Abstract
The pediatric small vessel vasculitides reviewed in this article are Henoch–Schönlein purpura (HSP) and the anti-neutrophil cytoplasmic antibody-associated vasculitides (AAV). The new classification criteria for HSP and Wegener’s granulomatosis are now validated and will facilitate the conduct of future epidemiological studies and clinical trials. The clinical manifestations of small vessel vasculitis in children are described, and current therapies discussed. There is a lack of good clinical trial data on which to base therapy for HSP. Similarly, data based on randomized controlled trials (RCTs) for pediatric AAV are lacking, although children with AAV are for the first time now included in a RCT of mycophenolate mofetil versus cyclophosphamide. Significant challenges remain in the field of pediatric small vessel vasculitis, including the development of validated disease outcome measures and biomarkers to be used in clinical trials. Lastly, long-term outcome data are lacking in survivors of pediatric small vessel vasculitis.
Keywords: ANCA-associated vasculitis, Child, Henoch–Schönlein purpura, Vasculitis
Introduction
This teaching article will review the main important small vessel vasculitides affecting children. These include Henoch–Schönlein purpura (HSP) and the anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV) Wegener’s granulomatosis (WG), microscopic polyangiitis (MPA), renal limited vasculitis, and Churg–Strauss syndrome (CSS). Other small vessel vasculitides, such as cutaneous leucytoclastic vasculitis, essential cryoglobulinemic vasculitis, Behçet’ disease, Cogan’s syndrome, and other rarer entities, will not be considered here.
Henoch–Schönlein purpura
Definition
Henoch–Schönlein purpura (HSP) is defined as a vasculitis with immunoglobulin (Ig)A-dominant immune deposits affecting small vessels and typically involving the skin, gut, and glomeruli and associated with arthralgias or arthritis [1]. It is the most common childhood primary systemic vasculitis [2]. The modifications of the classification criteria defining HSP described by Ozen et al. in 2005 [3] have recently been accepted following a formal validation study [4]. According to the new EULAR/PRINTO/PRES definition, a patient is classified as having HSP in the presence of purpura (commonly palpable) or petechiae with lower limb predominance (mandatory criterion) plus one of the four following criteria:
abdominal pain;
histopathology showing typical leukocytoclastic vasculitis with predominant IgA deposit or proliferative glomerulonephritis with predominant IgA deposit;
arthritis or arthralgia;
renal involvement (proteinuria or hematuria or presence of red blood cell casts).
In cases of purpura with atypical distribution, a demonstration of IgA is required at biopsy. This new definition provides sensitivity and specificity for the classification of HSP (using other forms of vasculitis as controls) of 100 and 87%, respectively [4].
Manifestations
Henoch–Schönlein purpura typically affects children between the age of 3 and 10 years [5]. Gardner-Medwin et al. reported a large population-based survey (1.1 million children aged <17 years) from a multi-ethnic region of the UK [2]. The annual incidence was estimated to be 20.4 per 100,000 children in the UK, with a greater incidence in children from the Indian subcontinent (24 per 100,000) compared with White Caucasians (17.8 per 100,000) and Blacks (predominantly Afro-Caribbean: 6.2 per 100,000) [2]. Other epidemiological studies from the Netherlands and the Czech Republic place the incidence between 6.1 and 10.2 per 100,000 children, respectively, possibly reflecting differences in ethnicity and/or methodological differences in data collection in these studies [6, 7]. Yang et al. reported that HSP has an annual incidence of 12.9 per 100,000 children in Taiwan [8]. In addition, a wide variety of infectious agents have been reported as potential triggers of HSP [9]. Several polymorphisms relating to disease susceptibility, severity, and/or risk of renal involvement have recently been described [10, 11]. Many of these polymorphisms relate to cytokines or cell adhesion molecules involved in the modulation of inflammatory responses and endothelial cell activation [9]. On the whole, studies of this nature have been hampered by relatively small patient numbers; consequently, they lack the power to be definitive or necessarily applicable to all racial groups.
Skin involvement is typically with purpura which is generally symmetrical, affecting the lower limbs and buttocks in the majority of cases; the upper extremities are involved less frequently. The abdomen, chest, and face are generally unaffected. New crops of purpura may develop for several months after disease onset, though these generally fade with time. Lesions can be induced by mild trauma. Angioedema and urticaria can also occur.
Around two-thirds of children have joint manifestations at presentation. The knees and ankles are most frequently involved. Symptoms, which take the form of pain, swelling, and decreased range of movement, tend to be fleeting and resolve without the development of permanent damage.
Three-quarters of children develop abdominal symptoms ranging from mild colic to severe pain with ileus and vomiting. Hematemesis and melena are sometimes observed. Other complications include intestinal perforation and intussusception. The latter may be difficult to distinguish from abdominal colic, and the incidence of intussusception is significant enough to warrant exclusion by ultrasound where suspected. Acute pancreatitis is also described, although it is a rare complication [12].
Other organs less frequently involved include the central nervous system (cerebral vasculitis), gonads (orchitis may be confused with torsion of the testis), and the lungs (pulmonary hemorrhage). Many cases follow an upper respiratory tract infection, and the onset of the disorder may be accompanied by systemic symptoms, including malaise and mild pyrexia. Multiple organ involvement may be present from the outset of the disease or, alternatively, an evolving pattern may develop, with different organs becoming involved at different time points over the course of several days to several weeks. One complication worth emphasizing for pediatric nephrologists is the rare but well-recognized complication of ureteric obstruction [13].
Around one-third of children have signs and symptoms for <14 days, one-third for 2–4 weeks, and one-third for >4 weeks [14]. Recurrence of symptoms occurs in around one-third of cases, generally within 4 months of resolution of the original symptoms. Recurrences are more frequent in those patients with renal involvement [14].
Reports of HSP nephritis indicate that between 20–61% of cases are affected with this complication [15, 16]. Renal involvement is normally manifest between a few days and a few weeks after first clinical presentation, but it can occur up to 2 months or (rarely) more following presentation. Patients with bloody stools appear to have an increased risk of renal disease [14]. Renal involvement can present with varying degrees of severity, including isolated microscopic hematuria, proteinuria with microscopic or macroscopic hematuria, acute nephritic syndrome (hematuria with at least two of hypertension, raised plasma creatinine and oliguria), nephrotic syndrome (usually with microscopic hematuria), or a mixed nephritic–nephrotic picture.
Diagnosis
Diagnosis is usually straightforward clinically, with the typical skin rash (predominantly lower limb purpura) being the main clue, often accompanied by abdominal pain and arthralgia or overt arthritis. No single laboratory test is available for the diagnosis of HSP. Immunological investigations including complement levels and anti-nuclear antibodies are normal. The IgA level is elevated in approximately one-half of children, and a small number exhibit ANCA positivity [17]. Coagulation studies are normal (although factor XIII may be low; see below), and platelet numbers are normal or occasionally increased. Where significant nephritis is present at presentation, renal function and electrolytes may be correspondingly abnormal.
Most children do not require a tissue (renal and/ or skin) biopsy diagnosis, with the exception being children with atypical skin findings or suspected severe renal involvement. The skin lesion of HSP is that of a leukocytoclastic vasculitis with perivascular accumulation of neutrophils and mononuclear cells. The term leukocytoclasis refers to the breakdown of white blood cells in lesional tissue and, in particular, to the characteristic nuclear debris (“nuclear dust”) that is observed; this breakdown is not specific for HSP. Immunofluorescence studies reveal vascular deposition of IgA and C3 in the affected skin, although similar changes may be observed in skin unaffected by the rash. The cause of HSP is unknown, but it is likely that IgA has a pivotal role in the pathogenesis of the disease, a hypothesis supported by the almost universal deposition of IgA in lesional vascular tissue. Galactose deficiency of O-linked glycans in the hinge region of IgA1 has recently been reported in adults with IgA nephropathy and children with HSP [18]. Of note, however, it is possible to fail to detect IgA deposition in cutaneous vascular tissue in some cases of HSP, especially if the biopsy was obtained from the middle of a lesion where the presence of proteolytic enzymes can result in negative staining for IgA [19].
It is recognized that disease activity may be linked to a rapid decline in factor XIII, particularly in patients with severe abdominal involvement and, in some cases, before the appearance of the classical skin rash [20–22]. There have been anecdotal reports of factor XIII replacement to treat severe abdominal symptoms [21]. The levels of factor XIII also declines prior to the recurrence of HSP.
The renal lesion of HSP nephritis is characteristically a focal and segmental proliferative glomerulonephritis. Severe cases with rapidly progressive glomerulonephritis usually demonstrate a high percentage of crescentic glomerular changes on renal biopsy. Indications for diagnostic renal biopsy in children with HSP are [23]:
nephritic/nephrotic presentation (urgent);
raised creatinine, hypertension or oliguria (urgent);
heavy proteinuria (urine albumin/urine creatinine ratio persistently >100 mg/mmol) on an early morning urine sample at 4 weeks; serum albumin not necessarily in the nephrotic range;
persistent proteinuria (not declining) after 4 weeks;
consider biopsy for persistent impaired renal function [glomerular filtration rate (GFR) <80 ml/min/1.73 m2].
The differential diagnosis of HSP includes sepsis (particularly meningococcal septicaemia), other systemic vasculitides [systemic lupus erythematosus (SLE), polyarteritis nodosa (PAN), WG, MPA, hypersensitivity vasculitis, and cutaneous leukocytoclastic vasculitis], all of which can present with similar clinical features [23]. Isolated cutaneous leukocyclastic vasculitis does not typically present with a history of a hypersensitivity reaction to drugs or infections and hence should be differentiated clinically from true hypersensitivity vasculitis [24]. Cutaneous leukocytoclastic vasculitis can occur with arthralgia and thus mimic HSP, but it is not associated with the other systemic features of HSP, such as renal involvement. Thus, the diagnosis of true cutaneous leukocytoclastic vasculitis is one of exclusion after screening for features of systemic features [24].
Familial Mediterranean fever can also mimic or occur in association with HSP in areas where this is endemic [25, 26].
Treatment
The large majority of cases of HSP require symptomatic treatment only. Arthropathy is managed with rest and analgesia.
The management of HSP nephritis has recently been reviewed by Zaffanello and Fanos [27]. At the conclusion of this extensive literature review, the authors emphasized that currently prescribed treatments for HSP nephritis are not adequately guided by evidence obtained in robust randomized placebo-controlled trials with outcome markers related to the progression to end stage renal disease. The following is a more detailed discussion.
Treatment to prevent renal disease Various treatment strategies and strategies with the aim of preventing disease complications, such as renal disease, have been reported to have variable effects. The efficacy of corticosteroids to prevent complications such as abdominal pain is still debated [28, 29]. Chartapisak et al. recently systematically reviewed all published randomized controlled trials (RCTs) for the prevention or treatment of renal involvement in HSP [30]. Meta-analyses of four RCTs that evaluated prednisone therapy at the presentation of HSP revealed that the risk of renal involvement developing or persisting at 1, 3, 6, and 12 months with prednisone treatment was not significantly different from that with placebo or no specific treatment. It is therefore becoming clearer that prophylactic corticosteroid does not prevent the onset of HSP nephritis. That said, there could still be a role for the early use of corticosteroids in patients with severe extrarenal symptoms and in those with renal involvement, as suggested by the findings of a study performed by Ronkainen et al. [29]. Prednisone treatment (1 mg/kg/day for 2 weeks, with weaning over the subsequent 2 weeks) was effective in reducing the intensity of abdominal pain and joint pain. Prednisone did not prevent the development of renal symptoms, but it was effective in treating them if present; renal symptoms resolved in 61% of the prednisone patients after treatment, compared with 34% of the placebo patients, although it should be noted that the renal involvement in the patients in this study was relatively mild [29].
Treatment of rapidly progressive glomerulonephritis There are good data indicating that crescents in >50% of glomeruli and nephrotic range proteinuria carry an unfavorable prognosis, thus highlighting the need for an effective intervention. Unfortunately, to date, there is only one published RCT that has evaluated the benefit of treatment, showing no difference in outcome using cyclophosphamide versus supportive therapy alone [31]. However, this study did not examine combined therapy, i.e. cyclophosphamide and steroid, which is a regimen used in most other severe small vessel vasculitides (see below). For patients with rapidly progressive glomerulonephritis (RPGN) with crescentic change on biopsy, uncontrolled data suggest that treatment may comprise aggressive therapy with corticosteroid, cyclophosphamide and, possibly, plasma exchange [27], as for other causes of crescentic nephritis. Other therapies, such as cyclosporine A, azathioprine, and cyclophosphamide, have been reported by some authors to be effective [27]. As HSP is probably the commonest cause of rapidly progressive glomerulonephritis in childhood, more aggressive therapeutic approaches have been employed in some cases. Shenoy et al. reported on 14 children with severe HSP nephritis treated successfully with plasma exchange alone [32]. These treatment options, whilst potentially important in select cases, are not yet supported by the outcomes of RCTs.
Treatment of HSP nephritis that is not rapidly progressive Such patients may exhibit the following features: less than 50% crescents on renal biopsy, sub-optimal GFR, and heavy proteinuria which is not necessarily in the nephrotic range [23]. There are no outcomes from robust clinical trials to guide the therapy of this type of presentation. Many would advocate corticosteroids. Others advocate the addition of cyclophosphamide to corticosteroids in HSP nephritis in which biopsy shows diffuse proliferative lesions or sclerosis, but with <50% crescentic change, in patients who have ongoing heavy proteinuria. A typical regimen would comprise 8 weeks of oral cyclophosphamide (2 mg/kg/day) with intravenous pulsed methylprednisolone [33], followed by daily prednisolone, and converting to alternate day prednisolone and azathioprine for a total of 12 months [23]. Published evidence for the efficacy of this approach is lacking, but this may be a reasonable option bearing in mind the adverse prognosis of children with HSP who have a nephritic/nephrotic phenotype, particularly if therapy is delayed. In patients with >6 months duration of proteinuria an angiotensin converting enzyme (ACE) inhibitor may be indicated to limit secondary glomerular injury, although again the evidence to support this therapy is lacking [27].
Outcome
The majority of children with HSP make a full and uneventful recovery with no evidence of ongoing significant renal disease. Renal involvement is the most serious long-term complication of HSP. A study of the long-term outcome of 78 subjects who had Henoch–Schönlein nephritis during childhood (mean of 23.4 years after onset) demonstrated overall that initial findings on renal biopsy correlated well with outcome but that they had a poor predictive value in individual patients [34]. Of the patients who had nephritic, nephrotic, or nephritic/nephrotic syndromes at onset, 44% had hypertension or impaired renal function, whereas 82% of those who presented with hematuria (with or without proteinuria) were normal [34]. Seven patients deteriorated clinically years after apparent complete clinical recovery. Of the 44 full-term pregnancies, 16 were complicated by proteinuria and/or hypertension, even in the absence of active renal disease [34].
Narchi systematically reviewed all published literature on long-term renal impairment in children with HSP [35]. Twelve studies with 1133 children were reviewed. Renal involvement occurred in 34% of children; 80% had isolated hematuria and/or proteinuria, while 20% had acute nephritis or nephrotic syndrome [35]. Renal complications, if they did occur, developed early—by 4 weeks in 85% of children and by 6 months in nearly all children [35]. Persistent renal involvement (hypertension, reduced renal function, nephrotic or nephritic syndrome) occurred in 1.8% of children overall, but the incidence varied with the severity of the kidney disease at presentation, occurring in 5% of children with isolated hematuria and/or proteinuria but in 20% who had acute nephritis and/or nephrotic syndrome in the acute phase [35]. Children with significant renal impairment at presentation and/or persistent proteinuria should undergo regular assessment of their GFR, such as 1, 3, and 5 years after the acute episode of HSP [23]. Some instances of hypertension have been reported many years after the normalization of renal function and urinalysis [36]. An increased incidence of pre-eclampsia has also been reported. Interestingly, in children who underwent repeat renal biopsies, the majority of children with HSP still had IgA years later [37], which could partly explain the later renal morbidity sometimes described.
Thus, it is clear that robust clinical trials for the treatment of moderate and severe HSP nephritis are urgently required.
Lastly, it is recognized that HSP can occur in renal allografts [38]. True recurrence should, however, be differentiated from IgA deposits that are sometimes seen in renal transplants in patients who did not have HSP or IgA nephropathy [14]. One worrying suggestion is that recurrence rates may be higher for living donor transplants, although data are limited in this area [39].
Anti-neutrophil cytoplasmic antibody-associated vasculitides
The AAV are Wegener’s granulomatosis, microscopic polyangiitis, Churg–Strauss syndrome, and so-called renal limited vasculitis (previously referred to as idiopathic crescentic glomerulonephritis) [40]. Patients with renal limited vasculitis present with pauci-immune necrotizing crescentic glomerulonephritis (NCGN) without extrarenal manifestations of systemic vasculitis. Most of these patients have ANCA directed against myeloperoxidase (MPO-ANCA); hence, the condition is considered to be a form of MPA limited to the kidney. Although rare, the AAV do occur in childhood, although CSS is extremely rare in children [41].
The most accepted model of pathogenesis proposes that ANCA activate cytokine-primed neutrophils, leading to bystander damage of endothelial cells and an escalation of inflammation with recruitment of mononuclear cells [42]. However, other concomitant exogenous factors and genetic susceptibility appear to be necessary for disease expression as well. The AAV are considered individually in the following sections.
Wegener’s granulomatosis
Definition
Wegener’s granulomatosis is defined according to the Chapel–Hill criteria as granulomatous inflammation involving the respiratory tract, with necrotizing vasculitis affecting small to medium-sized vessels (e.g. capillaries, venules, arterioles, and arteries); necrotizing glomerulonephritis is common [1].
The recently modified classification definition for WG requires the presence of three of the following six criteria:
renal involvement (proteinuria or hematuria or red blood cell casts);
positive histopathology (granulomatous inflammation within the wall of an artery or in the perivascular or extravascular area);
upper airway involvement (nasal discharge or septum perforation, sinus inflammation);
laryngotracheobronchial involvement (subglottic, tracheal or bronchial stenosis);
pulmonary involvement [chest X-ray or computed tomography (CT)];
ANCA positivity [by immunofluorescence, or by enzyme-linked immunosorbent assay (ELISA) proteinase 3 (PR3)-ANCA or MPO-ANCA.
The sensitivity and specificity of the new EULAR/ PRINTO/PRES criteria are 93 and 99%, respectively, for the classification of WG as distinct from other forms of vasculitis in the pediatric population ([43], Brogan et al., submitted).
Definition of other AAV
Microscopic polyangiitis (formerly microscopic polyarteritis) differs from classic PAN by the presence of extensive glomerular involvement. Microscopic polyangiitis may be defined as necrotizing vasculitis, with few or no immune deposits, affecting small vessels (i.e. capillaries, venules, or arterioles); necrotizing arteritis involving small and medium-sized arteries may be present. In addition, necrotizing glomerulonephritis is very common, and pulmonary capillaritis often occurs [1]. Clinically, MPA can be difficult to distinguish from WG and often presents with rapidly progressive pauci-immune glomerulonephritis [44] in association with perinuclear ANCA (pANCA) positivity [1].
Renal limited AAV describes those patients with rapidly progressive glomerulonephritis, often with ANCA positivity (usually MPO-ANCA) but without other organ involvement. This condition probably represents a forme fruste MPA.
Churg–Strauss syndrome was defined in the Chapel–Hill Consensus as an eosinophil-rich and granulomatous inflammation involving the respiratory tract and necrotizing vasculitis affecting small to medium-sized vessels; there is an association with asthma and eosinophilia [1].
Manifestations of WG
Wegener’s granulomatosis typically affects the upper and lower respiratory tract and is associated with glomerulonephritis, although the disease can affect any organ system in the body [45]. The typical pathological process is granulomatous inflammation with or without necrotizing small vessel vasculitis. From a clinical perspective, it may be useful to think of WG as having two forms: (1) a predominantly granulomatous form with mainly localized disease with a chronic course, and (2) a florid, acute small vessel vasculitic form characterized by severe pulmonary hemorrhage and/or rapidly progressive vasculitis or other severe vasculitic manifestation (see below). These two broad presentations may coexist or present sequentially in individual patients.
Symptoms and signs of upper respiratory tract involvement include epistaxis, otalgia, and hearing loss (conductive and sensorineural) [45]. Nasal septal involvement with cartilaginous collapse results in the characteristic saddle nose deformity, although this may not be present at initial presentation. Inspection inside the nares using an auriscope may reveal inflammation with bleeding and crusting. Chronic sinusitis may be observed. Glottic and subglottic polyps and/or large and medium-sized airway stenoses can result from granulomatous inflammation. Lower respiratory tract manifestations also include granulomatous pulmonary nodules with or without central cavitation (Fig. 1) as well as pulmonary hemorrhages that can be relatively asymptomatic but which result in evanescent pulmonary shadows on chest X-ray or catastrophic pulmonary hemorrhage from pulmonary capillaritis associated with respiratory failure and high mortality (Fig. 2).
Fig. 1.
Pulmonary nodule in Wegener’s granulomatosis (WG). Computed axial tomography scan of thorax revealing a pulmonary nodule in a 12-year-old girl with WG with multi-systemic involvement (see also Fig. 3)
Fig. 2.
Diffuse alveolar hemorrhage in WG. Chest X-ray from a 14-year-old girl with diffuse alveolar hemorrhage due to WG with high titre PR3-anti-neutrophil cytoplasmic antibody (ANCA)
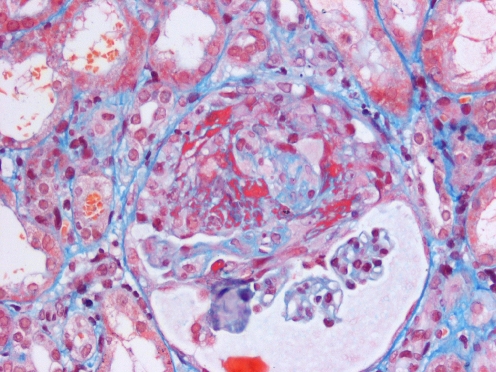
The typical renal lesion is a focal segmental necrotizing glomerulonephritis, with pauci-immune crescentic glomerular changes (Fig. 3). Clinical manifestations relating to this lesion include hypertension, significant proteinuria, nephritic and nephrotic syndrome and, ultimately, the protean clinical manifestations of renal failure.
Fig. 3.
Crescentic nephritis in WG. Focal segmental necrotizing glomerulonephritis with fibrocellular crescent formation in a 12-year-old girl with severe WG (same patient as shown in Fig. 1)
Other manifestations include orbital involvement with granuloma (Fig. 4), retinal vasculitis, peripheral gangrene with tissue loss, and vasculitis of the skin, gut, heart, central nervous system and/or peripheral nerves (mononeuritis multiplex), salivary glands, gonads, and breast. Non-specific symptoms, such malaise, fever, weight loss or growth failure, arthralgia, and arthritis, are relatively common.
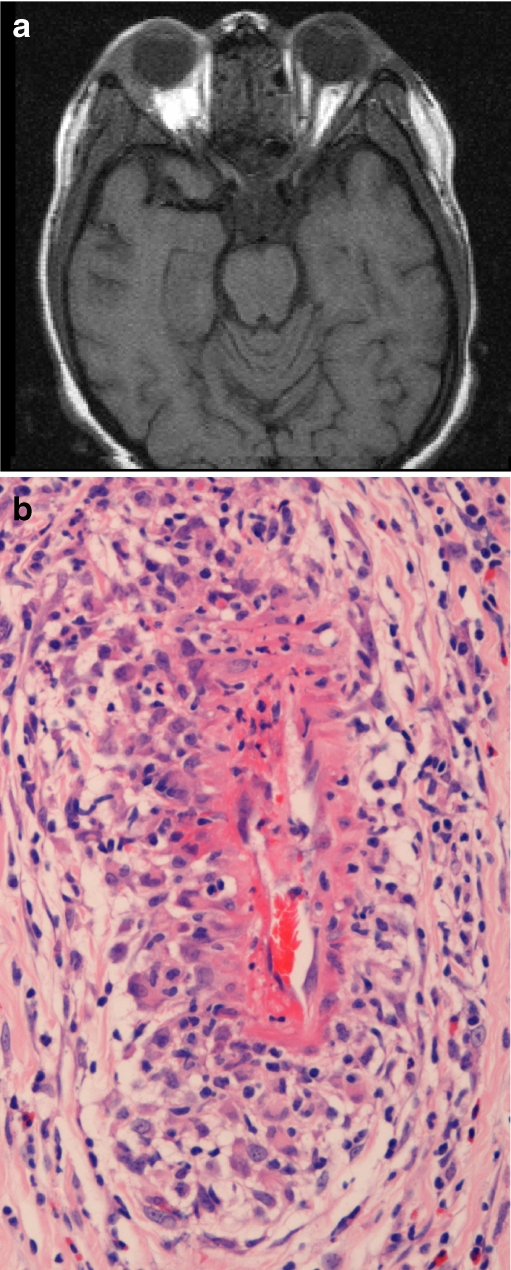
Fig. 4.
Orbital involvement in WG. a Magnetic resonance scan revealing bilateral soft tissue lesions involving the superolateral quadrants of both orbits in a 16-year-old girl with WG. b Orbital biopsy specimen from the same patient revealing perivascular granulomatous inflammation with fibrinoid necrosis of the vessel wall
The frequency of different system involvement in one published series of 17 children with WG was: respiratory, 87%; kidneys, 53%; sinuses, 35%; joints, 53%; eyes, 53%; nervous system, 12%; skin, 53% [46]. Another pediatric series of WG reported an even higher frequency of renal involvement, with 22/25 patients having glomerulonephritis at first presentation; only 1/11 patients who had renal impairment in that series recovered renal function with therapy [47]. Renal involvement in WG is recruited with increasing age, which could partly account for the variation in reported renal involvement in pediatric WG.
Manifestations of other AAV
Microscopic polyangiitis and renal limited AAV Microscopic polyangiitis was initially considered to be a “microscopic” form of polyarteritis nodosa, and the term MPA was first used to distinguish this small vessel vasculitis from classic PAN by the Chapel–Hill Consensus in 1994 [1], although recognition of the clinical differences between microscopic and classic PAN had been made long before time by others. The typical clinical manifestations are rapidly progressive glomerulonephritis and alveolar hemorrhage. Other possible symptoms resemble those encountered in PAN (see the review by Dillon et al. [43]). In adults, 75–80% of patients have pANCA/MPO-ANCA. The pathogenicity of MPO-ANCA has been established in animal models [48] and in at least two cases of transplacental transmission in humans resulting in affected neonates [49, 50]. Renal limited forms have been described in children and adults.
Churg–Strauss Syndrome In a recent review of 33 cases of childhood CSS, all patients had significant eosinophilia and asthma [41]. Furthermore, histological evidence of eosinophilia and/or vasculitis was present in virtually all patients. Anti-neutrophil cytoplasmic antibodies were found in only 25% of children with CSS.
Diagnosis
The diagnosis of AAV can be challenging and ANCA undoubtedly play an important role. Methodological improvements for the detection of ANCA have resulted in increased sensitivity and specificity. Both indirect immunofluorescence (IIF) and ELISA are used for routine diagnostic purposes. Typically, WG is associated with a cytoplasmic staining pattern of ANCA by IIF, and ELISA reveals specificity against PR3 (PR3-ANCA). However, pANCA with specificity directed against MPO (MPO-ANCA) can also be found in patients with WG. Microscopic polyangiitis and renal limited AAV are typically associated with pANCA by IIF and with MPO-ANCA by the ELISA.
Lastly, it should always be remembered that ANCA-negative forms of WG, MPA, renal limited vasculitis, and CSS are well described in children [41, 45].
Tissue diagnosis, in particular renal biopsy but also biopsy of skin, nasal septum, or other tissue, can be important diagnostically for diagnosing all of the AAV and can help stage the disease for therapeutic decision-making.
While the diagnostic value of ANCA is without question important, the value of ANCA for the longitudinal monitoring of disease activity is probably unreliable in many patients with WG [51]. The reasons for this unreliability are not fully understood, but they are likely to include methodological limitations of ANCA detection, the partial dissociation of ANCA levels and disease activity associated with the immunosuppressive therapy, and the as yet undefined complexity in the exact role of ANCA in the pathogenesis of WG [52].
Treatment of AAV
Renal morbidity and mortality are major concerns in the treatment of AAV and, consequently, therapy aimed at preserving renal function is a recurring theme when determining the therapeutic options [52]. Treatment for pediatric AAV is broadly similar to the approach used in adults, with corticosteroids, cyclophosphamide (usually six to ten intravenous doses of 500–1000 mg/m2 per dose, every three to four weeks; alternatively given orally at 2 mg/kg/day for 2–3 months), and plasma exchange (particularly for pulmonary capillaritis and/or rapidly progressive glomerulonephritis—“pulmonary-renal syndrome”) routinely employed to induce remission [52–54]. Intravenous pulsed cyclophosphamide is increasingly gaining favor over oral continuous cyclophosphamide in adults because of its reduced cumulative dose and lower incidence of neutropenic sepsis [55]. Consequently, it is being increasingly used to treat children with AAV as well, albeit without good pediatric evidence. This is followed by low dose corticosteroids and azathioprine (1.5–3 mg/kg/day) to maintain remission [56, 57]. Anti-platelet doses of aspirin (1–5 mg/kg once a day) can also be considered empirically on the basis of the increased risk of thrombosis associated with the disease process [58]. Methotrexate may have a role for inducing remission in patients with limited WG [59], but it is less commonly used as an induction agent in children with AAV. Co-trimoxazole is commonly added to therapeutic programs for the treatment of WG, particularly in those with upper respiratory tract involvement, serving both as prophylaxis against opportunistic infection and as a possible disease-modifying agent [60]. Recommendations regarding the duration of maintenance therapy are based on adult trial data which suggest that the strongest predictor of relapse is the withdrawal of therapy. These data indicate that maintenance therapy should be continued for several years [52]. As a general therapeutic measure, prophylaxis against osteoporosis, gastrointestinal ulceration, and infection (bacterial, protozoal, and fungal) are standard additional aspects of treatment for AAV [52].
As the use of cyclophosphamide contributes to morbidity and mortality [52, 56], with infection playing a prominent role [61], and disease relapses occur in 50% of the patients with AAV as the drugs are reduced or withdrawn, newer immunosuppressive agents and immunomodulatory strategies are being explored in both adults and children. Such treatments include mycophenolate mofetil (MMF) and rituximab, which have already been reported to be effective at inducing or maintaining remission in adults with AAV [62, 63] and are currently being evaluated in RCTs undertaken under the auspices of the European Vasculitis Study Group (EUVAS) and other groups worldwide. Of note is the EUVAS MYCYC trial (UK and Europe), which is comparing induction therapy of WG and MPA using cyclophosphamide (standard therapy) versus MMF (experimental therapy). This is the first EUVAS trial to include children as well as adults, and it is actively recruiting patients under the age of 17 years in the UK. At the time of writing this review, however, the rest of Europe was not yet set up to recruit children. For a full list of the past and present EUVAS trials for AVV, the reader is directed to: http://www.vasculitis.org/.
Increasingly, biologic therapy is being used to treat children with small vessel vasculitis, including AAV as well as ANCA-negative vasculitides. Such agents include rituximab, anti-tumor necrotizing factor-α (etanercept, infliximab, and adalimumab), and anakinra (recombinant interleukin 1 receptor antagonist) [64]. These therapies are mainly reserved for those who have failed to respond to standard treatment or for those patients for whom cumulative cyclophosphamide and/or corticosteroid toxicity is of particular concern [64]. Other novel therapies for AAV are reviewed elsewhere [52, 56].
Specialist-localized treatment of the upper and lower respiratory tract involvement of WG is beyond the scope of this review. The reader is therefore directed to the report of Hoffman et al. [65] and the recent report by White et al. [66].
Outcome of AAV
The AAV still carry considerable disease-related morbidity and mortality, particularly due to progressive renal failure or aggressive respiratory involvement, and therapy-related complications, such as sepsis.
The mortality for pediatric WG from one recent pediatric series was 12% over a 17-year period of study inclusion [46]. The largest pediatric series of patients with WG reported 40% of cases with chronic renal impairment at 33 months of follow-up [47] despite therapy. Thus, treatment has shifted WG from a disease associated with high mortality in the first year [67] to that of a chronic illness associated with relapsing course and high renal morbidity during pediatric follow-up.
Mortality in pediatric patients with MPA during pediatric follow-up has been reported to be between 0 and 14% [68, 69]. Two of seven children reported by Peco-Antic et al. [70] progressed to end stage renal disease, one developed chronic renal failure, and four normalized renal function. The outcome of a study on a selected population of 31 Japanese children with necrotizing pauci-immune glomerulonephritis and positive ANCA suggested a poor renal prognosis despite therapy [69]. In that series, at 43 months of follow-up 29% had developed end stage renal failure, 19.4% had reduced renal function, and only 48.4% had normal renal function. Using these data, the authors suggested that there was an overall renal survival of 75% at 39 months, which is comparable to the poor renal prognosis of MPA in adults [52].
For CSS in children, the most recent series quotes a related mortality of 18%, all attributed to disease rather than therapy [41].
Conclusion
Significant challenges remain to those concerned with the management of small vessel vasculitides in children. Randomized controlled trials involving pediatric multicenter collaboration are urgently required to guide therapy, particularly for the AAV, but also for HSP. Future basic science developments relating to the pathogenesis of pediatric small vessel vasculitis (and other vasculitides), including genetics, environmental factors, and host immunological responses, are anticipated to improve our understanding of these diseases and provide new therapeutic targets in the future. The development of validated disease outcome measures and robust biomarkers of disease activity will facilitate future clinical trials—and such developments are currently underway. Lastly, long-term follow-up data relating to such outcome variables as late mortality, cardiovascular health, malignancy, and impact on education are lacking for survivors of pediatric small vessel vasculitis.
Questions:
(answers appear at the end of the list of questions)
Regarding HSP, which of the following statements is TRUE?
HSP never recurs in renal allografts.
IgA is always found on immunostaining of biopsies of lesional tissue.
HSP rarely occurs after infections.
IgA may be elevated in sera.
Regarding Wegener’s granulomatosis in children, which of the following is TRUE?
The diagnosis can be excluded in children if ANCA are negative.
Serum ANCA levels correlate well with disease activity.
Despite lack of RCT evidence, there is still a role for plasma exchange in those with severe disease.
New therapies such as rituximab have replaced standard toxic therapies such as cyclophosphamide.
Regarding ANCA associated vasculitides, which of the following is TRUE?
Plasma exchange has proven efficacy for the treatment of WG in adults.
CSS in children only occurs as a result of therapy with leukotriene inhibitors.
The majority of children with CSS are ANCA positive.
Etanercept has proven efficacy for the treatment of WG in adults.
Regarding ANCA associated vasculitides, which of the following statements is FALSE?
Wegener’s granulomatosis never affects the aorta.
Intravenous cyclophosphamide is preferable to oral cyclophosphamide because it has a lower toxicity profile.
Intravenous cyclophosphamide can be given to patients with MESNA hypersensitivity.
Septrin (co-trimoxazole) may reduce relapses for patients with WG and respiratory tract involvement.
Regarding the following clinical trials of small vessel vasculitis, which of the following statements is TRUE?
Trials of novel therapies for small vessel vasculitis should always be conducted in adults before children.
Lack of good RCT data precludes the use of rituximab in the treatment of pediatric AAV.
RCT data suggest that early use of corticosteroids helps prevent the onset of HSP nephritis.
The Birmingham vasculitis activity score can be helpfully used in RCTs of therapy for small vessel vasculitis.
Answers:
d
c
a
a
d
References
- 1.Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, Hoffman GS, Hunder GG, Kallenberg CG. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 2.Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch-Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet. 2002;360:1197–1202. doi: 10.1016/S0140-6736(02)11279-7. [DOI] [PubMed] [Google Scholar]
- 3.Ozen S, Ruperto N, Dillon MJ, Bagga A, Barron K, Davin JC, Kawasaki T, Lindsley C, Petty RE, Prieur AM, Ravelli A, Woo P. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936–941. doi: 10.1136/ard.2005.046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozen S, Pistorio A, Iusan M, Bakkaloglu A, Herlin T, Brik R, Uziel S, Stabile A, Cantarini L, Norambuena X, Berkun Y, Olivieri AN, Djeddi D, Nuno L, Chasnyk V, Pruunsild C, Pagava K, Pederzoli S, Martini A, Ruperto N, Paediatric Rheumatology International Trials Organisation (PRINTO) The EULAR/ PRINTO/PRES criteria for Henoch-Schönlein purpura. Ann Rheum Dis. 2009;68(Suppl 3):712. [Google Scholar]
- 5.Gedalia A. Henoch-Schönlein purpura. Curr Rheumatol Rep. 2004;6:195–202. doi: 10.1007/s11926-004-0068-2. [DOI] [PubMed] [Google Scholar]
- 6.Dolezalova P, Telekesova P, Nemcova D, Hoza J. Incidence of vasculitis in children in the Czech Republic: 2-year prospective epidemiology survey. J Rheumatol. 2004;31:2295–2299. [PubMed] [Google Scholar]
- 7.Aalberse J, Dolman K, Ramnath G, Pereira RR, Davin JC. Henoch Schönlein purpura in children: an epidemiological study among Dutch paediatricians on incidence and diagnostic criteria. Ann Rheum Dis. 2007;66:1648–1650. doi: 10.1136/ard.2006.069187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang YH, Hung CF, Hsu CR, Wang LC, Chuang YH, Lin YT, Chiang BL. A nationwide survey on epidemiological characteristics of childhood Henoch-Schönlein purpura in Taiwan. Rheumatology (Oxford) 2005;44:618–622. doi: 10.1093/rheumatology/keh544. [DOI] [PubMed] [Google Scholar]
- 9.Brogan PA. What’s new in the aetiopathogenesis of vasculitis? Pediatr Nephrol. 2007;22:1083–1094. doi: 10.1007/s00467-007-0450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amoli MM, Thomson W, Hajeer AH, Calvino MC, Garcia-Porrua C, Ollier WE, Gonzalez-Gay MA. Interleukin 1 receptor antagonist gene polymorphism is associated with severe renal involvement and renal sequelae in Henoch-Schönlein purpura. J Rheumatol. 2002;29:1404–1407. [PubMed] [Google Scholar]
- 11.Gershoni-Baruch R, Broza Y, Brik R. Prevalence and significance of mutations in the familial Mediterranean fever gene in Henoch-Schönlein purpura. J Pediatr. 2003;143:658–661. doi: 10.1067/S0022-3476(03)00502-X. [DOI] [PubMed] [Google Scholar]
- 12.Cheung KM, Mok F, Lam P, Chan KH. Pancreatitis associated with Henoch-Schönlein purpura. J Paediatr Child Health. 2001;37:311–313. doi: 10.1046/j.1440-1754.2001.00606.x. [DOI] [PubMed] [Google Scholar]
- 13.Siomou E, Serbis A, Salakos C, Papadopoulou F, Stefanidis CJ, Siamopoulou A. Masked severe stenosing ureteritis: a rare complication of Henoch-Schönlein purpura. Pediatr Nephrol. 2008;23:821–825. doi: 10.1007/s00467-007-0698-5. [DOI] [PubMed] [Google Scholar]
- 14.Webb NJA, Brogan PA, Baildam EM. Renal manifestations of systemic disorders. In: Webb N, Postlethwaite R, editors. Clinical paediatric nephrology. Oxford: Oxford University Press; 2003. pp. 381–403. [Google Scholar]
- 15.Koskimies O, Rapola J, Savilahti E, Vilska J. Renal involvement in Henoch Schönlein purpura. Acta Pediatr Scand. 1974;63:357–363. doi: 10.1111/j.1651-2227.1974.tb04810.x. [DOI] [PubMed] [Google Scholar]
- 16.Stewart M, Savage JM, Bell B, McCord B. Long term renal prognosis of Henoch-Schönlein purpura in an unselected childhood population. Eur J Pediatr. 1988;147:113–115. doi: 10.1007/BF00442205. [DOI] [PubMed] [Google Scholar]
- 17.Lin JJ, Stewart CL, Kaskel FJ, Fine RN. IgG and IgA classes of anti-neutrophil cytoplasmic autoantibodies in a 13-year-old girl with recurrent Henoch-Schönlein purpura. Pediatr Nephrol. 1993;7:143–146. doi: 10.1007/BF00864379. [DOI] [PubMed] [Google Scholar]
- 18.Lau KK, Wyatt RJ, Moldoveanu Z, Tomana M, Julian BA, Hogg RJ, Lee JY, Huang WQ, Mestecky J, Novak J. Serum levels of galactose-deficient IgA in children with IgA nephropathy and Henoch-Schönlein purpura. Pediatr Nephrol. 2007;22:2067–2072. doi: 10.1007/s00467-007-0623-y. [DOI] [PubMed] [Google Scholar]
- 19.Shin JI, Kim JH, Lee JS. The diagnostic value of IgA deposition in Henoch-Schönlein purpura. Pediatr Dermatol. 2008;25:140–141. doi: 10.1111/j.1525-1470.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- 20.Gunasekaran TS, Berman J, Gonzalez M. Duodenojejunitis: is it idiopathic or is it Henoch-Schönlein purpura without the purpura? J Pediatr Gastroenterol Nutr. 2000;30:22–28. doi: 10.1097/00005176-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Kamitsuji H, Tani K, Yasui M, Taniguchi A, Taira K, Tsukada S, Iida Y, Kanki H, Fukui H. Activity of blood coagulation factor XIII as a prognostic indicator in patients with Henoch-Schönlein purpura. Efficacy of factor XIII substitution. Eur J Pediatr. 1987;146:519–523. doi: 10.1007/BF00441608. [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki K, Komura H, Nakahara Y, Shiraishi M, Higashida M, Ouchi K. Factor XIII in Henoch-Schönlein purpura with isolated gastrointestinal symptoms. Pediatr Int. 2006;48:413–415. doi: 10.1111/j.1442-200X.2006.02232.x. [DOI] [PubMed] [Google Scholar]
- 23.Rees L, Webb NJA, Brogan PA. Vasculitis. In: Rees L, Webb NJA, Brogan PA, editors. Paediatric nephrology (Oxford Handbook) Oxford: Oxford University Press; 2007. pp. 310–313. [Google Scholar]
- 24.Carlson JA, Chen KR. Cutaneous vasculitis update: small vessel neutrophilic vasculitis syndromes. Am J Dermatopathol. 2006;28:486–506. doi: 10.1097/01.dad.0000246646.45651.a2. [DOI] [PubMed] [Google Scholar]
- 25.Flatau E, Kohn D, Schiller D, Lurie M, Levy E. Schönlein-Henoch syndrome in patients with familial Mediterranean fever. Arthritis Rheum. 1982;25:42–47. doi: 10.1002/art.1780250107. [DOI] [PubMed] [Google Scholar]
- 26.Ozdogan H, Arisoy N, Kasapcapur O, Sever L, Caliskan S, Tuzuner N, Mat C, Yazici H. Vasculitis in familial Mediterranean fever. J Rheumatol. 1997;24:323–327. [PubMed] [Google Scholar]
- 27.Zaffanello M, Fanos V (2009) Treatment-based literature of Henoch-Schönlein purpura nephritis in childhood. Pediatr Nephrol 24:1901–1911 [DOI] [PubMed]
- 28.Huber AM, King J, McLaine P, Klassen T, Pothos M. A randomized, placebo-controlled trial of prednisone in early Henoch Schönlein Purpura [ISRCTN85109383] BMC Med. 2004;2:7. doi: 10.1186/1741-7015-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ronkainen J, Koskimies O, la-Houhala M, Antikainen M, Merenmies J, Rajantie J, Ormala T, Turtinen J, Nuutinen M. Early prednisone therapy in Henoch-Schönlein purpura: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2006;149:241–247. doi: 10.1016/j.jpeds.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 30.Chartapisak W, Opastiraku S, Willis NS, Craig JC, Hodson EM. Prevention and treatment of renal disease in Henoch-Schönlein purpura: a systematic review. Arch Dis Child. 2009;94:132–137. doi: 10.1136/adc.2008.141820. [DOI] [PubMed] [Google Scholar]
- 31.Tarshish P, Bernstein J, Edelmann CM., Jr Henoch-Schönlein purpura nephritis: course of disease and efficacy of cyclophosphamide. Pediatr Nephrol. 2004;19:51–56. doi: 10.1007/s00467-003-1315-x. [DOI] [PubMed] [Google Scholar]
- 32.Shenoy M, Ognjanovic MV, Coulthard MG. Treating severe Henoch-Schönlein and IgA nephritis with plasmapheresis alone. Pediatr Nephrol. 2007;22:1167–1171. doi: 10.1007/s00467-007-0498-y. [DOI] [PubMed] [Google Scholar]
- 33.Niaudet P, Habib R. Methylprednisolone pulse therapy in the treatment of severe forms of Schönlein-Henoch purpura nephritis. Pediatr Nephrol. 1998;12:238–243. doi: 10.1007/s004670050446. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein AR, White RH, Akuse R, Chantler C. Long-term follow-up of childhood Henoch-Schönlein nephritis. Lancet. 1992;339:280–282. doi: 10.1016/0140-6736(92)91341-5. [DOI] [PubMed] [Google Scholar]
- 35.Narchi H. Risk of long term renal impairment and duration of follow up recommended for Henoch-Schönlein purpura with normal or minimal urinary findings: a systematic review. Arch Dis Child. 2005;90:916–920. doi: 10.1136/adc.2005.074641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronkainen J, Nuutinen M, Koskimies O. The adult kidney 24 years after childhood Henoch-Schönlein purpura: a retrospective cohort study. Lancet. 2002;360:666–670. doi: 10.1016/S0140-6736(02)09835-5. [DOI] [PubMed] [Google Scholar]
- 37.Algoet C, Proesmans W. Renal biopsy 2–9 years after Henoch Schönlein purpura. Pediat Nephrol. 2003;18:471–473. doi: 10.1007/s00467-003-1132-2. [DOI] [PubMed] [Google Scholar]
- 38.Meulders Q, Pirson Y, Cosyns JP, Squifflet JP, Ypersele DS. Course of Henoch-Schönlein nephritis after renal transplantation. Report on ten patients and review of the literature. Transplantation. 1994;58:1179–1186. doi: 10.1097/00007890-199412150-00007. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa A, Kawamura T, Ito H, Hasegawa O, Ogawa O, Honda M, Ohara T, Hajikano H. Fate of renal grafts with recurrent Henoch-Schönlein purpura nephritis in children. Transplant Proc. 1989;21:2130–2133. [PubMed] [Google Scholar]
- 40.Radice A, Sinico RA. Antineutrophil cytoplasmic antibodies (ANCA) Autoimmunity. 2005;38:93–103. doi: 10.1080/08916930400022673. [DOI] [PubMed] [Google Scholar]
- 41.Zwerina J, Eger G, Englbrecht M, Manger B, Schett G. Churg-Strauss syndrome in childhood: a systematic literature review and clinical comparison with adult patients. Semin Arthritis Rheum. 2008 doi: 10.1016/j.semarthrit.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Harper L, Savage CO. Pathogenesis of ANCA-associated systemic vasculitis. J Pathol. 2000;190:349–359. doi: 10.1002/(SICI)1096-9896(200002)190:3<349::AID-PATH524>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 43.Dillon M, Eleftheriou D, Brogan P (2009) Medium-size vessel vasculitis. Pediatr Nephrol. doi:10.1007/s00467-009-1336-1 [DOI] [PMC free article] [PubMed]
- 44.Jardim HM, Leake J, Risdon RA, Barratt TM, Dillon MJ. Crescentic glomerulonephritis in children. Pediatr Nephrol. 1992;6:231–235. doi: 10.1007/BF00878354. [DOI] [PubMed] [Google Scholar]
- 45.Lindsley CB. Granulomatous vasculitis, giant cell arteritis, and sarcoidosis. In: Cassidy JT, Petty RE, editors. Textbook of pediatric rheumatology. Philadelphia: WB Saunders; 2001. pp. 604–627. [Google Scholar]
- 46.Belostotsky VM, Shah V, Dillon MJ. Clinical features in 17 paediatric patients with Wegener granulomatosis. Pediatr Nephrol. 2002;17:754–761. doi: 10.1007/s00467-002-0914-2. [DOI] [PubMed] [Google Scholar]
- 47.Akikusa JD, Schneider R, Harvey EA, Hebert D, Thorner PS, Laxer RM, Silverman ED. Clinical features and outcome of pediatric Wegener’s granulomatosis. Arthritis Rheum. 2007;57:837–844. doi: 10.1002/art.22774. [DOI] [PubMed] [Google Scholar]
- 48.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;10:955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bansal PJ, Tobin MC. Neonatal microscopic polyangiitis secondary to transfer of maternal myeloperoxidase-antineutrophil cytoplasmic antibody resulting in neonatal pulmonary hemorrhage and renal involvement. Ann Allergy Asthma Immunol. 2004;93:398–401. doi: 10.1016/S1081-1206(10)61400-7. [DOI] [PubMed] [Google Scholar]
- 50.Schlieben DJ, Korbet SM, Kimura RE, Schwartz MM, Lewis EJ. Pulmonary-renal syndrome in a newborn with placental transmission of ANCAs. Am J Kidney Dis. 2005;45:758–761. doi: 10.1053/j.ajkd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Finkielman JD, Merkel PA, Schroeder D, Hoffman GS, Spiera R, St Clair EW, Davis JC, Jr, McCune WJ, Lears AK, Ytterberg SR, Hummel AM, Viss MA, Peikert T, Stone JH, Specks U, WGET Research Group Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med. 2007;147:611–619. doi: 10.7326/0003-4819-147-9-200711060-00005. [DOI] [PubMed] [Google Scholar]
- 52.Jayne D. Review article: Progress of treatment in ANCA-associated vasculitis. Nephrology. 2009;14:42–48. doi: 10.1111/j.1440-1797.2009.01101.x. [DOI] [PubMed] [Google Scholar]
- 53.Brogan PA, Dillon MJ. The use of immunosuppressive and cytotoxic drugs in non-malignant disease. Arch Dis Child. 2000;83:259–264. doi: 10.1136/adc.83.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright E, Dillon MJ, Tullus K. Childhood vasculitis and plasma exchange. Eur J Pediatr. 2007;166:145–151. doi: 10.1007/s00431-006-0212-2. [DOI] [PubMed] [Google Scholar]
- 55.Groot K, Adu D, Savage CO. The value of pulse cyclophosphamide in ANCA-associated vasculitis: meta-analysis and critical review. Nephrol Dial Transplant. 2001;16:2018–2027. doi: 10.1093/ndt/16.10.2018. [DOI] [PubMed] [Google Scholar]
- 56.Dillon MJ. Vasculitis treatment-new therapeutic approaches. Eur J Pediatr. 2006;165:351–357. doi: 10.1007/s00431-005-0070-3. [DOI] [PubMed] [Google Scholar]
- 57.Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniene J, Ekstrand A, Gaskin G, Gregorini G, Groot K, Gross W, Hagen EC, Mirapeix E, Pettersson E, Siegert C, Sinico A, Tesar V, Westman K, Pusey C, European Vasculitis Study Group A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 58.Stassen PM, Derks RP, Kallenberg CG, Stegeman CA. Venous thromboembolism in ANCA-associated vasculitis-incidence and risk factors. Rheumatology (Oxford) 2008;47:530–534. doi: 10.1093/rheumatology/ken035. [DOI] [PubMed] [Google Scholar]
- 59.Groot K, Rasmussen N, Bacon PA, Tervaert JW, Feighery C, Gregorini G, Gross WL, Luqmani R, Jayne DR. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005;52:2461–2469. doi: 10.1002/art.21142. [DOI] [PubMed] [Google Scholar]
- 60.Stegeman CA, Tervaert JW, Jong PE, Kallenberg CG. Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. Dutch Co-Trimoxazole Wegener Study Group. N Eng J Med. 1996;335:16–20. doi: 10.1056/NEJM199607043350103. [DOI] [PubMed] [Google Scholar]
- 61.Beimler JH, Andrassy K. Cyclophosphamide treatment in systemic necrotizing vasculitis and lupus nephritis. How long? How much? Pediatr Nephrol. 2004;19:949–955. doi: 10.1007/s00467-004-1553-6. [DOI] [PubMed] [Google Scholar]
- 62.Joy MS, Hogan SL, Jennette JC, Falk RJ, Nachman PH. A pilot study using mycophenolate mofetil in relapsing or resistant ANCA small vessel vasculitis. Nephrol Dial Transplant. 2005;20:2725–2732. doi: 10.1093/ndt/gfi117. [DOI] [PubMed] [Google Scholar]
- 63.Smith KG, Jones RB, Burns SM, Jayne DR. Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: Remission, relapse, and re-treatment. Arthritis Rheum. 2006;54:2970–2982. doi: 10.1002/art.22046. [DOI] [PubMed] [Google Scholar]
- 64.Eleftheriou D, Melo M, Marks SD, Tullus K, Sills J, Cleary G, Dolezalova P, Ozen S, Pilkington C, Woo P, Klein N, Dillon MJ, Brogan PA. Biologic therapy in primary systemic vasculitis of the young. Rheumatology (Oxford) 2009;48:978–986. doi: 10.1093/rheumatology/kep148. [DOI] [PubMed] [Google Scholar]
- 65.Hoffman GS, Thomas-Golbanov CK, Chan J, Akst LM, Eliachar I. Treatment of subglottic stenosis, due to Wegener’s granulomatosis, with intralesional corticosteroids and dilation. J Rheumatol. 2003;30:1017–1021. [PubMed] [Google Scholar]
- 66.White JB, Shah RK. Wegener’s granulomatosis of the pediatric airway: a case demonstrating a conservative management approach. Am J Otolaryngol. 2009;30:212–215. doi: 10.1016/j.amjoto.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 67.Walton EW. Giant-cell granuloma of the respiratory tract (Wegener’s granulomatosis) Br Med J. 1958;2:265–270. doi: 10.1136/bmj.2.5091.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ozen S, Anton J, Arisoy N, Bakkaloglu A, Besbas N, Brogan P, Garcia-Consuegra J, Dolezalova P, Dressler F, Duzova A, Ferriani VP, Hilário MO, Ibáñez-Rubio M, Kasapcopur O, Kuis W, Lehman TJ, Nemcova D, Nielsen S, Oliveira SK, Schikler K, Sztajnbok F, Terreri MT, Zulian F, Woo P. Juvenile polyarteritis: results of a multicenter survey of 110 children. J Pediatr. 2004;145:517–522. doi: 10.1016/j.jpeds.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 69.Hattori M, Kurayama H, Koitabashi Y. Antineutrophil cytoplasmic autoantibody-associated glomerulonephritis in children. J Am Soc Nephrol. 2001;12:1493–1500. doi: 10.1681/ASN.V1271493. [DOI] [PubMed] [Google Scholar]
- 70.Peco-Antic A, Bonaci-Nikolic B, Basta-Jovanovic G, Kostic M, Markovic-Lipkovski J, Nikolic M, Spasojevic B. Childhood microscopic polyangiitis associated with MPO-ANCA. Pediatr Nephrol. 2006;21:46–53. doi: 10.1007/s00467-005-2063-x. [DOI] [PubMed] [Google Scholar]