Abstract
Purpose
The European Organization for Research and Treatment of Cancer (EORTC) scoring system and risk table were introduced in the 2008 European Association of Urology guidelines on TaT1 bladder cancer. We compared the recurrence and progression rate between EORTC risk tables and author's patients who underwent transurethral resection of bladder cancer (TURB) following intravesical Bacillus Calmette-Guerin (BCG) instillation.
Materials and Methods
The medical records of 251 patients who underwent TURB and were diagnosed with non-muscle-invasive bladder cancer from l993 to 2007 were analyzed. The patients were divided into 2 groups: the recurrence group and the progression group. According to the EORTC scoring system, the patients in each group were categorized in terms of number of tumors, tumor size, prior recurrence rate, T category, carcinoma in situ, and pathologic grade and the scores were summed. According to the summed scores, the recurrence group and the progression group were divided into 3 subgroups: low, intermediate, and high risk, respectively. The recurrence rate and progression rate of each group were compared with the EORTC risk tables.
Results
The recurrence rate and progression rate were almost similar to the EORTC risk tables. However, the recurrence rate was low in the intermediate-risk group.
Conclusions
Clinical utilization of the EORTC scoring system and risk tables is very effective in predicting the recurrence and progression of non-muscle-invasive bladder cancer and in selecting treatment.
Keywords: Urinary bladder neoplasms, Recurrence, BCG vaccine
INTRODUCTION
Transitional epithelial cell tumors of the bladder are the malignant tumors with the highest prevalence in the urinary system in our country, and around 70% of them are diagnosed as non-muscle-invasive bladder cancer [1,2]. Non-muscle-invasive bladder cancer recurs in 48% to 70% of patients after transurethral bladder tumor resections, and 10% to 48% of these patients progress into infiltrative cancer [2-4]. To prevent this, diverse drugs including Bacillus Calmette-Guerin (BCG) are injected into the bladder after transurethral resections, and many studies have been conducted to develop scales to predict the recurrence and progression of non-muscle-invasive bladder cancer [5-10].
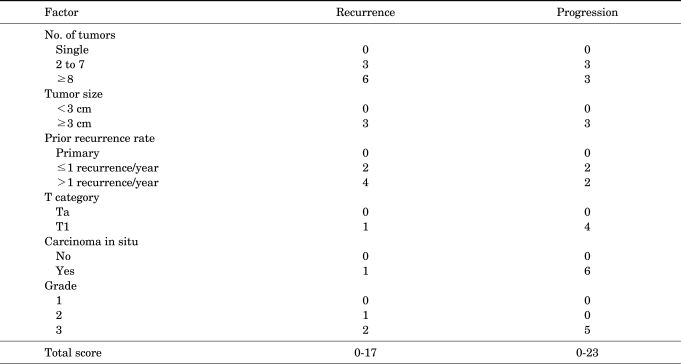
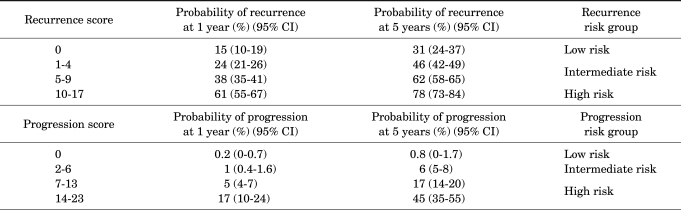
The European Organization for Research and Treatment of Cancer (EORTC) scoring system (Appendix 1) was introduced in 2008 in the European Association of Urology (EAU) guidelines on TaT1 bladder cancer. The system is a table that scores six clinical and pathological factors that are known to be prognostic factors in superficial bladder cancer, including the number of tumors, tumor size, prior recurrence rate, T category, presence of carcinoma in situ (CIS), and grade. The EORTC risk tables (Appendix 2) are tables that divide patients into four groups by score and three groups by risk (low risk, intermediate risk, and high risk) with the sums of the six factors to enable easy prediction of the recurrence and progression of non-muscle-invasive bladder cancer. These were developed to aid in the selection of treatment after performing transurethral bladder tumor resections [2].
We attempted to compare the results of treatment of those patients who were diagnosed as having non-muscle-invasive bladder cancer in our hospital and received BCG therapy with the recurrence and progression rates presented in the EORTC risk tables in order to examine differences.
MATERIALS AND METHODS
The medical records of 251 patients who were followed up for at least 1 year among those patients who had been diagnosed as having non-muscle-invasive bladder cancer after undergoing transurethral bladder tumor resections anytime between 1993 and 2007 and who thus had received BCG therapy were retrospectively analyzed. The BCG used by the authors was Oncotice®, which contained Tice BCG 2-8×108 CFU/ml. Intravesical BCG instillation was conducted once per week for the first 6 weeks after bladder transitional cell cancers were identified in pathological tissue examinations after transurethral resections and then once a month for 3 months.
The number of tumors, tumor size, the prior recurrence rate, T category, the presence of CIS, and grade were recorded for each patient according to the EORTC scoring system (Appendix 1), and the recurrence and progression of bladder cancers were examined. The patients were divided into low, intermediate, and high risk groups according to the EORTC risk tables (Appendix 2) on the basis of the summed scores. The recurrence rate and the progression rate of each group were compared with the values presented in the EORTC risk tables. In addition, the side effects of BCG therapy were examined.
RESULTS
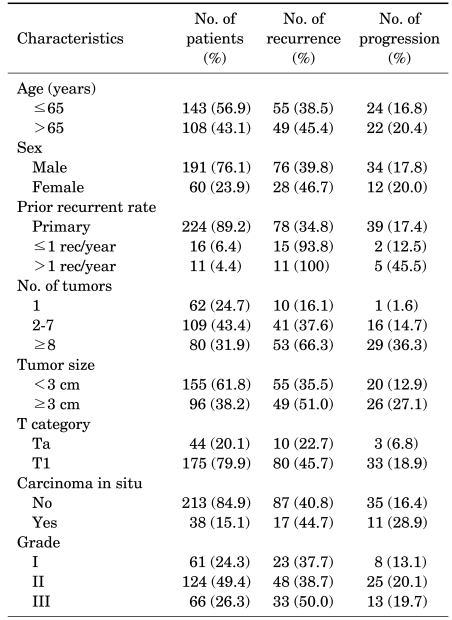
The average follow-up period was 68.9 months (range, 12-204 months). The distributions of the number of tumors, tumor size, the prior recurrence rate, T category, the presence of CIS, and grade and the recurrence and progression rates by factor are shown in Table 1.
TABLE 1.
Patient characteristics and recurrence and progression rates
Of the total 251 patients, disease recurred in 104 patients (41.4%) and progressed in 46 patients (18.3%). The average recurrence period was 13.6 months (range, 6-70 months) and the average progression period was 57.2 months (range, 12-188 months).
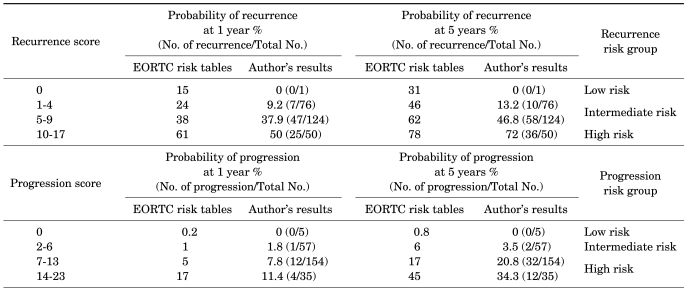
The recurrence rates and progression rates by risk group were compared with the EORTC risk tables (Table 2). There was no case of recurrence or progression in the low risk group with a score of 0. All of the 1-year and 5-year recurrence rates of the patients were lower than the values presented in the EORTC risk tables except for the 1-year recurrence rate of the intermediate risk group with recurrence scores of 5-9, which was 37.9% and thus almost the same as the value of 38% in the EORTC risk table. With regard to progression rates, 1-year progression rates in the intermediate risk group with scores of 2-6 and in the high risk group with scores of 7-13 were higher than those in the EORTC risk tables (1% vs. 1.8% and 5% vs. 7.8%, respectively), whereas the rate in the high risk group with scores of 14-23 in the EORTC risk table was 17% and that in our patients was 11.4%. Thus, the rate was lower in our patients. As for the 5-year progression rates, the rate in the high risk group with scores of 7-13 was higher in our patients (20.8% vs. 17%), whereas in the intermediate risk group with scores of 2-6 and in the high risk group with scores of 14-23, the rates were lower in our patients (3.5% vs. 6% and 34.4% vs. 45%, respectively).
TABLE 2.
Comparison of recurrence and progression rates according to total scores of both groups
EORTC: European organization for research and treatment of cancer
Side effects of BCG therapy appeared in 32 patients (13%). The symptoms included bladder-irritating symptoms such as urinary frequency, dysuria, burning sensation, and nocturia and symptoms of lower abdominal pain, temporary hematuria, fatigue, chill, and fever. No serious complications such as systemic sepsis developed in any case.
DISCUSSION
Non-muscle-invasive bladder cancer shows quite diverse natural histories and prognoses. It sometimes shows repeated superficial recurrences, but in some cases, it progresses into infiltrative or metastatic cancer despite diverse treatments. Many studies have been conducted to predict the recurrence and progression of non-muscle-invasive bladder cancer. The involved factors known so far include clinicopathologic factors, such as the size of the tumor, the degree of infiltration of the tumor, and the degree of differentiation of the tumor; DNA evaluation using chromosome markers and flow cytometry; and molecular biological markers such as tumor surface antigens (ABO, T, Lewis Ag), tumor-related antigens including Ɓ-2 microglobulin, and tumor genes including H-ras and p53 cell cycle regulatory protein [11-15]. However, authors report the prognostic importance of these multiple factors differently [5-15], and no certain method to predict prognosis has yet been found.
The EORTC scoring system and the risk tables of the 2008 EAU guidelines were made to aid future selections of treatments by providing a method to simply predict the possibility of recurrence and progression of non-muscle-invasive bladder cancer by using factors that can be easily applied clinically. The EORTC scoring system gives scores to factors such as the number of tumors, tumor size, the prior recurrence rate, T category, the presence of CIS, and grade and sums the scores. In the EORTC scoring system, different scores are used to predict recurrence rates and to predict progression rates. Factors that more severely affected recurrence in the EORTC trials were the number of tumors, the prior recurrence rate, and the degree of differentiation of tumor cells (WHO grade II), and thus higher scores were given to these factors. Factors that more severely affected progression were T category, existence of CIS, and the degree of differentiation of tumor cells (WHO grade III), and these factors were given relatively higher scores for predicting progression compared with other factors. The size of the tumors was shown to affect both recurrence and progression and thus the same score was given [2].
When we compared the recurrence rates in the EORTC risk tables with the results in our patients, the 1-year recurrence rate (9.2% vs. 24%) and the 5-year recurrence rate (13.2% vs. 46%) in the intermediate risk group with a recurrence score of 1 to 4 were lower in our patients. In addition, in the intermediate risk group with a recurrence score of 5 to 9, the 5-year recurrence rate was lower in our patients (46.8% vs. 62%). This result is suggested to be because of differences in subject groups. With regard to the population of the EORTC trial, BCG therapy was carried out in only 5.9% of the population and intravesical chemotherapy (thiotepa, epirubicin, mitomycin C, or pyridoxine) was carried out in the remaining patients. On the other hand, the subjects in our study received only BCG therapy, and this is considered to be the reason for the low recurrence rates and progression rates.
In general, treatment methods have been established for patients with low risks such as a single tumor, category Ta, and WHO grade I or patients with high risks such as CIS and WHO grade III; however, there is no clearly defined treatment method for patients with intermediate risks. In the past, intravesical chemotherapy was mainly used, but recently, BCG therapy has been preferred and many reports indicate that BCG therapy is more effective in preventing recurrences than is intravesical chemotherapy [16-18]. In a multicenter study conducted with 2,820 non-muscle-invasive bladder cancer patients, Malmström et al reported that, of 1,983 patients with intermediate risks, 49% were in a mitomycin-C therapy group and 51% were in a BCG therapy group, and the risk of recurrence decreased by 32% in the patients who received BCG maintenance therapy [19]. In a study by Böhle et al, of 2,749 patients, 1,421 patients received BCG therapy and 1,328 patients received mitomycin-C therapy, and the rate of recurrence in the BCG therapy group was 38.6%, which was lower than that in the mitomycin-C therapy group, which was 46.4% [20]. Also, in an analysis that classified the patients by risk groups, BCG was shown to be more effective than mitomycin-C in preventing recurrences in patients with intermediate or high risks; thus, it was emphasized that BCG maintenance therapy was an independent factor in preventing recurrences [20]. Cheng et al compared the 10-year recurrence-free survival rate between 102 non-muscle-invasive bladder cancer patients in a BCG therapy group and 107 patients in a epirubicin therapy group and reported a lower recurrence-free survival rate in the BCG therapy group than in the epirubicin therapy group, with a rate of 61% in the BCG therapy group and 32% in the epirubicin therapy group [21].
In the comparison of progression rates, there was no definite difference between the values presented in the EORTC risk tables and our results. In previous studies that compared BCG therapy and intravesical chemotherapy, many results indicating that BCG therapy and intravesical chemotherapy do not differ in preventing the progression of bladder cancer have been reported. Through a multicenter study, Shelley et al compared the non-muscle-invasive bladder cancer progression rate of a BCG therapy group with that of a mitomycin-C therapy group and reported that there was no difference between the two groups with a log risk rate of 0.044 (p=0.16) [22]. Also, Lundholm et al reported that non-muscle-invasive bladder cancer progressed in 12.2% of a BCG therapy group and 13% of a mitomycin-C therapy group and that there was thus no difference between BCG therapy and mitomycin-C therapy [23].
Although it is known that BCG therapy is helpful in preventing recurrences, doctors hesitate to use BCG therapy due to complications. The symptoms include urinary frequency, burning sensation, light muscle pain, and low fever; the most dangerous complications are systemic sepsis and hypersensitivity reactions, which are characterized by chill, fever, and progressive multiorgan damage. However, many studies suggest that the side effects of BCG therapy are minor. Lamm et al reported that, of 2,602 patients who received BCG therapy, less than 5% experienced side effects, and the symptoms were minor in most cases. The incidence of hypersensitivity reactions, which is a dangerous complication, has been reduced greatly from 0.4% in the early days to around 0.007% currently [24,25]. In this study, treatments were stopped due to complications in 5 patients out of 251 (2%), but there was no case in which serious complications like systemic sepsis developed. In general, compliance with BCG therapy was quite high and complications were shown to be minor. Thus, there was no problem in proceeding with the BCG maintenance therapy.
There are several limitations to simply comparing our results with the values in the EORTC risk tables of the EAU guidelines. First, the patient groups were treated mostly by intravesical chemotherapy in the EAU guidelines, whereas our patient groups consisted only of patients treated with BCG therapy. Because of this difference, patients were evenly distributed in the low, intermediate, and high risk groups in the EAU guidelines, whereas the number of patients in the low risk group was relatively small in our study. Second, it is difficult to concretely verify statistics because our study data were compared with data from the EORTC study, which was a multicenter study. Due to differences in subject patient groups, treatment methods, and the fact that the statistical support is not sufficient, it is not quite reasonable to directly compare the results of our study with the results of the EAU guidelines. However, it is noteworthy that our study compared and verified the EORTC scoring system and risk tables presented by the 2008 EAU guidelines on TaT1 Bladder Cancer for the first time in Korea. Also, because our patient groups were treated by BCG only, it is possible to compare the effect of BCG therapy with the effects of other treatment methods. The results of this study will therefore provide meaningful data for supporting the superiority of BCG therapy in typical intermediate risk groups as shown in previous studies.
CONCLUSIONS
The recurrence and progression of non-muscle-invasive bladder cancer in patients in our hospital were compared with those in the EORTC risk tables using the EORTC scoring system presented by the 2008 EAU guidelines on TaT1 Bladder Cancer. The results suggest that clinical utilization of the EORTC scoring system will be useful in predicting the recurrence and progression of non-muscle-invasive bladder cancer and in selecting treatments.
APPENDIX 1
EORTC scoring system: weights used to calculate the recurrence and progression scores
EORTC: European organization for research and treatment of cancer
APPENDIX 2
EORTC risk tables: probability of recurrence and progression according to total score
EORTC: European organization for research and treatment of cancer
Footnotes
This study was supported by a grant from the 2008 Keimyung University Graduate School Research Fund.
The authors have nothing to disclose.
References
- 1.Kim WJ, Chung JI, Hong JH, Kim CS, Jung SI, Yoon DK. Epidemiological study for urologic cancer in Korea (1998-2002) Korean J Urol. 2004;45:1081–1088. [Google Scholar]
- 2.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–477. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 3.Oosterlinck W, Lobel B, Jakse G, Malmström PU, Stöckle M, Sternberg C. Guidelines on bladder cancer. Eur Urol. 2002;41:105–112. doi: 10.1016/s0302-2838(01)00026-4. [DOI] [PubMed] [Google Scholar]
- 4.Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Algaba F, Vicente-Rodriguez J. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol. 2000;164:680–684. doi: 10.1016/s0022-5347(05)67280-1. [DOI] [PubMed] [Google Scholar]
- 5.Kurth KH, Denis L, Bouffioux C, Sylvester R, Debruyne FM, Pavone-Macaluso M, et al. Factors affecting recurrence and progression in superficial bladder tumours. Eur J Cancer. 1995;31A:1840–1846. doi: 10.1016/0959-8049(95)00287-s. [DOI] [PubMed] [Google Scholar]
- 6.Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Vicente-Rodriguez J. Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J Urol. 2000;163:73–78. doi: 10.1016/s0022-5347(05)67975-x. [DOI] [PubMed] [Google Scholar]
- 7.Solsona E, Iborra I, Dumont R, Rubio-Briones J, Casanova J, Almenar S. The 3-month clinical response to intravesical therapy as a predictive factor for progression in patients with high risk superficial bladder cancer. J Urol. 2000;164:685–689. doi: 10.1097/00005392-200009010-00016. [DOI] [PubMed] [Google Scholar]
- 8.Kaasinen E, Rintala E, Hellström P, Viitanen J, Juusela H, Rajala P, et al. Factors explaining recurrence in patients undergoing chemoimmunotherapy regimens for frequently recurring superficial bladder carcinoma. Eur Urol. 2002;42:167–174. doi: 10.1016/s0302-2838(02)00260-9. [DOI] [PubMed] [Google Scholar]
- 9.Kwak C, Ku JH, Park JY, Lee E, Lee SE, Lee C. Initial tumor stage and grade as a predictive factor for recurrence in patients with stage T1 grade 3 bladder cancer. J Urol. 2004;171:149–152. doi: 10.1097/01.ju.0000099825.98542.a8. [DOI] [PubMed] [Google Scholar]
- 10.Choi CK, Kim JS, Rim JS. Application of scoring system reflecting various prognostic factors to the prediction of recurrence in superficial bladder carcinoma. Korean J Urol. 1999;40:878–885. [Google Scholar]
- 11.Kwak DY, Ha JY, Chang HS, Choi MS, Park CH, Kim CI. Clinical implications of the expression of survivin and p53 in superficial transitional cell carcinoma of the bladder. Korean J Urol. 2009;50:12–17. [Google Scholar]
- 12.Maeng YH, Kang HW, Huh JS. The expression and clinical significance of the minichromosome maintenance (MCM) 7 proliferation markers in urothelial carcinomas of the bladder. Korean J Urol. 2008;49:12–17. [Google Scholar]
- 13.Choi YD, Park JA, Cho NH, Yang WJ, Cho KS, Lee HY, et al. Expression of p53 and p73 genes in human transitional cell carcinoma of the bladder. Korean J Urol. 2004;45:1209–1214. [Google Scholar]
- 14.Ha HK, Lee SD, Chung MK. The relationship between expression of hypoxia inducible factor-1α or vascular endothelial growth factor and histopathological characteristics in human transitional bladder cancer. Korean J Urol. 2004;45:7–13. [Google Scholar]
- 15.Hinz S, Kempkensteffen C, Christoph F, Krause H, Schrader M, Schostak M, et al. Expression parameters of the polycomb group proteins BMI1, SUZ12, RING1 and CBX7 in urothelial carcinoma of the bladder and their prognostic relevance. Tumour Biol. 2008;29:323–329. doi: 10.1159/000170879. [DOI] [PubMed] [Google Scholar]
- 16.Böhle A, Bock PR. Intravesical bacille Calmette-Guérin versus mitomycin C in superficial bladder cancer: formal meta-analysis of comparative studies on tumor progression. Urology. 2004;63:682–686. doi: 10.1016/j.urology.2003.11.049. [DOI] [PubMed] [Google Scholar]
- 17.Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–1970. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 18.Dalbagni G. Is intravesical bacillus Calmette-Guérin better than mitomycin for intermediate-risk bladder cancer? Eur Urol. 2009;56:257–258. doi: 10.1016/j.eururo.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 19.Malmström PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56:247–256. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 20.Böhle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169:90–95. doi: 10.1016/S0022-5347(05)64043-8. [DOI] [PubMed] [Google Scholar]
- 21.Cheng CW, Chan SF, Chan LW, Chan CK, Ng CF, Cheung HY, et al. Twelve-year follow up of a randomized prospective trial comparing bacillus Calmette-Guerin and epirubicin as adjuvant therapy in superficial bladder cancer. Int J Urol. 2005;12:449–455. doi: 10.1111/j.1442-2042.2005.01064.x. [DOI] [PubMed] [Google Scholar]
- 22.Shelley MD, Wilt TJ, Court J, Coles B, Kynaston H, Mason MD. Intravesical bacillus Calmette-Guérin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: a meta-analysis of randomized trials. BJU Int. 2004;93:485–490. doi: 10.1111/j.1464-410x.2003.04655.x. [DOI] [PubMed] [Google Scholar]
- 23.Lundholm C, Norlén BJ, Ekman P, Jahnson S, Lagerkvist M, Lindeborg T, et al. A randomized prospective study comparing long-term intravesical instillations of mitomycin C and bacillus Calmette-Guerin in patients with superficial bladder carcinoma. J Urol. 1996;156:372–376. doi: 10.1016/s0022-5347(01)65853-1. [DOI] [PubMed] [Google Scholar]
- 24.Lamm DL. Efficacy and safety of bacille Calmette-Guérin immunotherapy in superficial bladder cancer. Clin Infect Dis. 2000;31(Suppl 3):S86–S90. doi: 10.1086/314064. [DOI] [PubMed] [Google Scholar]
- 25.Lamm DL, van der Meijden PM, Morales A, Brosman SA, Catalona WJ, Herr HW, et al. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147:596–600. doi: 10.1016/s0022-5347(17)37316-0. [DOI] [PubMed] [Google Scholar]