partially restores Ex:GFP levels in discs that also express crbi (Fig. 3G). Expression of crbi did not stimulate endolysosomal routing of Ex:GFP as measured by the effect of treatment with the lysosomal inhibitor chloroquine on Ex:GFP localization (Fig. S3). Thus, it appears that Ex protein levels are antagonized by the proteasome, and that blocking proteasome activity can retard the effect of Crbi on Ex, although the rescue of crbi PCR by UAS-ex (see Fig. 3B) suggests that this mechanism cannot completely overcome the ability of overexpressed Ex to rescue PCR. Moreover, as in disc cells, the PBM domain is dispensable for the down-regulation of Ex in S2 cells, while constructs that retain the JM also retain the ability to regulate Ex.
Effects of crb loss on Ex protein
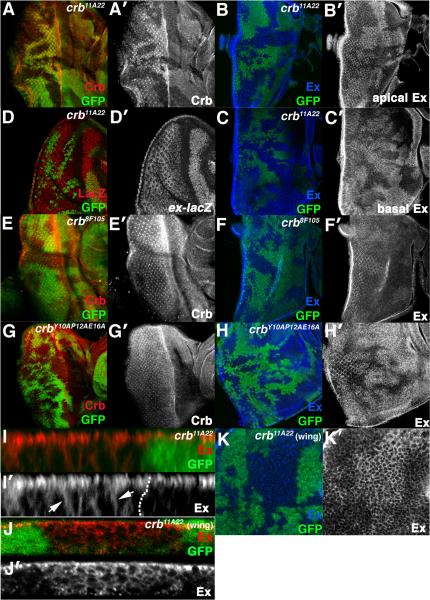
The effect of crbi on Ex suggests that under physiologic conditions crb might be required to restrict accumulation of Ex protein. Consistent with this, cells homozygous for the crb11A22 allele, which reduces endogenous Crb protein to background levels (Fig. 5A-A′), show elevated Ex levels (Fig. 5B-C) with no change in ex-lacZ expression (Fig. 5D). A similar effect occurs in crb11A22 wing clones (Fig. 5K). Levels and localization of the Dlg, Arm, and Mer protein are not affected by the crb11A22 allele (Fig. S1,K-M), confirming that the effect of crb loss on Ex is fairly specific. Optical sections through the apical and basal planes of the eye disc indicate that while a portion of the excess Ex in crb11A22 localizes apically, a portion also drops more basally (Fig, 5B′,C′). Lateral sections through crb11A22 eye and wing clones confirm that Ex accumulates in a linear manner along what appears to be the basolateral membrane of cells (Fig. 5I-I′, J-J′). Since the excess Ex that accumulates in core SWH mutants remains at the apical domain [e.g. 10]), this Ex ‘basal spreading’ phenotype is not a secondary effect of elevating Ex levels in crb cells, but rather appears to reflect a role for crb in localizing Ex.
Figure 5. Effect of crb loss on Ex in disc cells.
Confocal images of crb11A22 (A-D, I-K), crb8F105 (E-F), or crbY10AP12AE16A (G-H) clones in the eye (A-I) or wing (J-K) stained for Crb (A,E,G), Ex (B,C,F,H-K). (B) and (C) are apical and basal planes of the same disc. Arrowheads in (I) denote excess Ex in crb11A22 cells that fails to localize apically. Disc in (I) is imaged through apical portion of epithelium; disc in (J) is imaged through entire epithelium. (D) α-β-gal staining to detect activity of the ex-lacZ transgene in crb11A22 eye clones.
Site specific alleles support a role for the JM domain in the Ex-inhibitory role of crb: cells homozygous for the crb8F105 allele, which lacks the C-terminal PBM and the preceding 19 amino acids but maintains a largely intact JM [24], show a more mild effect on Ex levels than the crb11A22 allele (Fig. 5F-F′). This weaker phenotype could be due to a role for the PBM region in regulating the Ex-regulatory function of the JM, or to the loss of additional sequences between the JM and PBM that affect protein stability or function. The crb8F105 allele has been reported to reduce Crb levels and alter Crb protein distribution in embryonic epithelial cells [25] but no differences in Crb protein were detected in crb8F105 clones (Fig. 5E-E′). To more finely map the sequences within Crb that are required to restrict Ex in vivo, a genomic crb allele carrying three missense mutations in the JM region (crbY10AP12AE16E) [26] was tested for its effect on Ex. While the crbY10AP12AE16E allele has no obvious effect on Crb levels or localization (Fig. 5G-G′), it did elevate Ex levels in cells (Fig. 5H-H′). Thus amino acids in the Crb JM are both necessary and sufficient to restrict Ex levels in vivo.
Modification by Crb-interacting factors
The Crb JM interacts directly with the FERM-domain protein Yurt [27] and indirectly with the FERM-domain protein DMoe [19], both of which are structurally related to Ex. Although no evidence was found of a stable interaction between the Crb and Ex (see Discussion), genetic data suggest that the DMoe plays a role in the link between Crb and SWH activity. RNAi knockdown of DMoe [28] in en>crbi discs suppressed both enlarged PCR (Fig. 4F,G) and ex-lacZ expression (Fig. 4F′), but did not rescue the drop in endogenous Ex induced by crbi (Fig. S4,A) and had no independent effect on Ex:GFP (Fig. S4,B). Although this effect could be due to a non-specific effect of DMoe loss, the ability of DMoe-IR to uncouple Ex loss from Yki activation in crbi-expressing cells argues that DMoe may play a more specific role downstream of Ex in a Crb/Ex pathway, or that DMoe acts in a parallel pathway that converges on Yki.
The Crb cytoplasmic tail is phosphorylated by the Drosophila atypical protein kinase C (DaPKC), a component of the Par complex that regulates polarity and endocytosis [29]. These modifications are thought to be involved in the ability of Crb to in turn participate in the establishment of epithelial polarity. As in these prior studies, a dominant-negative DaPKC transgene effectively suppressed the cuticular disorganization of en>crbi wings (Fig 4E). However, it did not change en>crbi PCR (Fig. 4G), rescue the drop in Ex protein (Fig. S4,C-C″), or prevent induction of diap1-lacZ (Fig. 4E′). By contrast, a transgene encoding a dominant-negative form of the GTPase Cdc42 (dn-cdc42) [30], whose effectors include the Crb- and aPKC-interactor Par6, was an efficient suppressor of en>crbi posterior compartment growth (Fig. 4G). This variable effect could be due to differences in transgene strength or to functional differences between aPKC and cdc42, as observed in other studies [31]. Notably, the dn-cdc42 allele is able to suppress the crb-JM PCR phenotype (Fig. S4,F), indicating either that Cdc42 can act on the Crb tail independent of the PBM, or that Cdc42 acts by a distinct pathway to control wing size.
Effect of excess Ex in crb mutant cells
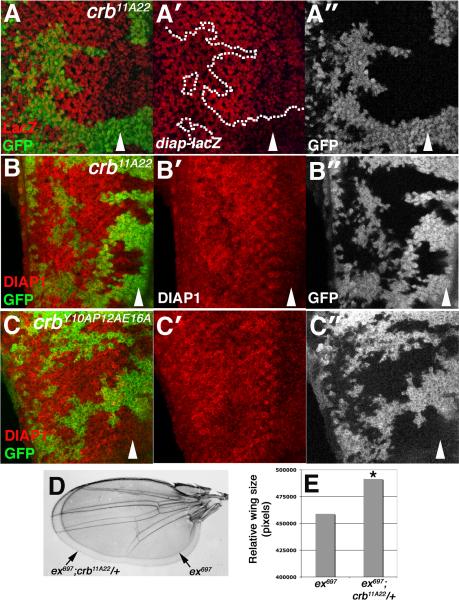
As transgenic overexpression of ex is growth-suppressive [22, 32], the excess Ex in crb mutant cells might be predicted to reduce Yki activity. However, RNAi knockdown of crb do not suppress organ overgrowth driven by a UAS-yki:YFP transgene [33] but rather enhanced them (Fig. S5,A-C). Expression of diap-lacZ, a sensitive readout of Yki activity [34], is also elevated in crb11A22 cells posterior to the MF (Fig. 6A); DIAP1 protein shows a similar pattern in crb11A22 and crbY10AP12AE16A clones (Fig. 6B-C). crb11A22 and crbY10AP12AE16A cells also display a clonal growth advantage in the eye relative to both control and crb8F105 chromosomes (Fig. S5,D-G). Thus, mutations in the JM are sufficient to deregulate DIAP1 levels and to confer a growth advantage in vivo. Moreover, the crb11A22 allele acts as a dominant enhancer of the increased wing-size phenotype associated with the ex697 hypomorphic allele (Fig. 6D-E), arguing that at a genetic level crb normally promotes ex activity, and that the effect of crb loss on Ex levels and localization may compromise signaling through the SWH pathway.
Figure 6. crb alleles interact with ex and elevate DIAP1 expression posterior to the furrow.
Images of (A-B) crb11A22 or (C) crbY10AP12AE16A clones (lacking GFP) in larval eye discs stained with (A) α-β-Gal to detect diap1-lacZ (red) or (B-C) α-DIAP1 (red). Arrowheads denote position of the MF. (posterior = left). Dotted line in (A) highlights a crb clone that projects posterior to the MF and expresses elevated diap1-lacZ. (D) Optical overlay and (E) size adult wings of the indicated genotypes. (* p<1.5×10-7 relative to ex697 wings).
Discussion
Crb as dual regulator of polarity and growth
Crb nucleates apical membrane formation in the embryonic epidermis and other epithelial cell types in Drosophila. It exerts these effects primarily through two motifs in its intracellular tail: the C-terminal PBM and the juxtamembrane FERM-binding motif (JM). We find that Crb also acts as a peripheral regulator of the SWH pathway in larval discs via a previously unappreciated role for the JM domain in controlling levels of the Ex protein. Known peripheral regulators of the SWH pathway modulate signaling in response to upstream inputs including planar cell polarity pathways, morphogen gradients, and adhesion molecules [8]. Our data extend this theme by suggesting that Crb may serve as an interface between apicobasal polarity signals and the SWH pathway. Overexpression and loss of crb have opposing effects on Ex levels, and sequences in the Crb JM are both necessary and sufficient to control Ex in vivo. The ability of the Crb-JM to deplete Ex from cells suggests that mutations in endocytic genes that block Crb turnover and produce dramatic tissue disorganization and overgrowth (e.g. avl and ept/tsg101) [4, 5] may elicit their phenotypes in part via effects on Ex and SWH signaling. Indeed, vps25 mutants have been shown to downregulate Ex levels [35]. A more complete analysis of the role of Crb and Ex in endocytic tumor mutants is required to understand this link more fully.
The link between crb loss and diap1 expression is at present not clear. Since localization has been suggested to be an important determinant of Ex function [22], our finding that a portion of the excess Ex found in crb cells is displaced basally suggests that the function of this fraction of Ex may be somehow altered or compromised. Prior work showing that loss of the tumor suppressor ft can also mislocalize Ex and compromise its function [36-38] provides precedent for this type of effect, but does not provide insight into what aspect of Ex function might be affected by crb loss. Moreover, since ft alleles elicit far stronger effects on Yki activity than do crb alleles, the consequences of Ex defects in each background would appear to be quite different. Future analysis of the effect of crb loss on the biochemical properties and subcellular localization of Ex may provide insight into this issue.
Ex stability appears to correlate inversely with expression of the Crb JM region, which is known to interact with FERM-domain proteins that are structurally similar to Ex. Attempts to detect a physical interaction between Ex and the Crb intracellular tail using multiple techniques have not been successful (BSR and KHM; unpub.). While this does not preclude a Crb:Ex complex, it does suggest that Crb controls Ex via unidentified intermediates. The modular structure of the Crb protein raises the possibility that factors that interact with the intracellular and extracellular portions of the intact protein may modulate the JM-dependent regulation of Ex. If so, then Crb-dependent changes in Ex levels might couple SWH activity to both changes in intracellular signals as cells begin to polarize their membranes, and to variations in extracellular adhesion during developmental tissue morphogenesis and wound repair. Given the ability of SWH pathway alleles to confer a proliferative advantage in cell competition scenarios [39], it may be that crb that plays a more significant role as a SWH regulator in these types of regenerative and homeostatic growth regulatory programs. Major goals of future studies will therefore be to identify the precise mechanistic details of how Crb controls Ex, and how the intrinsic Ex-regulatory activity of the JM domain is linked to other functional domains of the Crb molecule.
The differential effect of the Crb-JM and Crb-PBM on growth and tissue architecture appears to conflict with a requirement for the JM in rescue of polarity defects in crb mutant embryos [21]. However, this is not without precedent [40] and may be may be explained by the well-documented differences between the roles of Crb in the embryonic epidermis and disc epithelium: loss of Crb disrupts the polarity of embryonic ectoderm and compromises tissue integrity [16], whereas loss of Crb in larval discs has minimal effect on the architecture, polarity, or organization of undifferentiated cells [41].
crb as a growth suppressor
In addition to the circumstantial evidence of a growth advantage conferred by crb alleles,recent work has shown that reduced expression of the murine crb3 gene, a homolog of Drosophila crb, can promote tumorigenicity of kidney epithelial cells and relieve contact inhibition [42]. A pro-proliferative effect of crb loss might seem at odds with recent work showing that loss of crb did not detectably alter overgrowth driven by wts inactivation [43, 44]. In these studies crb was analyzed as a downstream target of the SWH pathway; the data here show that it is also upstream of Ex. Loss of wts is thus predicted to be epistatic to the effects of crb loss on Ex. Rather, our data showing a upregulation of diap1 expression in crb clones indicate that crb alleles might enhance the effects of wts loss, although this would in all likelihood have little effect in the background of wts loss. Rather crb alleles may be more likely to synergize with mutations in other peripheral regulators of the SWH pathway such as mer, which functions redundantly to ex [10, 45].
Multiple polarity links to SWH activity
The link between Crb and Ex reinforces emerging links between polarity control and the SWH pathway. The polarity gene Discs large (Dlg) suppresses tumor growth of ovarian follicle cells via a pathway involving warts but independent of ex, ft, and mer [46]. The polarity factor Scribble can also interact with the Fat2 protein in the developing zebrafish kidney nephron [47]. In an accompanying study to the work presented here, Grzeschik et. al. have found that loss of Drosophila lgl can activate diap1-lacZ; significantly, this occurs without a loss of Ex protein (Fig. S6). In view of the link uncovered here between crb and Ex levels in imaginal epithelial cells, it would thus seem that multiple mechanisms link polarity and the SWH pathway, and that multiple links can exist between apicobasal polarity factors and SWH activity even within a single cell type.
Experimental Procedures
Genetics
Crosses performed at 25°C unless noted. Larval wing discs were harvested from animals kept at 20°C during embryogenesis. Animals were maintained at 20°C for adult wing analysis. Alleles used: UAS-myc-crbintra; UAS-Ex:GFP; UAS-sav; ykiB5; banl(3)05967; sdETX4; ex697; thjc58; fj-lacZ; dpp-lacZ; ban-GFP; UAS-ex; FRT82B,crb11A22; FRT82B,crb8F105; crbY10P12AE16A; UAS-aPKCCAAX-DN; UAS-cdc42N17.3; UAS-moeIR-327-775; UAS-yki-YFP; UAS-crbIR-1 and UAS-crbIR-2 (VDRC #39178 and #39177); lgl4; E-spl(m)ß-CD2; UAS-appIR; UAS-crbJM (also UAS-Myc-IntraΔERLI); UAS-crbPBM (also UAS–Myc–IntraY10A/E16A); UAS-crbΔΔ (also UAS–Myc–IntraY10A/E16A/ΔERLI); eyFLP;ubi-GFP,FRT80B; eyFLP;ubi-GFP,FRT82B; eyFLP;Act>CD2>Gal4;Rps174,FRT80B; eyFLP;tub-Gal4;FRT80B,tub-Gal80; w;UAS-GFP,UAS-crbi;FRT80B.
Cell Culture & FACS
S2 cells were cultured under standard conditions. Constructs used: pAc5.1-HA-Ex (G. Halder), pMT-VSV-G-crb-intra and pMT-VSV-G-crb-8F105 (A. Le Bivic). Transfected cells were analyzed 24-36 hours post-transfection (Cellfectin II, Invitrogen); where appropriate, CuSO4 (0.5mm) was added for the final 12 hours. MG-132 (Sigma) was used at 50μM. Discs were treated with 100μM chloroquine (Sigma) as described previously [48]. Trypsin-dissociated discs were stained with 20 μM DRAQ-5 (Biostatus Limited), analyzed on a BD-LSR II cytometer via a 755 nM laser with a 780/60 nM BP collection filter, and analyzed on FlowJo (TreeStar).
Immunohistochemistry & Immunoblotting
Immunostaining, confocal microscopy, and immunoblotting performed as described previously [49]. Antibodies: mouse α-β-gal 1:1000 (Promega); mouse α-Wg 1:800 (DSHB); rabbit α-yki 1:1000 (K. Irvine); mouse α-HP1 1:20 (DSHB); 1:500 rat α-Crb (H. Bellen); mouse α-Dlg 1:20 (DSHB); rabbit α-GFP (Molecular Probes); guinea pig α-Ex 1:5000 (R. Fehon); rabbit α-Ex 1:200 (K. Irvine); goat α-ß-tub 1:10000 (Santa Cruz); mouse α-HA 1:1000 (Sigma); goat α-VSV-G 1:1000 (Bethyl Labs); mouse α-Arm 1:20 (DSHB); mouse α-DIAP1 1:50 (B. Hay); mouse α-rat CD2 1:100 (Research Diagnostics, Inc.); mouse-α-Notch 1:10 (DSHB, clone 9C6); guinea pig α-Mer 1:7500 (R. Fehon), rabbit α-Fat 1:200 [50] and rat α-Elav 1:200 (DHSB); Alexa-488 phalloidin, 1:100, and YOYO-1, 1:20,000 (Molecular Probes).
Wing/Eye Measurements
Eyes/wings were imaged on a Leica DFC500 CCD camera and quantified with Adobe Photoshop. Posterior compartment ratio (PCR) = posterior compartment size/total wing size.
Supplementary Material
Supplemental Data
Figure S1. Lack of effect of crb alleles on the apical proteins Notch, Fat, Merlin, Dlg, and Arm. (A-A′) Confocal section of en>crbi,GFP wing disc stained with α-Arm (red) to visualize AJs. Activity of the Notch reporter E(spl)m-β-CD2 in (B) en>+ and (C) en>crbi wings, and in (D) crb11A22 mosaic eye discs as assessed by α-CD2 staining (red). Confocal images of Mer (E), Fat (F) and Notch (G) in en>crbi larval wing discs. Posterior is to the right of the dashed line. α-β-gal staining to detect the Fat-pathway reporter fj-lacZ in (H) en>+ and (I) en>crbi wing discs. (J) Quantitative analysis of the PCR in indicated genotypes. A minimum of 10 wings was counted per genotype. No statistical difference was observed between en>crbi and en>crbi,appIR PCR. (K-L) Lateral confocal section of crb11A22 clones marked by the absense of GFP stained for Dlg (K) and Arm (L). (M) Merged confocal sections of crb11A22 mosaic eye discs marked by the absence of GFP stained for Mer (blue).
Figure S2. Expression of crbi phenocopies loss of ex. Representative images of adult wings of (A) ex697/ ex697 and (B) transgenic en>crbi flies and adult eyes taken from control (C) ey>+ and (D-E) ey>crbi flies. α-β-gal staining of (F) ey>+ and (G) ey>crbi larval eye discs to visualize the MF marker dpp-lacZ. Arrow in G indicates ectopic furrow formation. (H) Expression for the neuronal marker Elav in en>crbi eye discs. (I) Confocal image of α-Dlg staining to mark apical cell profiles, in 48hr pupal discs. Image captures a boundary between normal cells and a MARCM-generated crbi-expressing clone (right of dotted line). Note the excess interommatidial cells in the crbi-overexpressing clone as first reported by [51].
Figure S3. Loss of Ex following expression of Crbi is not accompanied by routing of Ex:GFP into a chloroquine-sensitive lysosomal compartment. Lateral optical images of (A-B) en>+ or (C-D) en>crbi larval wing discs either (A,C) untreated or (B,D) treated with chloroquine and stained for Notch (red) or Ex (blue). Arrows in (B) and (D) denote intracellular Notch-positive puncta.
Figure S4. Domain specific phenotypes and functional interactions with putative Crb interactors. (A) Lateral image of endogenous Ex protein in an en>crbi+DMoe-IR disc. Note absence of Ex in posterior to the right of the dotted line. (B) Ex:GFP fusion protein in posterior domain of an en>DMoe-IR disc. Lateral image of nuclei (green) and Dlg (red) in posterior domain of (C) en>crb-JM and (D) en>crb-PBM wing discs. Apical is orientated up in A-D. (E) Confocal image of an en>crbi,DN-DaPKC larval wing disc stained for Ex (blue) and Ci (red) to mark the boundary between Ci-positive anterior cells and Ci-negative posterior cells that express the crbi transgene. (F) Quantitative analysis of the PCR in indicated genotypes. A minimum of 10 wings was counted per genotype. Asterisk denotes a statistically significant interaction between dn-cdc42 and crb-JM relative to controls as determined by two-way ANOVA analysis.
Figure S5. Growth phenotypes associated with loss of Crb. Representative images of (A) GMR>yki:YFP and (B) GMR-yki:YFP,crbIR-2 adult heads (images to scale), and (C) quantification of en face adult female eye size of GMR>yki:YFP, GMR-yki:YFP,crbIR-1, and GMR-yki:YFP,crbIR-2 flies (minimum of 10 eyes was analyzed per genotype; Asterisk: p<0.05 compared to GMR>yki:YFP eyes. Representative images of (D) FRT82B, (E) FRT82B,crb11A22, (F) FRT82B,crb8F105, and (G) FRT82B,crbY10AP12AE16A adult mosaic eyes. Mutant tissue is marked by the absence of pigment (red).
Figure S6. The lgl4 allele affects diap1 transcription without altering Ex protein levels or localization. (A-C) Confocal sections of clones of lgl4 mutant eye disc cells marked by the absence of GFP and stained with α-β-Gal to detect (A′) diap1-lacZ expression, (B′) DIAP1 protein, and (C′) Ex protein levels. Arrowheads denote location of the MF.
Acknowledgements
We apologize whose work we could not cite due to space constraints. We thank G. Halder, H. McNeill, D. Pan, J. Jiang, K. Irvine, K. Harvey, N. Tapon, R. Fehon, T. Xu, U. Tepass, E. Knust, A. LeBivic, and K.W. Choi for gifts of reagents and stocks, the BDSC, VDRC, and DSHB for fly stocks and antibodies, and H. Richardson for sharing data prior to publication. This work was supported by RO1 CA123368 (KHM) and NIH T32 GM008367 (BSR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- 2.Humbert PO, Grzeschik NA, Brumby AM, Galea R, Elsum I, Richardson HE. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene. 2008;27:6888–6907. doi: 10.1038/onc.2008.341. [DOI] [PubMed] [Google Scholar]
- 3.Wodarz A, Hinz U, Engelbert M, Knust E. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell. 1995;82:67–76. doi: 10.1016/0092-8674(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 4.Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol. 2005;7:1132–1139. doi: 10.1038/ncb1324. [DOI] [PubMed] [Google Scholar]
- 5.Moberg KH, Schelble S, Burdick SK, Hariharan IK. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev Cell. 2005;9:699–710. doi: 10.1016/j.devcel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Bulgakova NA, Knust E. The Crumbs complex: from epithelial-cell polarity to retinal degeneration. J Cell Sci. 2009;122:2587–2596. doi: 10.1242/jcs.023648. [DOI] [PubMed] [Google Scholar]
- 7.Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- 8.Badouel C, Garg A, McNeill H. Herding Hippos: regulating growth in flies and man. Curr Opin Cell Biol. 2009;21:837–843. doi: 10.1016/j.ceb.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Boedigheimer M, Bryant P, Laughon A. Expanded, a negative regulator of cell proliferation in Drosophila, shows homology to the NF2 tumor suppressor. Mech Dev. 1993;44:83–84. doi: 10.1016/0925-4773(93)90058-6. [DOI] [PubMed] [Google Scholar]
- 10.Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 11.Meignin C, Alvarez-Garcia I, Davis I, Palacios IM. The salvador-warts-hippo pathway is required for epithelial proliferation and axis specification in Drosophila. Curr Biol. 2007;17:1871–1878. doi: 10.1016/j.cub.2007.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polesello C, Tapon N. Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr Biol. 2007;17:1864–1870. doi: 10.1016/j.cub.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 13.Yu J, Poulton J, Huang YC, Deng WM. The hippo pathway promotes Notch signaling in regulation of cell differentiation, proliferation, and oocyte polarity. PLoS One. 2008;3:e1761. doi: 10.1371/journal.pone.0001761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herranz H, Stamataki E, Feiguin F, Milan M. Self-refinement of Notch activity through the transmembrane protein Crumbs: modulation of gamma-secretase activity. EMBO Rep. 2006;7:297–302. doi: 10.1038/sj.embor.7400617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Celis JF, Tyler DM, de Celis J, Bray SJ. Notch signalling mediates segmentation of the Drosophila leg. Development. 1998;125:4617–4626. doi: 10.1242/dev.125.23.4617. [DOI] [PubMed] [Google Scholar]
- 16.Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–799. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- 17.Rogulja D, Rauskolb C, Irvine KD. Morphogen control of wing growth through the Fat signaling pathway. Dev Cell. 2008;15:309–321. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matakatsu H, Blair SS. The DHHC palmitoyltransferase approximated regulates Fat signaling and Dachs localization and activity. Curr Biol. 2008;18:1390–1395. doi: 10.1016/j.cub.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medina E, Williams J, Klipfell E, Zarnescu D, Thomas G, Le Bivic A. Crumbs interacts with moesin and beta(Heavy)-spectrin in the apical membrane skeleton of Drosophila. J Cell Biol. 2002;158:941–951. doi: 10.1083/jcb.200203080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCartney BM, Kulikauskas RM, LaJeunesse DR, Fehon RG. The neurofibromatosis-2 homologue, Merlin, and the tumor suppressor expanded function together in Drosophila to regulate cell proliferation and differentiation. Development. 2000;127:1315–1324. doi: 10.1242/dev.127.6.1315. [DOI] [PubMed] [Google Scholar]
- 21.Klebes A, Knust E. A conserved motif in Crumbs is required for E-cadherin localisation and zonula adherens formation in Drosophila. Curr Biol. 2000;10:76–85. doi: 10.1016/s0960-9822(99)00277-8. [DOI] [PubMed] [Google Scholar]
- 22.Badouel C, Gardano L, Amin N, Garg A, Rosenfeld R, Le Bihan T, McNeill H. The FERM-domain protein Expanded regulates Hippo pathway activity via direct interactions with the transcriptional activator Yorkie. Dev Cell. 2009;16:411–420. doi: 10.1016/j.devcel.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Belote JM, Fortier E. Targeted expression of dominant negative proteasome mutants in Drosophila melanogaster. Genesis. 2002;34:80–82. doi: 10.1002/gene.10131. [DOI] [PubMed] [Google Scholar]
- 24.Jurgens G, Wieschaus E, Nusslein-Volhard C, Kluding H. Mutations affecting the pattern of larval cuticle in Drosophila melanogaster. Roux's Arch. of Dev Biol. 1984;II:283–295. doi: 10.1007/BF00848157. [DOI] [PubMed] [Google Scholar]
- 25.Wodarz A, Grawe F, Knust E. CRUMBS is involved in the control of apical protein targeting during Drosophila epithelial development. Mech Dev. 1993;44:175–187. doi: 10.1016/0925-4773(93)90066-7. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Zhou W, Dong W, Watson AM, Hong Y. From the Cover: Directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering. Proc Natl Acad Sci U S A. 2009;106:8284–8289. doi: 10.1073/pnas.0900641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laprise P, Beronja S, Silva-Gagliardi NF, Pellikka M, Jensen AM, McGlade CJ, Tepass U. The FERM protein Yurt is a negative regulatory component of the Crumbs complex that controls epithelial polarity and apical membrane size. Dev Cell. 2006;11:363–374. doi: 10.1016/j.devcel.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karagiosis SA, Ready DF. Moesin contributes an essential structural role in Drosophila photoreceptor morphogenesis. Development. 2004;131:725–732. doi: 10.1242/dev.00976. [DOI] [PubMed] [Google Scholar]
- 29.Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 30.Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- 31.Harris KP, Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol. 2008;183:1129–1143. doi: 10.1083/jcb.200807020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boedigheimer MJ, Nguyen KP, Bryant PJ. Expanded functions in the apical cell domain to regulate the growth rate of imaginal discs. Dev Genet. 1997;20:103–110. doi: 10.1002/(SICI)1520-6408(1997)20:2<103::AID-DVG3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Milton CC, Humbert PO, Harvey KF. Transcriptional output of the Salvador/warts/hippo pathway is controlled in distinct fashions in Drosophila melanogaster and mammalian cell lines. Cancer Res. 2009;69:6033–6041. doi: 10.1158/0008-5472.CAN-08-4592. [DOI] [PubMed] [Google Scholar]
- 34.Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Herz HM, Chen Z, Scherr H, Lackey M, Bolduc C, Bergmann A. vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis. Development. 2006;133:1871–1880. doi: 10.1242/dev.02356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng Y, Irvine KD. Fat and expanded act in parallel to regulate growth through warts. Proc Natl Acad Sci U S A. 2007;104:20362–20367. doi: 10.1073/pnas.0706722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H. The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr Biol. 2006;16:2081–2089. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Bennett FC, Harvey KF. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr Biol. 2006;16:2101–2110. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 39.Tyler DM, Li W, Zhuo N, Pellock B, Baker NE. Genes affecting cell competition in Drosophila. Genetics. 2007;175:643–657. doi: 10.1534/genetics.106.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izaddoost S, Nam SC, Bhat MA, Bellen HJ, Choi KW. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature. 2002;416:178–183. doi: 10.1038/nature720. [DOI] [PubMed] [Google Scholar]
- 41.Pellikka M, Tanentzapf G, Pinto M, Smith C, McGlade CJ, Ready DF, Tepass U. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 2002;416:143–149. doi: 10.1038/nature721. [DOI] [PubMed] [Google Scholar]
- 42.Karp CM, Tan TT, Mathew R, Nelson D, Mukherjee C, Degenhardt K, Karantza-Wadsworth V, White E. Role of the polarity determinant crumbs in suppressing mammalian epithelial tumor progression. Cancer Res. 2008;68:4105–4115. doi: 10.1158/0008-5472.CAN-07-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamaratoglu F, Gajewski K, Sansores-Garcia L, Morrison C, Tao C, Halder G. The Hippo tumor-suppressor pathway regulates apical-domain size in parallel to tissue growth. J Cell Sci. 2009;122:2351–2359. doi: 10.1242/jcs.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Genevet A, Polesello C, Blight K, Robertson F, Collinson LM, Pichaud F, Tapon N. The Hippo pathway regulates apical-domain size independently of its growth-control function. J Cell Sci. 2009;122:2360–2370. doi: 10.1242/jcs.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pellock BJ, Buff E, White K, Hariharan IK. The Drosophila tumor suppressors Expanded and Merlin differentially regulate cell cycle exit, apoptosis, and Wingless signaling. Dev Biol. 2007;304:102–115. doi: 10.1016/j.ydbio.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao M, Szafranski P, Hall CA, Goode S. Basolateral junctions utilize warts signaling to control epithelial-mesenchymal transition and proliferation crucial for migration and invasion of Drosophila ovarian epithelial cells. Genetics. 2008;178:1947–1971. doi: 10.1534/genetics.108.086983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skouloudaki K, Puetz M, Simons M, Courbard JR, Boehlke C, Hartleben B, Engel C, Moeller MJ, Englert C, Bollig F, et al. Scribble participates in Hippo signaling and is required for normal zebrafish pronephros development. Proc Natl Acad Sci U S A. 2009;106:8579–8584. doi: 10.1073/pnas.0811691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rives AF, Rochlin KM, Wehrli M, Schwartz SL, DiNardo S. Endocytic trafficking of Wingless and its receptors, Arrow and DFrizzled-2, in the Drosophila wing. Dev Biol. 2006;293:268–283. doi: 10.1016/j.ydbio.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moberg KH, Mukherjee A, Veraksa A, Artavanis-Tsakonas S, Hariharan IK. The Drosophila F box protein archipelago regulates dMyc protein levels in vivo. Curr Biol. 2004;14:965–974. doi: 10.1016/j.cub.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 50.Milton CC, Zhang X, Albanese NO, Harvey KF. Differential requirement of Salvador-Warts-Hippo pathway members for organ size control in Drosophila melanogaster. Development. 137:735–743. doi: 10.1242/dev.042309. [DOI] [PubMed] [Google Scholar]
- 51.Grzeschik NA, Knust E. IrreC/rst-mediated cell sorting during Drosophila pupal eye development depends on proper localisation of DE-cadherin. Development. 2005;132:2035–2045. doi: 10.1242/dev.01800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data
Figure S1. Lack of effect of crb alleles on the apical proteins Notch, Fat, Merlin, Dlg, and Arm. (A-A′) Confocal section of en>crbi,GFP wing disc stained with α-Arm (red) to visualize AJs. Activity of the Notch reporter E(spl)m-β-CD2 in (B) en>+ and (C) en>crbi wings, and in (D) crb11A22 mosaic eye discs as assessed by α-CD2 staining (red). Confocal images of Mer (E), Fat (F) and Notch (G) in en>crbi larval wing discs. Posterior is to the right of the dashed line. α-β-gal staining to detect the Fat-pathway reporter fj-lacZ in (H) en>+ and (I) en>crbi wing discs. (J) Quantitative analysis of the PCR in indicated genotypes. A minimum of 10 wings was counted per genotype. No statistical difference was observed between en>crbi and en>crbi,appIR PCR. (K-L) Lateral confocal section of crb11A22 clones marked by the absense of GFP stained for Dlg (K) and Arm (L). (M) Merged confocal sections of crb11A22 mosaic eye discs marked by the absence of GFP stained for Mer (blue).
Figure S2. Expression of crbi phenocopies loss of ex. Representative images of adult wings of (A) ex697/ ex697 and (B) transgenic en>crbi flies and adult eyes taken from control (C) ey>+ and (D-E) ey>crbi flies. α-β-gal staining of (F) ey>+ and (G) ey>crbi larval eye discs to visualize the MF marker dpp-lacZ. Arrow in G indicates ectopic furrow formation. (H) Expression for the neuronal marker Elav in en>crbi eye discs. (I) Confocal image of α-Dlg staining to mark apical cell profiles, in 48hr pupal discs. Image captures a boundary between normal cells and a MARCM-generated crbi-expressing clone (right of dotted line). Note the excess interommatidial cells in the crbi-overexpressing clone as first reported by [51].
Figure S3. Loss of Ex following expression of Crbi is not accompanied by routing of Ex:GFP into a chloroquine-sensitive lysosomal compartment. Lateral optical images of (A-B) en>+ or (C-D) en>crbi larval wing discs either (A,C) untreated or (B,D) treated with chloroquine and stained for Notch (red) or Ex (blue). Arrows in (B) and (D) denote intracellular Notch-positive puncta.
Figure S4. Domain specific phenotypes and functional interactions with putative Crb interactors. (A) Lateral image of endogenous Ex protein in an en>crbi+DMoe-IR disc. Note absence of Ex in posterior to the right of the dotted line. (B) Ex:GFP fusion protein in posterior domain of an en>DMoe-IR disc. Lateral image of nuclei (green) and Dlg (red) in posterior domain of (C) en>crb-JM and (D) en>crb-PBM wing discs. Apical is orientated up in A-D. (E) Confocal image of an en>crbi,DN-DaPKC larval wing disc stained for Ex (blue) and Ci (red) to mark the boundary between Ci-positive anterior cells and Ci-negative posterior cells that express the crbi transgene. (F) Quantitative analysis of the PCR in indicated genotypes. A minimum of 10 wings was counted per genotype. Asterisk denotes a statistically significant interaction between dn-cdc42 and crb-JM relative to controls as determined by two-way ANOVA analysis.
Figure S5. Growth phenotypes associated with loss of Crb. Representative images of (A) GMR>yki:YFP and (B) GMR-yki:YFP,crbIR-2 adult heads (images to scale), and (C) quantification of en face adult female eye size of GMR>yki:YFP, GMR-yki:YFP,crbIR-1, and GMR-yki:YFP,crbIR-2 flies (minimum of 10 eyes was analyzed per genotype; Asterisk: p<0.05 compared to GMR>yki:YFP eyes. Representative images of (D) FRT82B, (E) FRT82B,crb11A22, (F) FRT82B,crb8F105, and (G) FRT82B,crbY10AP12AE16A adult mosaic eyes. Mutant tissue is marked by the absence of pigment (red).
Figure S6. The lgl4 allele affects diap1 transcription without altering Ex protein levels or localization. (A-C) Confocal sections of clones of lgl4 mutant eye disc cells marked by the absence of GFP and stained with α-β-Gal to detect (A′) diap1-lacZ expression, (B′) DIAP1 protein, and (C′) Ex protein levels. Arrowheads denote location of the MF.