Abstract
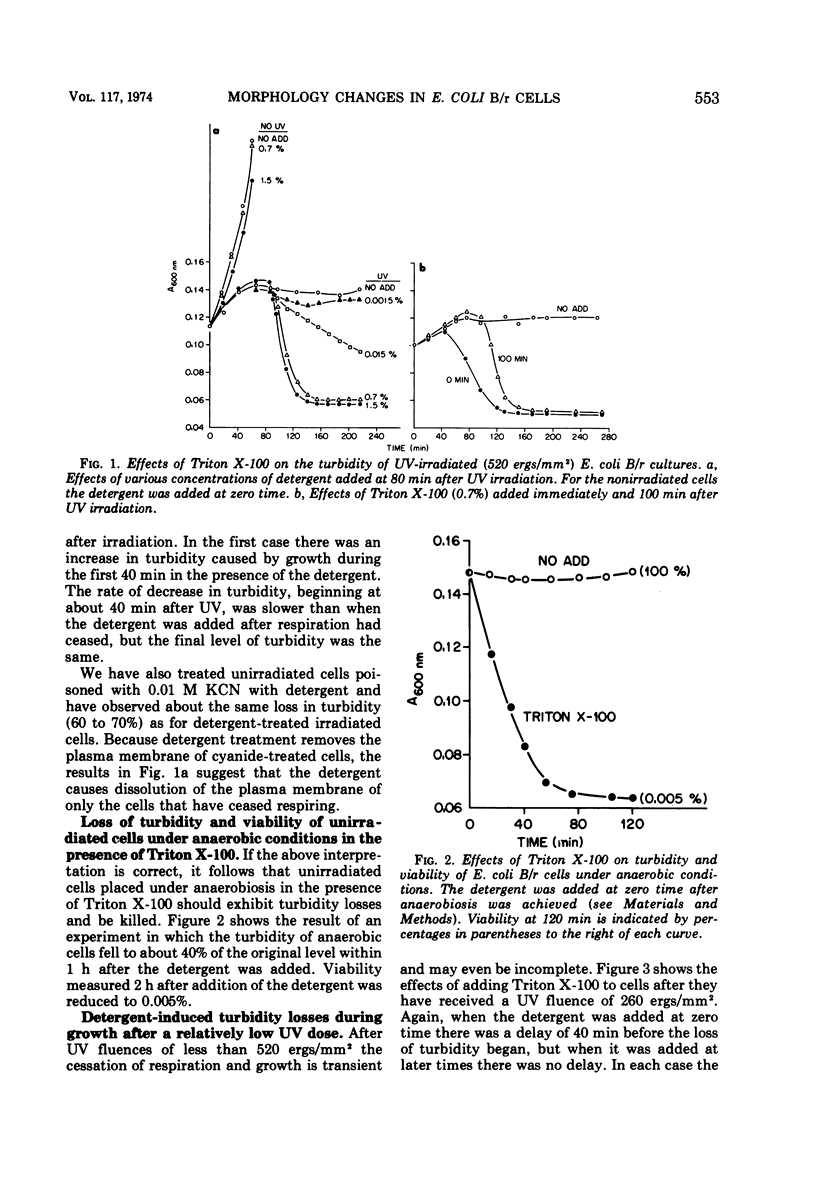
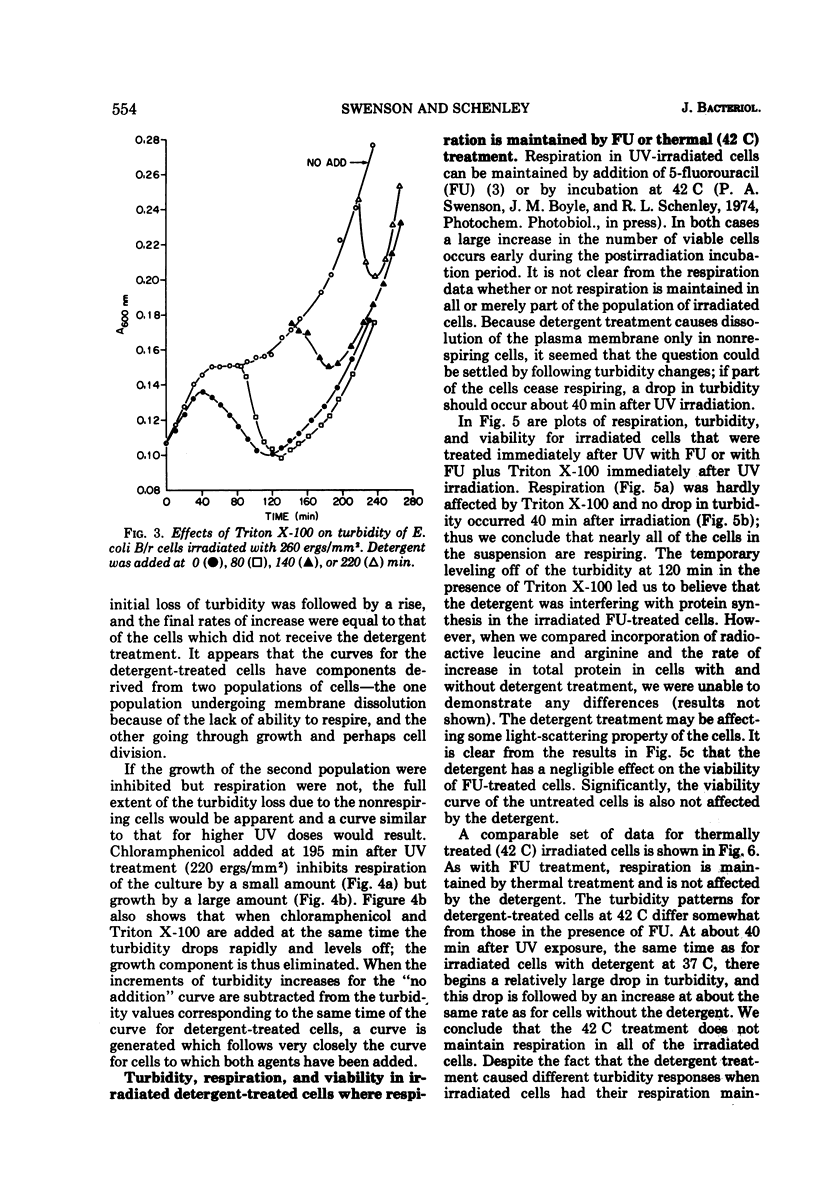
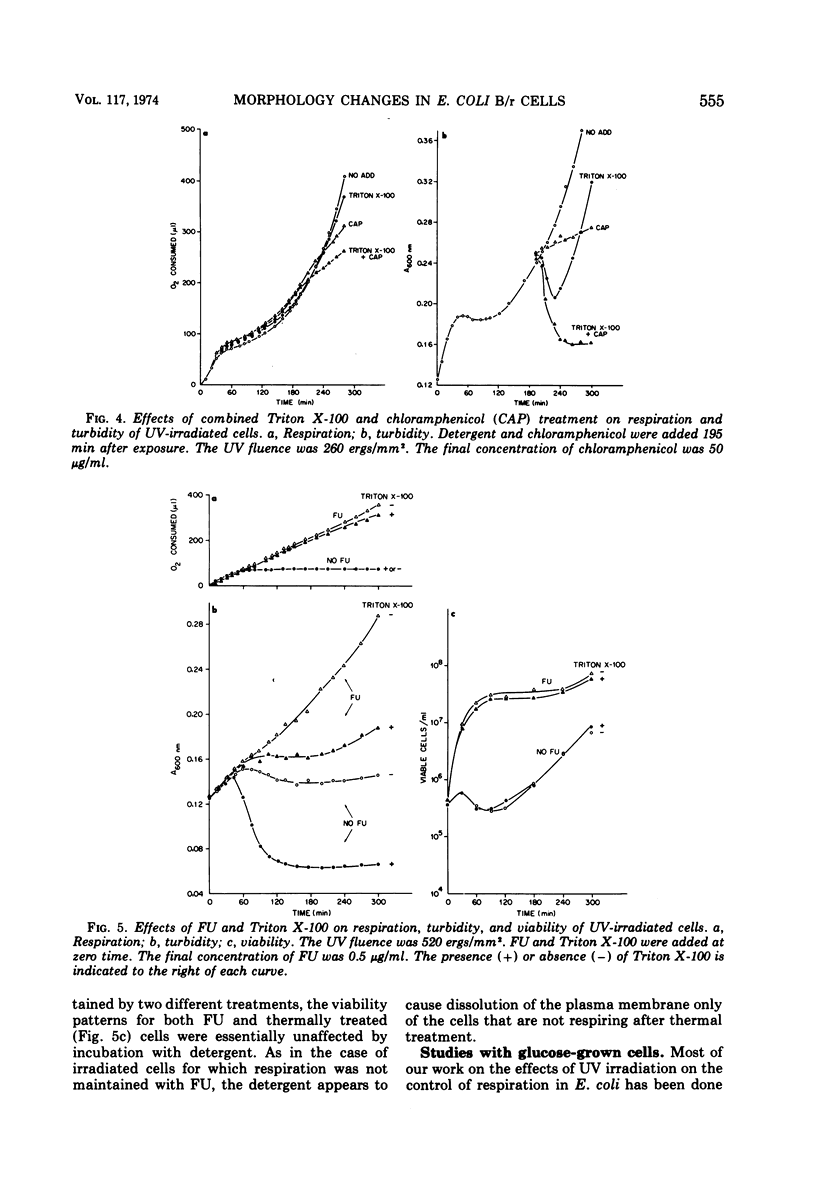
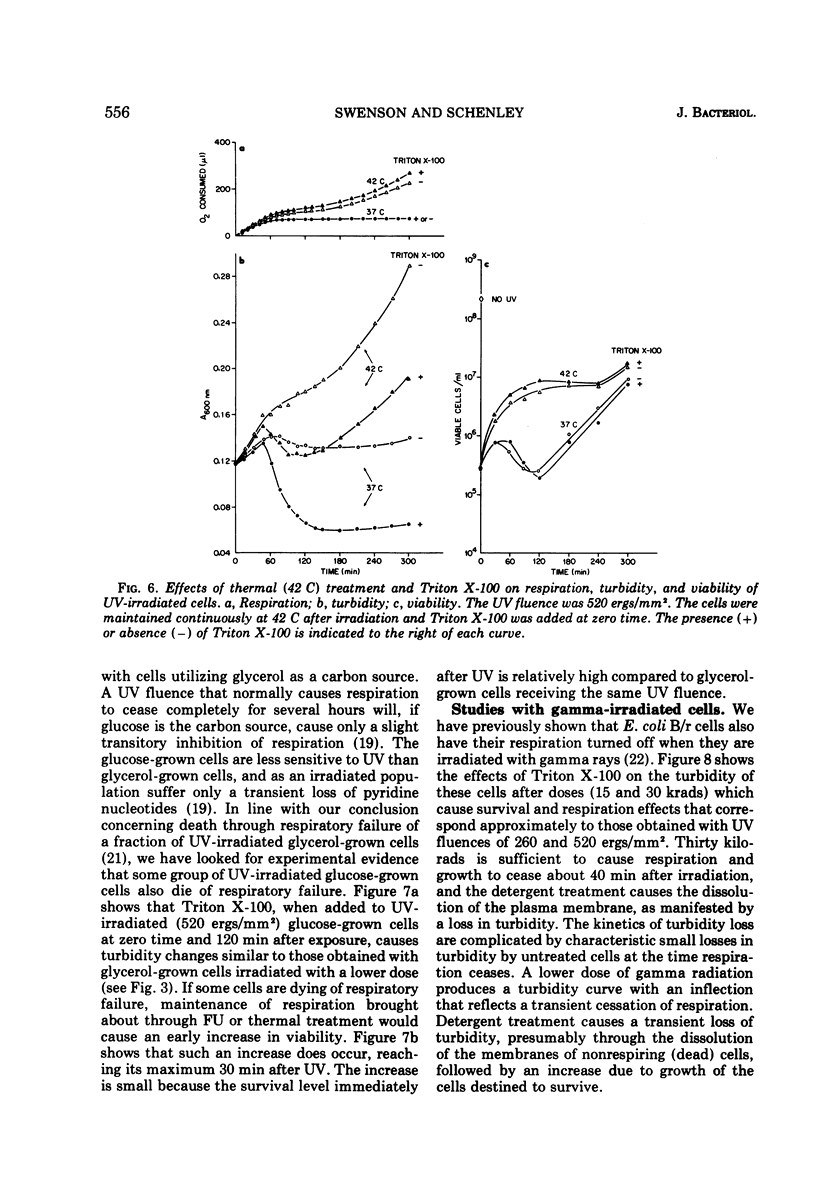
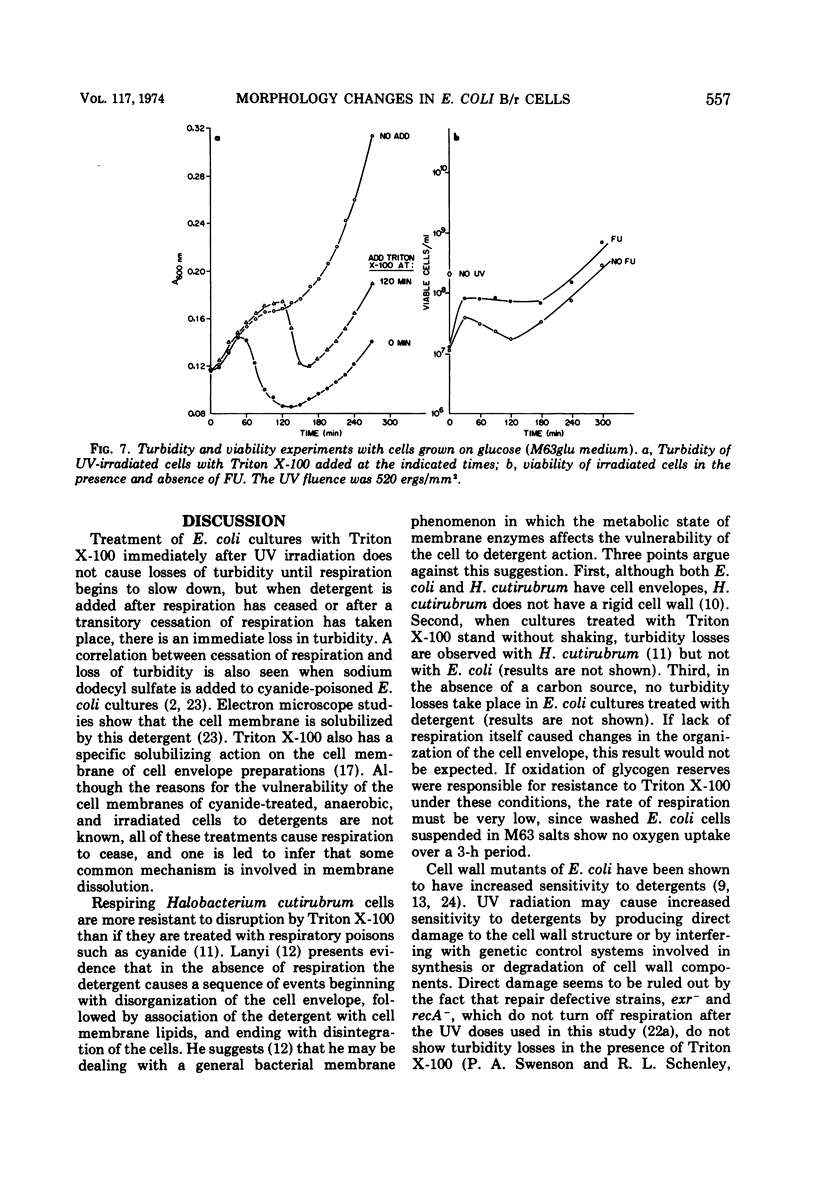
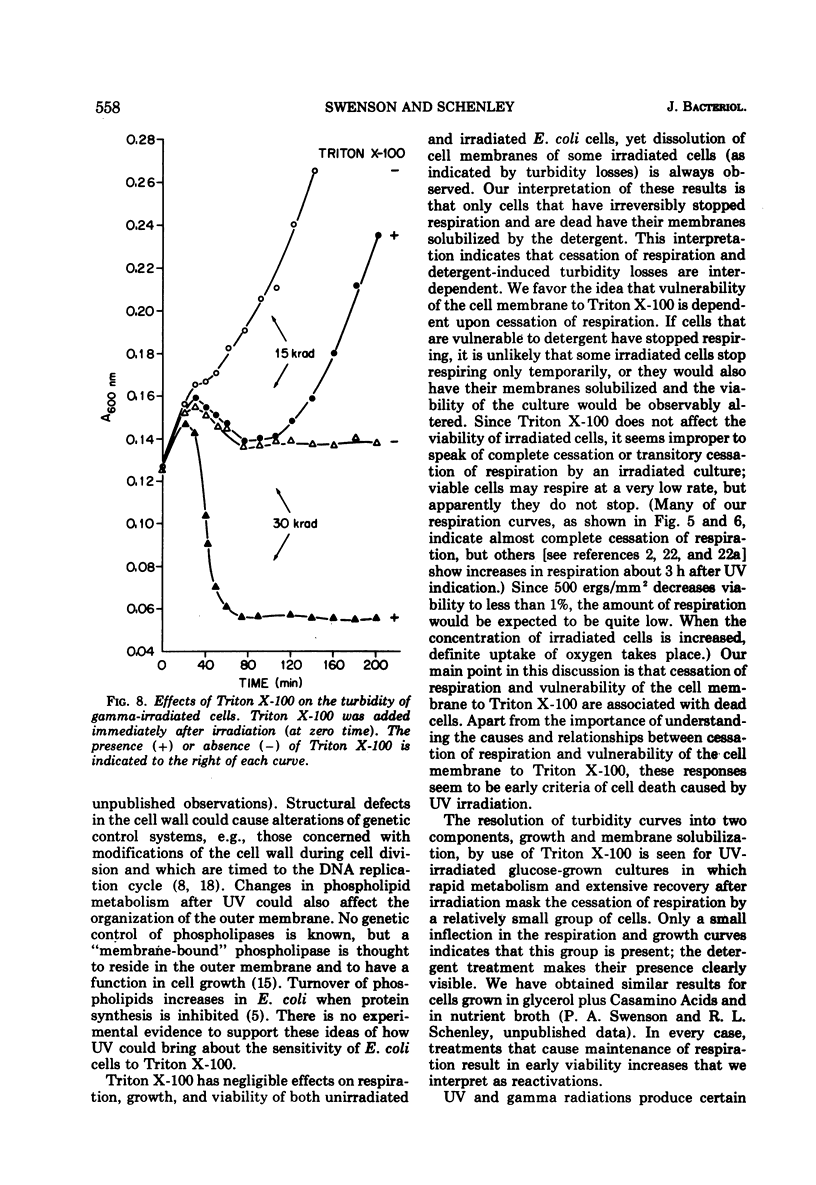
Ionic and nonionic detergents have little effect on respiring bacteria, but in cultures poisoned with KCN rapid solubilization of the cell membrane, as indicated by turbidity losses, takes place. Ultraviolet radiations cause Escherichia coli cells grown in minimal medium with glycerol as a carbon source to cease respiring and growing about 1 h after irradiation. We tested the effect of the nonionic detergent Triton X-100 on growth and cell membrane dissolution (both measured by turbidity changes), respiration, and viability of unirradiated and irradiated E. coli B/r cells. When the detergent was added to cells immediately after irradiation, a decrease in turbidity occurred only when respiration was about to cease; when it was added after cessation of respiration, the turbidity loss was immediate. In both cases the turbidity loss was about 60%, and disintegration of the cell walls did not take place. 5-Fluorouracil (FU) and thermal (42 C) treatments cause respiration of irradiated cells to be maintained and also cause viability increases. Irradiated cells treated with FU and detergent show no turbidity loss just prior to the time respiration normally ceases, but a loss does occur in irradiated cells incubated with detergent at 42 C. We conclude that FU maintains respiration for all of the cells, but that thermal treatment maintains respiration for only part of the cells. In all cases the detergent had only a negligible effect on the respiration and viability of unirradiated and irradiated cells. We conclude that Triton X-100 causes solubilization of cell membranes of only nonrespiring cells that are not destined to survive.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLE A., KELLENBERGER E. Etude de l'action du laurylsulfate de sodium sur E. coli. Schweiz Z Pathol Bakteriol. 1958;21(3):714–740. [PubMed] [Google Scholar]
- Birdsell D. C., Cota-Robles E. H. Lysis of spheroplasts of Escherichia coli by a non-ionic detergent. Biochem Biophys Res Commun. 1968 May 10;31(3):438–446. doi: 10.1016/0006-291x(68)90496-8. [DOI] [PubMed] [Google Scholar]
- Boyle J. M., Schenley R. L., Swenson P. A. Role of pyridine nucleotides in 5-fluorouracil-mediated reactivation of ultraviolet radiation damage. J Bacteriol. 1971 Jun;106(3):896–903. doi: 10.1128/jb.106.3.896-903.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. doi: 10.1111/j.1432-1033.1970.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Crowfoot P. D., Esfahani M., Wakil S. J. Relation between protein synthesis and phospholipid synthesis and turnover in Escherichia coli. J Bacteriol. 1972 Dec;112(3):1408–1415. doi: 10.1128/jb.112.3.1408-1415.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L. Dissociation and reassembly of Escherichia coli outer membrane and of lipopolysaccharide, and their reassembly onto flagellar basal bodies. J Bacteriol. 1971 Mar;105(3):1184–1199. doi: 10.1128/jb.105.3.1184-1199.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N., Sinsheimer R. L. Lysis of Escherichia coli with a neutral detergent. Biochim Biophys Acta. 1967 Dec 19;149(2):476–488. doi: 10.1016/0005-2787(67)90175-x. [DOI] [PubMed] [Google Scholar]
- Hoffmann B., Messer W., Schwarz U. Regulation of polar cap formation in the life cycle of Escherichia coli. J Supramol Struct. 1972;1(1):29–37. doi: 10.1002/jss.400010105. [DOI] [PubMed] [Google Scholar]
- Holland I. B., Threlfall E. J., Holland E. M., Darby V., Samson A. C. Mutants of Escherichia coli with altered surface properties which are refractory to colicin E2, sensitive to ultraviolet light and which can also show recombination deficiency, abortive growth of bacteriophage lambda and filament formation. J Gen Microbiol. 1970 Aug;62(3):371–382. doi: 10.1099/00221287-62-3-371. [DOI] [PubMed] [Google Scholar]
- KUSHNER D. J., BAYLEY S. T., BORING J., KATES M., GIBBONS N. E. MORPHOLOGICAL AND CHEMICAL PROPERTIES OF CELL ENVELOPES OF THE EXTREME HALOPHILE, HALOBACTERIUM CUTIRUBRUM. Can J Microbiol. 1964 Jun;10:483–497. doi: 10.1139/m64-058. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Influence of electron transport on the interaction between membrane lipids and Triton X-100 in Halobacterium cutirubrum. Biochemistry. 1973 Mar 27;12(7):1433–1438. doi: 10.1021/bi00731a025. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Studies of the electron transport chain of extremely halophilic bacteria. 8. Respiration-dependent detergent dissolution of cell envelopes. Biochim Biophys Acta. 1972 Sep 1;282(1):439–446. doi: 10.1016/0005-2736(72)90351-3. [DOI] [PubMed] [Google Scholar]
- Lazdunski C., Shaprio B. M. Relationship between permeability, cell division, and murein metabolism in a mutant of Escherichia coli. J Bacteriol. 1972 Aug;111(2):499–509. doi: 10.1128/jb.111.2.499-509.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative bacteria by lysozyme. Biochim Biophys Acta. 1956 Oct;22(1):189–191. doi: 10.1016/0006-3002(56)90240-2. [DOI] [PubMed] [Google Scholar]
- Scandella C. J., Kornberg A. A membrane-bound phospholipase A1 purified from Escherichia coli. Biochemistry. 1971 Nov 23;10(24):4447–4456. doi: 10.1021/bi00800a015. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Swenson P. A., Schenley R. L., Boyle J. M. Interference with respiratory control by ionizing radiations in Escherichia coli B-r. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;20(3):213–223. doi: 10.1080/09553007114551111. [DOI] [PubMed] [Google Scholar]
- Swenson P. A., Schenley R. L. Death through respiratory failure of a fraction of ultraviolet-irradiated Escherichia coli b-r cells. J Bacteriol. 1972 Sep;111(3):658–663. doi: 10.1128/jb.111.3.658-663.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson P. A., Schenley R. L. Evidence for the control of respiration by DNA in ultraviolet-irradiated Escherichia coli B-r cells. Mutat Res. 1970 May;9(5):443–453. doi: 10.1016/0027-5107(70)90028-x. [DOI] [PubMed] [Google Scholar]
- Swenson P. A., Schenley R. L. Respiration, growth and viability of repair-deficient mutants of Escherichia coli after ultraviolet irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1974 Jan;25(1):51–60. doi: 10.1080/09553007414550051. [DOI] [PubMed] [Google Scholar]
- Swenson P. A., Schenley R. L. Role of Pyridine Nucleotides in the Control of Respiration in Ultraviolet-Irradiated Escherichia coli B/r Cells. J Bacteriol. 1970 Dec;104(3):1230–1235. doi: 10.1128/jb.104.3.1230-1235.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh C. L., van Iterson W. Effects of treatment with sodium dodecyl sulfate on the ultrastructure of Escherichia coli. J Bacteriol. 1972 Sep;111(3):801–813. doi: 10.1128/jb.111.3.801-813.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C. Isolation and characterization of an Escherichia coli mutant with alteration in the outer membrane porteins of the cell envelope. Biochim Biophys Acta. 1972 Dec 1;290(1):274–289. doi: 10.1016/0005-2736(72)90070-3. [DOI] [PubMed] [Google Scholar]



