Abstract
Purpose
To evaluate the accuracy of radiologic tumor size for making decisions regarding nephron-sparing surgery of localized renal cell carcinomas (RCCs), we compared tumor size measured by a preoperative radiologic modality with that measured in the pathologic specimen.
Materials and Methods
Between January 2003 and December 2007, a total of 186 patients with pT1 or pT2 RCC underwent radical or partial nephrectomy at our institute. We excluded 11 patients who had preoperative arterial embolization (n=9) or positive surgical margins (n=2), and a total of 175 patients were included in this study. Radiologic size was defined as the largest diameter on computed tomography (CT), and pathologic size was defined as the largest diameter of the surgical specimen of the tumor. We retrospectively analyzed the difference between radiologic and pathologic tumor size.
Results
The radiologic and pathologic tumor sizes did not significantly differ (4.98±2.82 cm vs. 4.55±2.70 cm, respectively, p=0.152). In the subgroup analysis, the size difference was statistically significant only for tumor sizes of less than 6 cm. The size difference was largest in tumors of 3 to 4 cm, for which mean the radiologic size was 0.63±1.19 cm larger than the mean pathologic size (p=0.002). Histologic type had no significant influence on the difference between radiologic and pathologic size.
Conclusions
The tumor size of RCCs in preoperative CT seems to correlate well with pathologic tumor size. However, CT imaging may overestimate the size of a tumor in the small mass group (less than 6 cm). These results should be considered when making decisions about nephron-sparing surgery.
Keywords: Renal cell carcinoma, Nephrectomy, Radiology
INTRODUCTION
Tumor size is known as an important prognostic factor in localized renal cell carcinoma (RCC), and staging and selection of an appropriate treatment depend on the size of the tumor [1]. Most research on the prognostic value of tumor size has been based on pathologic size rather than radiologic size [2,3]. On the other hand, treatment including nephron-sparing surgery (NSS) is decided upon according to radiologic size. Novick reported that, with the accumulation of longer-term data, a size criterion has gained gradual acceptance for elective NSS [4]. He also reported that radical nephrectomy and NSS are equally effective curative treatments for patients who present with a single, small (<4 cm), and clearly localized RCC (4-6 cm). In his opinion, although the long-term functional advantage of NSS when there is a normal opposite kidney remains to be shown definitively, the benefits of maximal nephron preservation may include a decreased risk of progression to chronic renal insufficiency and end-stage renal disease. Therefore, it is important to define the correlation and agreement between radiologic size and pathologic size, especially for patients who are candidates for NSS of a localized RCC. We studied the relationship between radiologic tumor size, as determined by preoperative computed tomography (CT), and pathologic tumor size from a renal surface series.
MATERIALS AND METHODS
The medical records of 186 patients treated by radical nephrectomy or partial nephrectomy for localized RCC from January 2003 to December 2007 were retrospectively reviewed. We excluded 11 patients who had preoperative arterial embolization (n=9) or positive surgical margins (n=2), and a total of 175 patients were included in this study.
All patients underwent an intravenous contrast-enhanced abdominal CT scan before surgery, and the results of that were interpreted by a single experienced abdominal imaging radiologist.
The CT was the 16-channel multidetector type and the slice thickness was 0.5 mm. The size of the tumor on CT was measured in four axes: superior to inferior, anterior to posterior, oblique, and left to right. The largest of these four measurements was defined as the radiologic tumor size. The pathologic tumor size was defined as the largest diameter of the tumor examined just after extraction of the specimen without formalin fixation. In patients with multiple unilateral tumors of the same histologic subtype, the largest tumor was included. The tumors were divided into size ranges by radiologic size. The mean values of radiologic sizes, pathologic sizes, and the difference were calculated. Student's t-test was used to compare the mean values. A 5% level of significance was used for all statistical testing, and all statistical tests were two-sided.
RESULTS
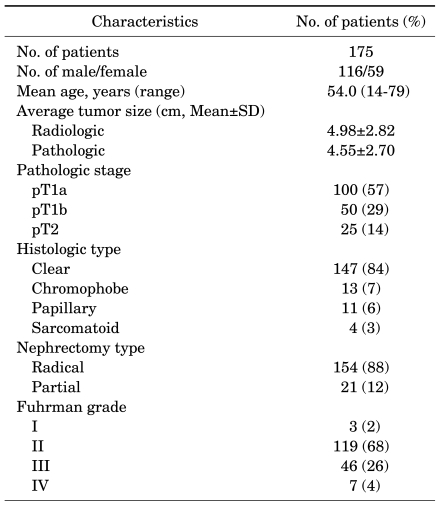
The mean patient age was 54.0 years (range, 14-79 years); of 175 patients, 116 were men and 59 were women. The characteristics of the 175 patients with localized RCCs are shown in Table 1.
TABLE 1.
Characteristics of the 175 patients with localized renal cell carcinomas
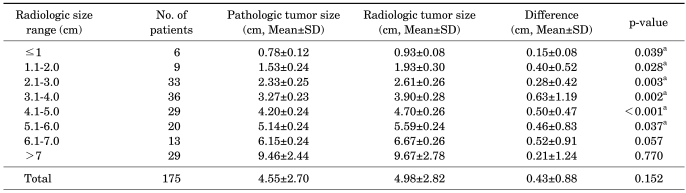
The mean radiologic and pathologic tumor sizes for all 175 patients did not differ significantly (4.98±2.82 cm vs. 4.55±2.70 cm, respectively, p=0.152). Table 2 lists the mean radiologic and pathologic sizes of the tumors, which were divided into 1 cm ranges by radiologic size. The mean radiologic tumor size was larger than the pathologic tumor size for all sizes. In particular, in tumors less than 6 cm, the difference between radiologic and pathologic tumor size was statistically significant (p<0.05). The largest difference was for tumors 3 to 4 cm in size, for which the mean radiologic size was 0.63±1.19 cm larger than the mean pathologic size (p=0.002). No significant difference was seen between the radiologic and pathologic tumor size for tumors larger than 6 cm (Table 2).
TABLE 2.
Differences in radiologic and pathologic tumor size according to radiologic size range
SD: standard deviation, a: statistically significant
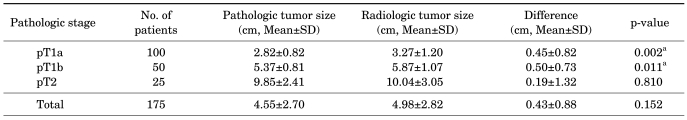
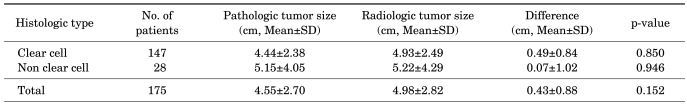
Table 3 shows the mean radiologic and pathologic sizes of tumors divided into pT1a, pT1b, and pT2 groups. In pT1a and pT1b tumors, the difference between mean radiologic and pathologic tumor size was statistically significant (0.45±0.82 cm and 0.50±0.73 cm, respectively; p=0.002, p=0.011). However, in pT2 tumors, no significant difference was seen between radiologic and pathologic tumor size (p=0.810) (Table 3). Table 4 lists the mean radiologic and pathologic sizes of tumors divided by histologic type into the clear cell type and non clear cell type. In both groups, the mean radiologic tumor size was larger than the pathologic tumor size (0.49±0.84 cm vs. 0.07±1.02 cm), but there was no significant difference between the two groups (p=0.850, p=0.946) (Table 4).
TABLE 3.
Differences in radiologic and pathologic tumor size according to pathologic stage
SD: standard deviation, a: statistically significant
TABLE 4.
Differences in radiologic and pathologic tumor size according to histologic type
SD: standard deviation
DISCUSSION
The increased usage of advanced imaging techniques such as ultrasonography or CT has led to an increase in incidental tumors, and the size of incidental tumors tends to be smaller [5,6]. Lightfoot et al reported that incidental RCC was only 17.5% in the period of 1970 to 1981, but 82.8% in the period of 1982 to 1993, when ultrasonography and CT were introduced in clinical practice [7]. Recently, it was reported that the rate of incidental tumors has increased about 40.1% to 46.4%, even in Korea [8-10]. With this increase in incidental localized RCC and decrease in tumor size, the treatment modality of RCC has changed. The frequency of NSS, such as partial nephrectomy or cryoablation, and radio frequency ablation, has increased as opposed to radical nephrectomy.
Previously, NSS had only been performed if radical nephrectomy was not indicated absolutely or relatively, such as with a bilateral RCC, solitary kidney, severe medical disease, or renal stone, and successful outcomes were reported in some cases [11,12]. Hafez et al recommended that tumor size be used as an indication for NSS. They reported that patients with renal tumors less than 4 cm have better outcomes than do those with tumors greater than 4 cm when NSS is performed [13]. However, Leibovich et al reported that there were no significant differences in recurrence or distant metastases between patients treated with NSS for RCCs less than 4 cm or RCCs of 4 to 7 cm that were exophytic or did not reach the collecting system [14]. Manikandan et al reported that NSS seems to be as effective as radical nephrectomy in patients with RCCs up to 4 cm [15]. Nam et al reported that there were no significant differences in complications, recurrence, or metastasis between patients treated with NSS for RCCs less than 4 cm or RCCs of 4 to 7 cm [16].
The radiologic size of renal tumors is an important factor in the decision for NSS; thus, several studies have examined the relationship between radiologic and pathologic tumor sizes, with varying results. Herr prospectively investigated 50 patients treated with partial nephrectomy and found that the radiologic tumor size was 0.63 cm larger than the pathologic size and attributed this difference to decreased tumor vascularity after renal artery clamping [17]. Schlomer et al reported that a significant difference was noted in tumors less than 5 cm, although the mean radiologic and pathologic tumor size for all 133 RCC patients was not significantly different [18]. They also found that the largest difference was for tumors in the range of 4 to 5 cm, which may affect decisions to perform NSS in certain patients. In our study, the mean radiologic tumor size was larger than the pathologic size for all 175 patients, but not significantly so. In tumors in the range of less than 6 cm, mean radiologic tumor size was significantly larger than mean pathologic size, and the difference was largest in tumors of 3 to 4 cm.
Kanofsky et al retrospectively reviewed 236 patients with RCC treated with radical or partial nephrectomy and found that radiologic tumor size was commonly overestimated; this was more frequently observed for clear cell type tumors than for other tumor types, such as papillary or chromophobe type [19]. Yaycioglu et al also reported similar results [20]. In our study, we found that mean radiologic tumor size was larger than mean pathologic tumor size for both clear cell and non-clear cell type tumors, but there was no significant difference between the two groups. This study had the disadvantage of a relatively small number of patients, because the research was conducted at a single center. However, as discussed in the Introduction, investigating the relationship between radiologic and pathologic tumor size has implications for determining with assurance when to perform NSS, especially for patients who are candidates for NSS of a localized RCC.
Mindful of the results of our study, we are confirming the plan for surgical management whether patients with T1 stage RCC in our cancer center undergo radical nephrectomy or NSS. Additional study should be taken into consideration when interpreting the relationship between radiologic and pathologic tumor size. We are making progress in a standardized prospective study analyzing radiologic and pathologic tumor characteristics along with prognosis, which will help to more definitively characterize the relationship between clinical and pathologic tumor size.
CONCLUSIONS
Preoperative CT imaging may overestimate tumor size in RCCs of less than 6 cm. This result may enable us to perform NSS with assurance in certain patients with localized RCC. A prospective study that includes a comparison of prognosis is needed to definitively characterize the proper use of clinical tumor size when making decisions regarding NSS.
Footnotes
The authors have nothing to disclose.
References
- 1.Guinan P, Sobin LH, Algaba F, Badellino F, Kameyama S, MacLennan G, et al. Union International Contre Ie Cancer (UICC) and the American Joint Committee on Cancer (AJCC) TNM staging of renal cell carcinoma: Workgroup No. 3. Cancer. 1997;80:992–993. doi: 10.1002/(sici)1097-0142(19970901)80:5<992::aid-cncr26>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 2.Belldegrun A, Tsui KH, deKernion JB, Smith RB. Efficacy of nephron-sparing surgery for renal cell carcinoma: analysis based on the new 1997 tumor-node-metastasis staging system. J Clin Oncol. 1999;17:2868–2875. doi: 10.1200/JCO.1999.17.9.2868. [DOI] [PubMed] [Google Scholar]
- 3.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000;163:442–445. [PubMed] [Google Scholar]
- 4.Novick AC. Laparoscopic and partial nephrectomy. Clin Cancer Res. 2004;10:6322S–6327S. doi: 10.1158/1078-0432.CCR-050003. [DOI] [PubMed] [Google Scholar]
- 5.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51:203–205. doi: 10.1016/s0090-4295(97)00506-2. [DOI] [PubMed] [Google Scholar]
- 6.Smith SJ, Bosniak MA, Megibow AJ, Hulnick DH, Horii SC, Raghavendra BN. Renal cell carcinoma: earlier discovery and increased detection. Radiology. 1989;170:699–703. doi: 10.1148/radiology.170.3.2644658. [DOI] [PubMed] [Google Scholar]
- 7.Lightfoot N, Conlon M, Kreiger N, Bissett R, Desai M, Warde P, et al. Impact of noninvasive imaging on increased incidental detection of renal cell carcinoma. Eur Urol. 2000;37:521–527. doi: 10.1159/000020188. [DOI] [PubMed] [Google Scholar]
- 8.Lee HW, Cho KS, Jeong H, Yoon SJ, Jo MK, Lee ES, et al. Clinical analysis of incidentally found renal cell carcinoma: experiences of recent 8 years. Korean J Urol. 1998;39:982–987. [Google Scholar]
- 9.Seong BM, Kim DS, Yoon DK. Clinical characteristics of incidentally detected renal cell carcinoma. Korean J Urol. 1997;38:245–249. [Google Scholar]
- 10.Rhew HY, Kang JS, Jo SS, Lee CK. Clinical characteristics of incidentally detected renal cell carcinoma: incidentaloma. Korean J Urol. 2000;41:1195–1201. [Google Scholar]
- 11.Smith RB, deKernion JB, Ehrlich RM, Skinner DG, Kaufman JJ. Bilateral renal cell carcinoma and renal cell carcinoma in the solitary kidney. J Urol. 1984;132:450–454. doi: 10.1016/s0022-5347(17)49687-x. [DOI] [PubMed] [Google Scholar]
- 12.Schiff M, Jr, Bagley DH, Lytton B. Treatment of solitary and bilateral renal carcinomas. J Urol. 1979;121:581–586. doi: 10.1016/s0022-5347(17)56888-3. [DOI] [PubMed] [Google Scholar]
- 13.Hafez KS, Fergany AF, Novick AC. Nephron sparing surgery for localized renal cell carcinoma: impact of tumor size on patient survival, tumor recurrence and TNM staging. J Urol. 1999;162:1930–1933. doi: 10.1016/S0022-5347(05)68071-8. [DOI] [PubMed] [Google Scholar]
- 14.Leibovich BC, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004;171:1066–1070. doi: 10.1097/01.ju.0000113274.40885.db. [DOI] [PubMed] [Google Scholar]
- 15.Manikandan R, Srinivasan V, Rané A. Which is the real gold standard for small-volume renal tumors? Radical nephrectomy versus nephron-sparing surgery. J Endourol. 2004;18:39–44. doi: 10.1089/089277904322836659. [DOI] [PubMed] [Google Scholar]
- 16.Nam JK, Cha CS, Chung MK. The treatment outcomes of a partial nephrectomy in the management of renal cell carcinomas. Korean J Urol. 2004;45:1100–1105. [Google Scholar]
- 17.Herr HW. Radiographic vs surgical size of renal tumours after partial nephrectomy. BJU Int. 2000;85:19–21. doi: 10.1046/j.1464-410x.2000.00357.x. [DOI] [PubMed] [Google Scholar]
- 18.Schlomer B, Figenshau RS, Yan Y, Bhayani SB. How does the radiographic size of a renal mass compare with the pathologic size? Urology. 2006;68:292–295. doi: 10.1016/j.urology.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Kanofsky JA, Phillips CK, Stifelman MD, Taneja SS. Impact of discordant radiologic and pathologic tumor size on renal cancer staging. Urology. 2006;68:728–731. doi: 10.1016/j.urology.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 20.Yaycioglu O, Rutman MP, Balasubramaniam M, Peters KM, Gonzalez JA. Clinical and pathologic tumor size in renal cell carcinoma: difference, correlation, and analysis of the influencing factors. Urology. 2002;60:33–38. doi: 10.1016/s0090-4295(02)01668-0. [DOI] [PubMed] [Google Scholar]