Abstract
Purpose of the Review
β2-adrenoceptor (AR) agonists are the most effective bronchodilators known, and play important roles in every step of asthma therapy. The intrinsic efficacy is an important pharmacological property that differentiates the clinical effects and safety profile of ß2AR agonists. We review the role of ß2-AR agonist intrinsic efficacy in asthma treatment focusing on recent literature.
Recent Findings
In acute asthma, a full agonist (high intrinsic efficacy) offers a clinical advantage over a partial agonist (low intrinsic efficacy) but with the potential of inducing dose-dependent adverse effects. The chronic use of ß2-AR agonists may be associated with several adverse outcomes including loss of asthma control and even increased mortality. Recently, the role of βAR inverse agonists (beta-blockers) which have a negative intrinsic efficacy was studied. While contraindicated in acute asthma, preliminary data suggest that the chronic use of these agents may be associated with attenuation of airway hyperresponsiveness in patients with mild asthma. Studies in a murine model of asthma suggest that such effects may be related to decreased airway inflammation and mucous metaplasia.
Summary
Rational choice among β2AR agonists in acute and chronic asthma should be influenced by differences in intrinsic efficacy among these agents. In acute severe asthma, a full agonist offers a clinical advantage over a partial agonist. While the use of inverse agonists in the treatment of asthma is still experimental and needs further exploration in future trials, preliminary studies suggest that their chronic use is safe and is associated with decreased airway hyperresponsiveness.
Keywords: asthma, ß-blocker, ß-adrenoceptor inverse agonist, ß2-adrenoceptor agonist, chronic
Introduction
Asthma is a chronic inflammatory disorder of the airways characterized by airway hyperresponsiveness (AHR) and airflow obstruction. The National Asthma Education and Prevention Program (NAEPP) guidelines established a number of asthma management goals that focus on achieving and maintaining asthma control (1). Asthma control is achieved by minimizing impairment and reducing risk of exacerbations, lung function decline and adverse effects (1). To achieve these goals, a six-step pharmacological algorithm for treatment of asthma has been recommended. β2AR agonists are the most powerful known bronchodilators and play a pivotal role in every step of this algorithm. While these agents were first used thousands of years ago, progress in drug development has resulted in safer, longer-acting and β2AR-specific agents(2). β2AR agonists act by binding to the β2AR, which is a member of the seven transmembrane domain, G protein-coupled family of receptors (GPCRs). Although β2ARs are present in high density in airway smooth muscle cells, they are also present in a multitude of other tissues and cell types including submucosal glands, airway epithelial cells, vascular endothelium, mast cells, circulating inflammatory cells such as eosinophils and lymphocytes, type II pneumocytes and cholinergic ganglia. Therefore, these agents may have other potential non-bronchodilator effects such as anti-inflammatory properties (3), although the clinical implications of such effects are still debatable(4). Numerous β2AR agonists of differing pharmacologic properties are available for clinical use and several more are currently in development for use either as stand-alone drugs (5) or in combination with inhaled corticosteroids (6). Clinicians typically base their choice of a particular agent on parameters of receptor selectivity, onset and duration of action, but rarely consider another very important pharmacologic characteristic - the intrinsic efficacy.
Pharmacologic Differences Among βAR Agonists
β2AR agonists are classified by their onset and duration of action, receptor selectivity, affinity, potency and efficacy(2). The onset of action of inhaled β2AR agonists is primarily influenced by their lipophilicity (the higher the lipophilicity the slower onset of action) and kinetics of binding. Among the agents currently in use, albuterol and formoterol have a more rapid onset of action than salmeterol. Duration of action is similarly influenced by lipophilicity and binding kinetics, as well as by resistance to clearance. Both salmeterol and formoterol have a longer duration of action than albuterol as their lipophilicity produces a depot effect at the cell membrane allowing for their twice daily administration. Receptor selectivity is also an important pharmacologic characteristic, however all currently used β2AR agonists are moderately to highly selective β2AR. Affinity refers to the attraction between the agonist and its receptor and is most commonly expressed as the dissociation constant between agonist and receptor. Potency refers to the concentration of a drug that achieves the half maximal response of which that drug is capable (EC50), and is dependent upon the affinity and intrinsic efficacy of the drug.
Intrinsic Efficacy refers to the ability of a drug to activate its receptor, without regard for drug concentration or receptor numbers as it is defined as efficacy/total receptor number (2). Receptor activation may be measured as a conformational change by physical techniques, as a biochemical response to activation of the signal transduction pathway downstream of the receptor, or as a physiologic response. Measured efficacy depends on variable factors in the target cell, such as receptor number or the presence of functional antagonism (i.e., activation of an opposing signal transduction pathway). In a cell with high receptor numbers, activation of only a small fraction of receptors suffices to generate a full response. Conversely, in a cell with low receptor number or in the presence of functional antagonism, activation of even a high fraction of receptors may not yield a full relaxation response.
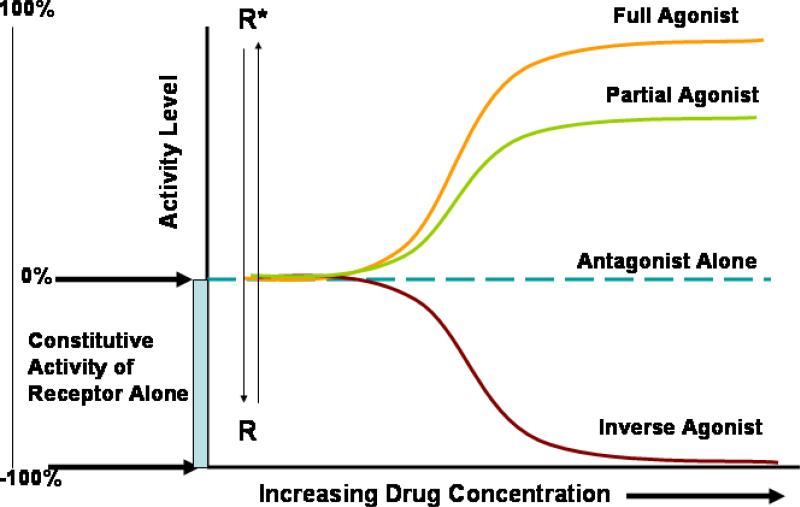
To better understand the differences between full, partial and inverse agonists, it is useful to consider the two-state receptor model. G-protein-coupled receptors are thought to exist in an equilibrium between an inactive conformation (R), and a spontaneously active conformation (R*), Classic agonists have a high affinity for R* relative to their affinity for R and increase the concentration of R*. In this model intrinsic efficacy is defined as the relative affinity preference of ligands for R and R* (Figure 1). Highly efficacious drugs (full agonists) have much higher affinity for R* than R, while agonists with low intrinsic efficacy (partial agonists) have a relatively small affinity preference for R* relative to R. Among currently available ß2AR agonists, epinephrine has the highest intrinsic efficacy followed by formoterol then by albuterol and finally salmeterol which has the lowest intrinsic efficacy. It is of interest that almost all novel ß2AR agonists under development have a high intrinsic efficacy comparable with that of formoterol (5). In this model, inverse agonists (negative intrinsic efficacy) have a high affinity for the R conformation relative to the R* and decrease the concentration of the R*, exerting the exact opposite effects of agonists. Finally, neutral competitive antagonists have equal affinity for R and R* and do not displace the equilibrium but competitively antagonize the effects of both agonists and inverse agonists (Figure 1)(7).
Figure 1. Schematic representation of the effects of a full agonist, a partial agonist and an inverse agonist.
Some receptors have measurable constitutive activity even in the absence of an agonist. The addition of an agonist to the system shifts the receptor conformation to the active conformation (R*). This is more pronounced with a full agonist than with a partial agonist. When an antagonist is added (in the absence of an agonist), it produces no effect (intrinsic efficacy = 0). However if an inverse agonist is added, it inhibits the constitutive (basal) activity of the receptor (negative intrinsic efficacy) and shifts the receptor away from the active conformation (R*) to the inactive conformation (R).
Clinical Implications of Full vs. Partial β2AR Agonists in Asthma
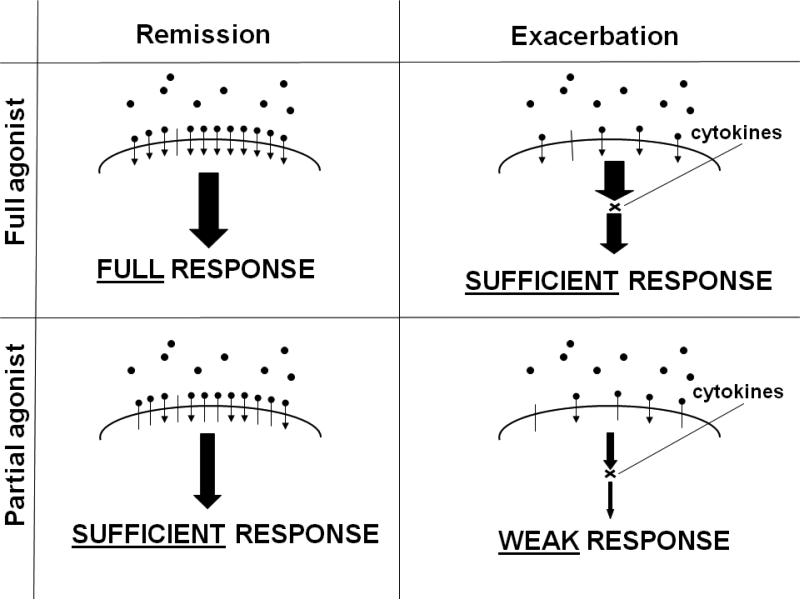
Because β2AR agonists can act rapidly and are the most effective bronchodilators available, their use is a cornerstone of the initial management of acute asthma exacerbations. The severity of acute asthma ranges from mild exacerbations that readily respond to initial therapy in the emergency department, to severe, life-threatening exacerbations requiring intubation and admission to the intensive care unit (8). Therefore, a single agent, a standard dose, and a particular route of delivery are not appropriate for all settings. In the majority of cases, an inhaled rescue drug such as albuterol, given more frequently and in higher doses than for simple rescue, will suffice. However, in a patient with impending respiratory failure despite the administration of high doses of a rescue medication when β2 AR are desensitized by prior use of β2-agonists and functionally antagonized by inflammatory mediators that are present during an acute exacerbation, a full agonist has advantages over a partial agonist (Figure 2) (9-11). In the chronic setting, agonists of high intrinsic efficacy such as formoterol have dose-dependent effects on bronchoprotection an effect that is not seen with salmeterol (12).
Figure 2.
Schematic Representation for the Potential Differential Effects Between Full and Partial Beta-Agonists on Airway Smooth Muscles During Remission and During an Acute Severe Exacerbation of Asthma (modified from (9)
Safety of β2AR Agonists in Asthma
Adverse effects of β2AR agonists are largely due to activation of β2AR in non-target tissues. Cardiac stimulation leads to tachycardia and arrhythmias, while skeletal muscle stimulation leads to tremor and hypokalemia. Non-target tissues, such as skeletal muscle and the heart, have a lower ß2AR density than airway smooth muscle. This accounts, in part, for the excellent side-effect profile of partial agonists such as albuterol, because there are sufficient spare receptors in the target tissue for full cell activation by a partial agonist but not in non-target tissues(2). Furthermore, the desensitization that occurs during the first few days of regular use of a ß2AR agonist results in further reduction in the responsiveness of non-target tissues, accounting for the commonly observed resolution of side effects, such as tachycardia and tremor, after the first few doses. In view of the low receptor density of non-target tissues, it might be expected that full ß2AR agonists would elicit a response greater than partial agonists in non-target tissues, and this is indeed the case. In dose-response studies, full agonists with high intrinsic efficacy have been demonstrated to be capable of causing more adverse effects, such as greater tachycardia and reduction in serum potassium, than partial agonists(12-14). Tolerance to the bronchoprotective properties of β2AR agonists and modest but non-progressive tolerance to their bronchodilator effects have been seen. The regular use of short-acting β2AR agonists has been associated with increased airway hyperresponsiveness, loss of asthma control and even increased asthma mortality (15). Recent data show similar associations with regular use of long-acting β2AR. It is of interest that the most dramatic spikes in asthma mortality were observed with the use of high-dose formulations of β2AR agonists of high intrinsic efficacy, isoproterenol and fenoterol (15). These adverse effects may be more pronounced in certain individuals with homozygous arginine genotype at position 16 of the β2AR (one sixth of Caucasians and one fifth of African Americans in the U.S.)(16;17).
More recently, the safety of regular use of long-acting β2AR agonists (both low and high intrinsic efficacy agents) has also been questioned (18-21). This issue was amplified by the results of a study (SMART) which suggested an association between the regular use of salmeterol with an increase asthma-related deaths and life-threatening experience (22). Other studies questioned the safety of formoterol in asthma (23) and a recent metanalysis did not have enough power to rule out any signal of increased mortality with formoterol (24). On the other hand, several observational studies failed to confirm these findings in patients treated with long acting β2AR agonists who are taking inhaled corticosteroids (25-27) (28;29).
The exact mechanisms for this safety issue with chronic use of β2AR agonists in asthma remain unknown. Potential mechanisms include the increase in airway inflammation (30), airway hyperresponsiveness (31;32), competitive antagonism of the β2AR causing subsensitivity to albuterol (33) and the increase in brain-derived neurotrophic factor (BDNF) (32). Furthermore, although some studies suggested that adverse effects occur more frequently in some patients with homozygous arginine 16 genotype (34) (35) (36), other studies failed to confirm this observation with the use of long-acting β2AR agonists (37;38).
Inverse βAR Agonists (β-blockers) in Asthma
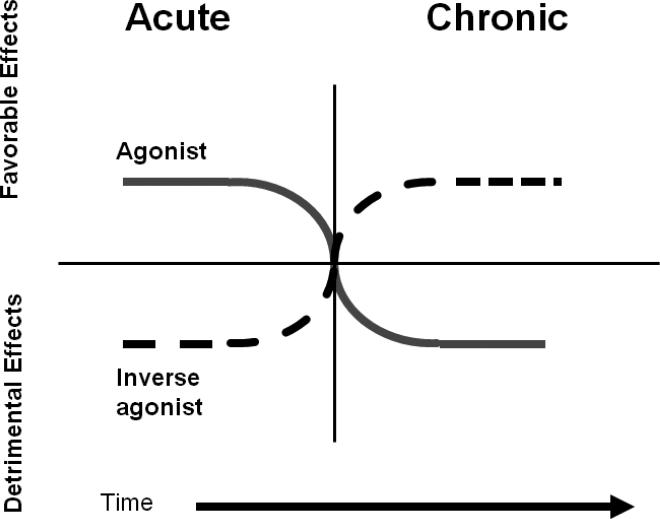
Inverse agonists exert opposite effects to agonists. However, the clinical effects of agonists and inverse agonists are highly dependent on the duration of therapy (39)(Figure 3). This paradox was first described in congestive heart failure (CHF) where the acute administration of a β2AR agonist is beneficial; however its chronic administration is deleterious to cardiac function and increases mortality. The opposite occurs with the use of an inverse agonist (β-blocker) which has an acute deleterious effect but chronically beneficial effect on survival (40). The same analogy is observed with β2AR agonists in asthma where their acute administration is beneficial to lung function and symptoms, but their chronic use is associated loss of asthma control and increased mortality. Inverse agonists are currently contraindicated in asthma and thus are underutilized in this population, even in those patients with cardiac risk factors who may benefit from them (41). This results from the fact that the acute administration of these drugs can produce bronchoconstriction and worsening of asthma symptoms (42). However, several recent reports demonstrated the safety and potential beneficial effects of cardioselective beta-blockers when administered to treat cardiac comorbidities in patients with asthma or COPD (43-46). The effects of chronic administration of inverse β-agonists in the treatment of asthma has remained unknown until recently. Recent studies using a murine model of asthma showed that while acute (single dose) administration of βAR inverse agonists increased airway hyperresponsiveness (AHR), their chronic (28 day) administration had an opposite effect and decreased AHR (47). Furthermore, chronic treatment with βAR inverse agonists produces broad anti-inflammatory effects, and especially dramatic effects on airway epithelium and mucous metaplasia (48;49). These results have been confirmed using β2AR null mice demonstrate a pivotal role of the β2AR for full development of mucous metaplasia and other features of asthma (50). In human asthma, the effect of chronic administration of the inverse βAR agonist, nadolol, on airway hyperresponsiveness in 10 patients with mild asthma was recently reported (51). Nadolol produced a dose-dependent increase in the PC20 methacholine. The significance of these above findings in animal models and humans subjects, if confirmed in additional studies, may provide a paradigm shift in the chronic management of asthma (52-54).
Figure 3. Effect of Duration of Exposure for an Agonist or an Inverse Agonist on Clinical Effects.
β-agonists can be acutely beneficial but chronically can induce desensitization of the receptor, while inverse agonists (beta-blockers) are acutely detrimental but chronically beneficial (adapted from (39).
Conclusion
Intrinsic efficacy is a key pharmacologic parameter that differs dramatically among available ß-agonists. β2AR agonists of high Intrinsic efficacy offer advantages over weak agonists in emergency management of asthma though their use is associated with dose-dependent adverse effects. The long-term use of both short- and long-acting β2AR agonists, especially when used without concomitant steroids, can be associated with worsening asthma control, increased airway hyperresponsiveness and increased mortality. βAR inverse agonists may have beneficial effects in the chronic treatment of asthma but this needs further evaluation.
Acknowledgements
This work was supported partly by grants from NIH (5K23HL079054) and the American Asthma Foundation Research Program.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
* Of special interest
** Of outstanding interest
Reference List
- 1.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Hanania NA, Sharafkhaneh A, Barber R, Dickey BF. Beta-agonist intrinsic efficacy: measurement and clinical significance. Am J Respir Crit Care Med. 2002;165:1353–58. doi: 10.1164/rccm.2109060. [DOI] [PubMed] [Google Scholar]
- 3.Hanania NA, Moore RH. Anti-inflammatory activities of beta2-agonists. Curr Drug Targets Inflamm Allergy. 2004;3:271–77. doi: 10.2174/1568010043343598. [DOI] [PubMed] [Google Scholar]
- 4 *.Sindi A, Todd DC, Nair P. Antiinflammatory effects of long-acting beta2-agonists in patients with asthma: a systematic review and metaanalysis. Chest. 2009;136:145–54. doi: 10.1378/chest.08-2149. [A recent review of the non-bronchodilatory effects of beta2-agonists which failed to show significant anti-inflammatory effects for these drugs in asthma] [DOI] [PubMed] [Google Scholar]
- 5 *.Cazzola M, Matera MG. Emerging inhaled bronchodilators: an update. Eur Respir J. 2009;34:757–69. doi: 10.1183/09031936.00013109. [A recent review of novel beta-2 adrenoceptor agonists currently in development] [DOI] [PubMed] [Google Scholar]
- 6.Mansfield LE. The future of the long-acting beta-adrenergic bronchodilators in the treatment of asthma. Allergy Asthma Proc. 2008;29:103–8. doi: 10.2500/aap.2008.29.3088. [DOI] [PubMed] [Google Scholar]
- 7.Parra S, Bond RA. Inverse agonism: from curiosity to accepted dogma, but is it clinically relevant? Curr Opin Pharmacol. 2007;7:146–50. doi: 10.1016/j.coph.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigo GJ, Rodrigo C, Hall JB. Acute asthma in adults: a review. Chest. 2004;125:1081–102. doi: 10.1378/chest.125.3.1081. [DOI] [PubMed] [Google Scholar]
- 9 **.Hanania NA, Moore RH, Zimmerman JL, Miller CT, Bag R, Sharafkhaneh A, et al. The role of intrinsic efficacy in determining response to a beta2-agonist in acute severe asthma. Respir Med. 2007;101:1007–14. doi: 10.1016/j.rmed.2006.08.023. [A clinical study that clearly demonstrates the superiority of full agonists over partial agonists in acute severe asthma] [DOI] [PubMed] [Google Scholar]
- 10.Bremner P, Siebers R, Crane J, Beasley R, Burgess C. Partial vs full beta-receptor agonism. A clinical study of inhaled albuterol and fenoterol. Chest. 1996;109:957–62. doi: 10.1378/chest.109.4.957. [DOI] [PubMed] [Google Scholar]
- 11.Lipworth BJ, Newnham DM, Clark RA, Dhillon DP, Winter JH, McDevitt DG. Comparison of the relative airways and systemic potencies of inhaled fenoterol and salbutamol in asthmatic patients. Thorax. 1995;50:54–61. doi: 10.1136/thx.50.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmqvist M, Ibsen T, Mellen A, Lotvall J. Comparison of the relative efficacy of formoterol and salmeterol in asthmatic patients. Am J Respir Crit Care Med. 1999;160:244–49. doi: 10.1164/ajrccm.160.1.9901063. [DOI] [PubMed] [Google Scholar]
- 13.Bremner P, Woodman K, Burgess C, Crane J, Purdie G, Pearce N, et al. A comparison of the cardiovascular and metabolic effects of formoterol, salbutamol and fenoterol. Eur Respir J. 1993;6:204–10. [PubMed] [Google Scholar]
- 14.Bremner P, Burgess C, Beasley R, Woodman K, Marshall S, Crane J, et al. Nebulized fenoterol causes greater cardiovascular and hypokalaemic effects than equivalent bronchodilator doses of salbutamol in asthmatics. Respir Med. 1992;86:419–23. doi: 10.1016/s0954-6111(06)80009-0. [DOI] [PubMed] [Google Scholar]
- 15 **.Pearce N. The use of beta agonists and the risk of death and near death from asthma. J Clin Epidemiol. 2009;62:582–87. doi: 10.1016/j.jclinepi.2009.01.004. [A comprehensive paper that summarizes the safety issues of beta2-adrenoceptor agonists in asthma including epidemiologic and clinical data] [DOI] [PubMed] [Google Scholar]
- 16.Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack R, Craig TJ, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004;364:1505–12. doi: 10.1016/S0140-6736(04)17273-5. [DOI] [PubMed] [Google Scholar]
- 17.Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, et al. Effect of polymorphism of the beta(2)-adrenergic receptor on response to regular use of albuterol in asthma. Int Arch Allergy Immunol. 2001;124:183–86. doi: 10.1159/000053705. [DOI] [PubMed] [Google Scholar]
- 18.Drazen JM, O'Byrne PM. Risks of long-acting beta-agonists in achieving asthma control. N Engl J Med. 2009;360:1671–72. doi: 10.1056/NEJMe0902057. [DOI] [PubMed] [Google Scholar]
- 19 *.Kramer JM. Balancing the benefits and risks of inhaled long-acting beta-agonists--the influence of values. N Engl J Med. 2009;360:1592–95. doi: 10.1056/NEJMp0810561. [This paper summarizes the recent issues with the safety of beta2-adrenoceptor agonists in asthma and the issues reviewed by the U.S. FDA] [DOI] [PubMed] [Google Scholar]
- 20.Taylor DR. The beta-agonist saga and its clinical relevance: on and on it goes. Am J Respir Crit Care Med. 2009;179:976–78. doi: 10.1164/rccm.200901-0055CC. [DOI] [PubMed] [Google Scholar]
- 21.Sears MR. Safety of long-acting beta-agonists: are new data really required? Chest. 2009;136:604–7. doi: 10.1378/chest.09-1214. [DOI] [PubMed] [Google Scholar]
- 22.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- 23.Mann M, Chowdhury B, Sullivan E, Nicklas R, Anthracite R, Meyer RJ. Serious asthma exacerbations in asthmatics treated with high-dose formoterol. Chest. 2003;124:70–74. doi: 10.1378/chest.124.1.70. [DOI] [PubMed] [Google Scholar]
- 24 *.Sears MR, Ottosson A, Radner F, Suissa S. Long-acting beta-agonists: a review of formoterol safety data from asthma clinical trials. Eur Respir J. 2009;33:21–32. doi: 10.1183/09031936.00145006. [A review of safety of formoterol clinical trials] [DOI] [PubMed] [Google Scholar]
- 25.Wijesinghe M, Perrin K, Harwood M, Weatherall M, Beasley R. The risk of asthma mortality with inhaled long acting beta-agonists. Postgrad Med J. 2008;84:467–72. doi: 10.1136/pgmj.2007.067165. [DOI] [PubMed] [Google Scholar]
- 26.Cates CJ, Lasserson TJ, Jaeschke R. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database Syst Rev. 2009:CD006922. doi: 10.1002/14651858.CD006922.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Cates CJ, Lasserson TJ, Jaeschke R. Regular treatment with formoterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database Syst Rev. 2009:CD006924. doi: 10.1002/14651858.CD006924.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Jaeschke R, O'Byrne PM, Nair P, Mejza F, Lesniak W, Brozek J, et al. The safety of formoterol among patients with asthma using inhaled corticosteroids. Systematic review and meta-analysis. Pol Arch Med Wewn. 2008;118:627–35. [PubMed] [Google Scholar]
- 29 **.Rodrigo GJ, Moral VP, Marcos LG, Castro-Rodriguez JA. Safety of regular use of long-acting beta agonists as monotherapy or added to inhaled corticosteroids in asthma. A systematic review. Pulm Pharmacol Ther. 2009;22:9–19. doi: 10.1016/j.pupt.2008.10.008. [A systematic review of the evidence for safety of long-acting beta2-agonists as stand- alone medications or in conjunction with inhaled corticosteroids] [DOI] [PubMed] [Google Scholar]
- 30.Nielson CP, Hadjokas NE. Beta-adrenoceptor agonists block corticosteroid inhibition in eosinophils. Am J Respir Crit Care Med. 1998;157:184–91. doi: 10.1164/ajrccm.157.1.9704070. [DOI] [PubMed] [Google Scholar]
- 31.Callaerts-Vegh Z, Evans KL, Dudekula N, Cuba D, Knoll BJ, Callaerts PF, et al. Effects of acute and chronic administration of beta-adrenoceptor ligands on airway function in a murine model of asthma. Proc Natl Acad Sci U S A. 2004;101:4948–53. doi: 10.1073/pnas.0400452101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32 *.Lommatzsch M, Lindner Y, Edner A, Bratke K, Kuepper M, Virchow JC. Adverse effects of salmeterol in asthma: a neuronal perspective. Thorax. 2009;64:763–69. doi: 10.1136/thx.2008.110916. [A recent clinical trial suggestion that the safety signal of salmeterol is driven by neuronal pathways] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipworth BJ. Antagonism of long-acting beta2-adrenoceptor agonism. Br J Clin Pharmacol. 2002;54:231–45. doi: 10.1046/j.1365-2125.2002.01651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer CN, Lipworth BJ, Lee S, Ismail T, Macgregor DF, Mukhopadhyay S. Arginine-16 beta2 adrenoceptor genotype predisposes to exacerbations in young asthmatics taking regular salmeterol. Thorax. 2006;61:940–944. doi: 10.1136/thx.2006.059386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wechsler ME, Israel E. beta-adrenergic receptor genotype and response to salmeterol. J Allergy Clin Immunol. 2007;120:218–19. doi: 10.1016/j.jaci.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 36.Lee DK, Currie GP, Hall IP, Lima JJ, Lipworth BJ. The arginine-16 beta2-adrenoceptor polymorphism predisposes to bronchoprotective subsensitivity in patients treated with formoterol and salmeterol. Br J Clin Pharmacol. 2004;57:68–75. doi: 10.1046/j.1365-2125.2003.01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bleecker ER, Postma DS, Lawrance RM, Meyers DA, Ambrose HJ, Goldman M. Effect of ADRB2 polymorphisms on response to longacting beta2-agonist therapy: a pharmacogenetic analysis of two randomised studies. Lancet. 2007;370:2118–25. doi: 10.1016/S0140-6736(07)61906-0. [DOI] [PubMed] [Google Scholar]
- 38.Bleecker ER, Yancey SW, Baitinger LA, Edwards LD, Klotsman M, Anderson WH, et al. Salmeterol response is not affected by beta2-adrenergic receptor genotype in subjects with persistent asthma. J Allergy Clin Immunol. 2006;118:809–16. doi: 10.1016/j.jaci.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 39.Dudekula N, Arora V, Callaerts-Vegh Z, Bond RA. The temporal hormesis of drug therapies. Dose Response. 2005;3:414–24. doi: 10.2203/dose-response.003.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bond RA, Spina D, Parra S, Page CP. Getting to the heart of asthma: can “beta blockers” be useful to treat asthma? Pharmacol Ther. 2007;115:360–374. doi: 10.1016/j.pharmthera.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 41 *.Olenchock BA, Fonarow GG, Pan W, Hernandez A, Cannon CP. Current use of beta blockers in patients with reactive airway disease who are hospitalized with acute coronary syndromes. Am J Cardiol. 2009;103:295–300. doi: 10.1016/j.amjcard.2008.09.081. [A study that reveals the underuse of beta-bockers in patients with asthma even in those who may benefit from them] [DOI] [PubMed] [Google Scholar]
- 42.Bond RA, Spina D, Parra S, Page CP. Getting to the heart of asthma: can “beta blockers” be useful to treat asthma? Pharmacol Ther. 2007;115:360–374. doi: 10.1016/j.pharmthera.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Thottathil P, Acharya J, Moss AJ, Jons C, McNitt S, Goldenberg I, et al. Risk of cardiac events in patients with asthma and long-QT syndrome treated with beta(2) agonists. Am J Cardiol. 2008;102:871–74. doi: 10.1016/j.amjcard.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashrafian H, Violaris AG. Beta-blocker therapy of cardiovascular diseases in patients with bronchial asthma or COPD: the pro viewpoint. Prim Care Respir J. 2005;14:236–41. doi: 10.1016/j.pcrj.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salpeter SR, Ormiston TM, Salpeter EE. Cardioselective beta-blockers in patients with reactive airway disease: a meta-analysis. Ann Intern Med. 2002;137:715–25. doi: 10.7326/0003-4819-137-9-200211050-00035. [DOI] [PubMed] [Google Scholar]
- 46.van Gestel YR, Hoeks SE, Sin DD, Welten GM, Schouten O, Witteveen HJ, et al. Impact of cardioselective beta-blockers on mortality in patients with chronic obstructive pulmonary disease and atherosclerosis. Am J Respir Crit Care Med. 2008;178:695–700. doi: 10.1164/rccm.200803-384OC. [DOI] [PubMed] [Google Scholar]
- 47.Callaerts-Vegh Z, Evans KL, Dudekula N, Cuba D, Knoll BJ, Callaerts PF, et al. Effects of acute and chronic administration of beta-adrenoceptor ligands on airway function in a murine model of asthma. Proc Natl Acad Sci U S A. 2004;101:4948–53. doi: 10.1073/pnas.0400452101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48 **.Lin R, Peng H, Nguyen LP, et al. Changes in beta 2-adrenoceptor and other signaling proteins produced by chronic administration of ‘beta-blockers’ in a murine asthma model. Pulm Pharmacol Ther. 2008;21:115–24. doi: 10.1016/j.pupt.2007.06.003. [A study in the murine asthma model demonstrating anti-inflammatory effects with the chornic administration of nadolol] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49 **.Nguyen LP, Omoluabi O, Parra S, et al. Chronic exposure to beta-blockers attenuates inflammation and mucin content in a murine asthma model. Am J Respir Cell Mol Biol. 2008;38:256–62. doi: 10.1165/rcmb.2007-0279RC. [A study from the murine asthma model demonstrating significant effects of nadolol on mucous metaplasia] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50 *.Nguyen LP, Lin R, Parra S, Omoluabi O, et al. {beta}2-Adrenoceptor signaling is required for the development of an asthma phenotype in a murine model. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0810902106. [A study from the murine asthma model demonstrating the pivotal role for β2AR on the development of asthma phenotype] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51 **.Hanania NA, Singh S, El-Wali R, et al. The safety and effects of the beta-blocker, nadolol, in mild asthma: an open-label pilot study. Pulm Pharmacol Ther. 2008;21:134–41. doi: 10.1016/j.pupt.2007.07.002. [First clinical trial that tested the efficacy and safety of nadolol treatment in mild asthma. Airway hyperresponsiveness was significantly reduced with chronic dosing] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lipworth BJ, Williamson PA. Beta blockers for asthma: a double-edged sword. Lancet. 2009;373:104–5. doi: 10.1016/S0140-6736(09)60018-0. [DOI] [PubMed] [Google Scholar]
- 53.Penn RB. Agonizing over agonism: should asthmatics turn their beta-receptors on or off? Proc Natl Acad Sci U S A. 2009;106:2095–96. doi: 10.1073/pnas.0812935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chupp GL. Say what, beta-blockers for asthma? Am J Respir Cell Mol Biol. 2008;38:249–50. doi: 10.1165/rcmb.2008-0001ED. [DOI] [PubMed] [Google Scholar]