Abstract
Purpose
We performed a comparative analysis of the plasma levels of antithrombin (AT) III, plasminogen, fibrinogen, and D-dimer among patients with and without clinically localized prostate cancer to investigate the clinical significance of the coagulation profile in prostate cancer.
Materials and Methods
A prospective study was performed in which plasma levels of AT III, plasminogen, fibrinogen, and D-dimer were assessed in patients before they underwent prostate biopsy. According to the results of the biopsy, the patients were categorized into the cancer group or the control group. Levels of the four coagulation factors were then compared between the cancer and control groups. Also, levels of the four coagulation factors were correlated with tumor stage and grade in the cancer group.
Results
The cancer group had significantly lower levels of AT III activity and higher plasma D-dimer levels than did the control group (p=0.007 and p=0.018, respectively). Within the cancer group, no significant differences were observed in the levels of AT III, plasminogen, fibrinogen, or D-dimer between those with a pathological Gleason score of ≥7 and otherwise. Regarding pathologic stage of prostate cancer, the subjects with organ-confined disease and those with extraprostatic extension of a tumor demonstrated no significant differences in the preoperative levels of the four coagulation factors analyzed.
Conclusions
Our results suggest that plasma levels of AT III and D-dimer are altered in patients with prostate cancer. Further study is needed to elucidate the underlying mechanism and clinical significances of such a phenomenon among patients with clinically localized prostate cancer.
Keywords: Prostatic neoplasms, Blood coagulation tests
INTRODUCTION
Abnormalities of the coagulation system have long been recognized in cancer patients, in whom the plasma levels of several coagulation factors have been observed to be altered [1,2]. Moreover, thromboembolism has been reported as being the second most frequent cause of death in cancer patients [3]. Even without obvious thrombosis, patients with solid tumors commonly present with subclinical prothrombotic or hypercoagulable conditions [4,5]. However, it is still unclear whether such abnormalities in coagulation bear any significance in the pathogenesis or progression of the malignant process. Meanwhile, the extent of activation of coagulation and fibrinolysis, as measured via plasma levels of antithrombin (AT) III and D-dimer, has been reported to correlate with tumor stage, aggressiveness, and prognosis in various malignancies, including colorectal, breast, ovarian, and lung cancer [6-9].
As for prostate cancer, a growing body of evidence suggests that the components of the coagulation and fibrinolysis system are involved in the pathogenesis and progression of this disease as well. For example, AT, a major plasma inhibitor of coagulating proteases, has been reported to be widely expressed in prostate cancer tissue but is gradually lost with increasing tumor grade [10]. Also, plasma AT levels in prostate cancer patients are observed to be increased after radical prostatectomy (RP) [11]. In addition, D-dimer, a stable end-product of fibrin degradation that has been known to be a useful prognostic marker for several solid tumors, has also been reported to be significantly increased in prostate cancer patients compared with healthy controls [12]. Meanwhile, compared with other cancers, a paucity of data exists regarding the clinical significance of these coagulation factors regarding clinically localized prostate cancer. Thus, we performed a comparative analysis of the plasma levels of AT III, plasminogen, fibrinogen, and D-dimer among patients with and without clinically localized prostate cancer to elucidate the clinical significance of the coagulation profile in prostate cancer.
MATERIALS AND METHODS
From July to December 2008, a prospective study was performed at our institution upon approval from our institutional review board (IRB no. B-0803-055-004) in which blood samples were collected from men before they underwent transrectal ultrasound (TRUS) guided multi-core biopsy of the prostate under the suspicion of prostate cancer. Among the 369 patients who gave written informed consent to participate in the study, 129 (34.9%) were diagnosed with prostate cancer from the biopsy. Of those who were not diagnosed as having prostate cancer, 56 men with an initial prostate-specific antigen (PSA) level of <10 ng/ml were selected and designated as the control group. Of those with prostate cancer, 93 who underwent RP were selected and included in the cancer group.
Blood sampling was performed before the scheduled prostate biopsy in each subject. Blood from a clean venipuncture was mixed with 0.129 mol/l trisodium citrate (Merck) containing 0.21 mol/l HEPES (Sigma) in cold plastic tubes. The blood was immediately spun at 2,000 g for 15 minutes in a refrigerated centrifuge. All coagulation parameters were measured within 2 hours of sample collection. The following assays were performed: fibrinogen (Clauss) with international standards from the National Institute for Biological Standards and Control (NIBSC), Hempstead, England; D-dimer via ELISA (Diagnostic Stago, France); ATIII activity via chromogenic substrate assay (S2238) with plasma standard calibration made against international standards (Chromogenix, Sweden); and plasminogen activity via hydrolysis of a chromogenic substrate (Coamatic, Chromogenix Instr. Lab. SpA, Milano, Italy).
Various clinicopathological data were also assessed in the aforementioned subjects of the cancer and control groups: patient age at the time of surgery, body mass index (BMI), preoperative PSA level, prostate volume assessed via preoperative TRUS exam, pathologic Gleason grade, pathologic stage, and surgical margin status. First, we compared the coagulation profiles of the cancer and control groups. Also, the coagulation profiles of the cancer group were correlated with cancer-related profiles of the patients.
The SPSS software package version 15.0 (Statistical Package for Social Sciences™, Chicago, USA) was used for statistical analysis. The profiles of the cancer and control groups were compared via independent-sample T tests. Comparison of continuous variables in different subgroups was performed by Mann-Whitney U-test or Kruskal-Wallis test. Relationships between categorical variables were compared by using chi-square test. A p-value less than 0.05 was considered statistically significant.
RESULTS
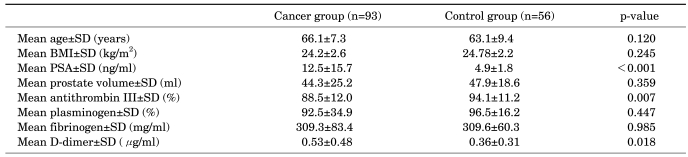
The characteristics of the subjects are listed in Table 1. As can be seen, the cancer and control groups did not differ significantly regarding age, BMI, or prostate volume measured via TRUS. The mean serum PSA levels of the cancer and control groups were 12.5±15.7 ng/ml and 4.9±1.8 ng/ml, respectively, demonstrating a significant difference (p<0.001). As for the coagulation profiles analyzed, the two groups demonstrated no significant differences with regard to plasminogen activity (p=0.447) or fibrinogen level (p=0.985). On the other hand, AT III activity was significantly lower and the plasma D-dimer level was significantly higher in the cancer group than in the control group (p=0.007 and p=0.018, respectively).
TABLE 1.
Patient characteristics
SD: standard deviation, BMI: body mass index, PSA: prostate-specific antigen
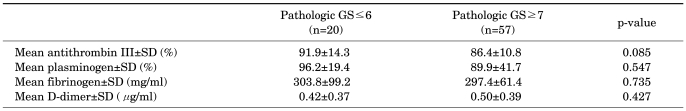
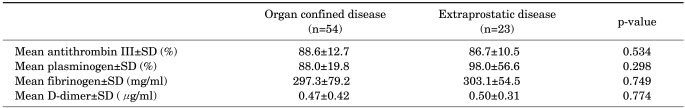
Within the cancer group, those with pathological Gleason score of ≥7 demonstrated lower preoperative ATIII activity than did the other patients, but the difference only approached significance (p=0.085) (Table 2). Concerning the other coagulation-related parameters, the subjects with a pathologic Gleason score of ≤6 and those with a pathologic Gleason score of ≥7 showed no significant differences in preoperative levels of plasminogen activity (p=0.547), fibrinogen (p=0.735), or D-dimer (p=0.427). When the same analysis was performed by using a pathologic Gleason score cutoff of 8, the results were the same. With regard to pathologic stage of prostate cancer, the subjects with organ-confined disease and those with extraprostatic extension of a tumor confirmed on pathologic examination of RP specimens demonstrated no significant differences in preoperative levels of AT III (p=0.534), plasminogen (p=0.298), fibrinogen (p=0.749), or D-dimer (p=0.774) (Table 3).
TABLE 2.
Coagulation profiles according to pathologic Gleason score
GS: Gleason score, SD: standard deviation
TABLE 3.
Coagulation profiles according to pathologic stage of prostate cancer
SD: standard deviation
DISCUSSION
In the current study, we observed that the plasma level of AT III was significantly decreased, whereas the D-dimer level was significantly increased, in patients with clinically localized prostate cancer compared with those without evidence of cancer on prostate biopsy. Such findings imply that, regarding prostate cancer, the coagulation system may well be associated with carcinogenesis. On the other hand, among our subjects with prostate cancer, preoperative levels of AT III, plasminogen, fibrinogen, and D-dimer had no significant associations with tumor stage or grade, which are established prognostic predictors of prostate cancer.
Previously, preoperative levels of AT III and D-dimer were reported to be useful predictors of prognosis in various cancers. Koh et al suggested that plasma levels of AT III and D-dimer, together with those of fibrinogen and von Willebrand factor, should be used as prognostic markers for ovarian cancer [8]. Also, Oya et al reported that an elevated plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer [13]. They observed via multivariate analysis that the preoperative plasma D-dimer level was the third strongest independent prognostic factor, exceeded in importance only by lymph node status and carcinoembryonic antigen (CEA) level. Similar associations have been reported between preoperative plasma D-dimer levels and postoperative survival in patients with lung cancer. Unsal et al reported that high D-dimer and low AT III levels were correlated with advanced tumor stage and short survival in patients with lung cancer [9]. Meanwhile, Dirix et al observed that plasma D-dimer levels correlated with tumor volume, progression rate, and survival in patients with breast cancer [7]. Thus, evidence has accumulated in the literature to suggest that plasma levels of AT III and D-dimer may be significantly associated with known prognostic indicators and the survival of patients with various malignancies.
As for prostate cancer, some researchers have also previously reported that AT III and D-dimer may have clinical significance. Cao et al found AT to be widely expressed in tissues of prostate adenocarcinoma and also observed that the expression was gradually lost in tumors of high Gleason grade [10]. Beecken et al observed from their series of 40 consecutive patients with prostate cancer that plasma levels of AT were significantly reduced before RP, only to recover to normal levels within 2 weeks after RP [11]. Meanwhile, Caine et al reported that plasma levels of D-dimer and fibrinogen were significantly elevated in prostate cancer patients compared with healthy controls [12]. These reports can be considered supportive of our finding, as we also observed significant differences in the levels of AT III and D-dimer between those with and without prostate cancer. Further study is needed to elucidate whether the observed levels of AT III and D-dimer are the contributors or the results of the carcinogenesis regarding prostate cancer. Still, our results suggest that levels of AT III and D-dimer are significantly altered in patients with prostate cancer compared with their healthy counterparts.
Meanwhile, Langer et al reported that the plasma D-dimer level was significantly higher in patients with less differentiated prostate cancers and also showed a trend of being higher in those with locally advanced disease [14]. Furthermore, the plasma level of urokinase-type plasminogen activator (uPA), which transfers plasminogen to plasmin, was reported to be associated with features of tumor aggressiveness and progression in prostate cancer. In our study, no significant association of the coagulation factors analyzed with tumor stage or grade was observed among the patients with prostate cancer [15]. The preoperative level of AT III was lower among those with relatively higher (≥7) pathologic Gleason score, but the difference only approached significance. Such discrepancy between our results and other published data may be due to the relatively smaller number of subjects included in our study. With longer follow-up, the coagulation profiles assessed in our study may demonstrate prognostic significance when correlated with parameters such as biochemical recurrence-free or overall survival of cancer patients. Thus, relevant investigations would be needed in the future regarding our subjects with prostate cancer.
In prostate cancer, chronic disseminated intravascular coagulation (DIC) leading to decreases in coagulation factors, which in turn may elicit thromboembolic complications, is considered a potential problem [16]. DIC results in the production of fibrin, which has been reported to cover cancer lesions at the invasion front. Tumors are known to actually use fibrin as a support, and fibrin appears to attract new blood vessels. Such a situation demonstrates a close interaction between tumors and the hemostatic system, eventually leading to tumor growth and invasion. It has also been suggested that tumor cells themselves could convert fibrinogen to fibrin. D-dimer, a fibrinogen degradation product, was shown to be associated with tumor progression in previously reported studies, and an increased plasma level of D-dimer may reflect ongoing fibrinogen metabolism within actively remodeling tumor stroma [17]. Furthermore, a reduction of AT, which is a major plasma inhibitor of coagulating protease, would lead to exaggerated production of fibrin in the case of the activation of the coagulation cascade commonly seen in malignancies [18]. Moreover, AT has been known to inhibit the angiogenic activity of tumors [8,19]. Also, AT may act additionally as a local inhibitor of tumor invasion because of its ability to form complexes with proteases, including plasma kallikreins and trypsin. Thus, reductions in the AT level may well lead to carcinogenesis, tumor growth, and progression in prostate cancer. In our study, we observed a relatively lower level of AT III among patients with a higher pathologic Gleason score. This finding is supportive of the aforementioned hypothesis of the potential role of AT in prostate cancer. Although no prognostic significance of AT III or D-dimer was observed in our study, our findings indicate that both coagulation factors may well be associated with pathogenesis or progression of prostate cancer.
Our study may be limited by the relatively small number of subjects analyzed. Furthermore, coagulation parameters were checked only once in each patient. Also, we focused on AT III and D-dimer, and plasma levels of various other hemostatic factors, such as uPA, were not analyzed. We could not assess the effects of factors such as cardiovascular comorbidities that may also impact the coagulative system. Also, due to the apparent lack of adequate follow-up, the significance of coagulation factors regarding the disease-specific survival of subjects, which we are planning to analyze in the future, could not be assessed in the current study. Still, we believe that the results of our study justify the need for further investigation, basic or clinical, on the prognostic significance of various coagulation factors in prostate cancer.
CONCLUSIONS
Our results suggest that plasma levels of AT III and D-dimer are altered in patients with clinically localized prostate cancer. Further study is needed to elucidate the underlying mechanism and clinical significance of such a phenomenon among patients with prostate cancer.
Footnotes
This study was supported by a grant from the 2008 Seoul National University Bundang Hospital Research Fund (02-2008-028).
References
- 1.Bick RL. Coagulation abnormalities in malignancy: a review. Semin Thromb Hemost. 1992;18:353–372. doi: 10.1055/s-2007-1002575. [DOI] [PubMed] [Google Scholar]
- 2.Constantini V, Zacharski LR. Fibrin and cancer. Thromb Haemost. 1993;69:406–414. [PubMed] [Google Scholar]
- 3.Donati MB, Falanga A. Pathogenetic mechanisms of thrombosis in malignancy. Acta Haematol. 2001;106:18–24. doi: 10.1159/000046585. [DOI] [PubMed] [Google Scholar]
- 4.Rickles FR, Levine M, Edwards RL. Hemostatic alterations in cancer patients. Cancer Metastasis Rev. 1992;11:237–248. doi: 10.1007/BF01307180. [DOI] [PubMed] [Google Scholar]
- 5.Falanga A, Rickles FR. Pathophysiology of the thrombophilic state in the cancer patient. Semin Thromb Hemost. 1999;25:173–182. doi: 10.1055/s-2007-994919. [DOI] [PubMed] [Google Scholar]
- 6.Kilic M, Yoldas O, Keskek M, Ertan T, Tez M, Gocmen E, et al. Prognostic value of plasma D-dimer levels in patients with colorectal cancer. Colorectal Dis. 2008;10:238–241. doi: 10.1111/j.1463-1318.2007.01374.x. [DOI] [PubMed] [Google Scholar]
- 7.Dirix LY, Salgado R, Weytjens R, Colpaert C, Benoy I, Huget P, et al. Plasma fibrin D-dimer levels correlate with tumour volume, progression rate and survival in patients with metastatic breast cancer. Br J Cancer. 2002;86:389–395. doi: 10.1038/sj.bjc.6600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koh SC, Tham KF, Razvi K, Oei PL, Lim FK, Roy AC, et al. Hemostatic and fibrinolytic status in patients with ovarian cancer and benign ovarian cysts: could D-dimer and antithrombin III levels be included as prognostic markers for survival outcome? Clin Appl Thromb Hemost. 2001;7:141–148. doi: 10.1177/107602960100700211. [DOI] [PubMed] [Google Scholar]
- 9.Unsal E, Atalay F, Atikcan S, Yilmaz A. Prognostic significance of hemostatic parameters in patients with lung cancer. Respir Med. 2004;98:93–98. doi: 10.1016/j.rmed.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Cao Y, Lundwall A, Gadaleanu V, Lilja H, Bjartell A. Anti-thrombin is expressed in the benign prostatic epithelium and in prostate cancer and is capable of forming complexes with prostate-specific antigen and human glandular kallikrein 2. Am J Pathol. 2002;161:2053–2063. doi: 10.1016/S0002-9440(10)64484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beecken WD, Bentas W, Engels K, Glienke W, Urbschat A, Jonas D, et al. Reduced plasma levels of coagulation factors in relation to prostate cancer. Prostate. 2002;53:160–167. doi: 10.1002/pros.10142. [DOI] [PubMed] [Google Scholar]
- 12.Caine GJ, Lip GY, Stonelake PS, Ryan P, Blann AD. Platelet activation, coagulation and angiogenesis in breast and prostate carcinoma. Thromb Haemost. 2004;92:185–190. doi: 10.1160/TH03-11-0679. [DOI] [PubMed] [Google Scholar]
- 13.Oya M, Akiyama Y, Okuyama T, Ishikawa H. High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jpn J Clin Oncol. 2001;31:388–394. doi: 10.1093/jjco/hye075. [DOI] [PubMed] [Google Scholar]
- 14.Langer F, Chun FK, Amirkhosravi A, Friedrich M, Leuenroth S, Eifrig B, et al. Plasma tissue factor antigen in localized prostate cancer: distribution, clinical significance and correlation with haemostatic activation markers. Thromb Haemost. 2007;97:464–470. [PubMed] [Google Scholar]
- 15.Shariat SF, Roehrborn CG, McConnell JD, Park S, Alam N, Wheeler TM, et al. Association of the circulating levels of the urokinase system of plasminogen activation with the presence of prostate cancer and invasion, progression, and metastasis. J Clin Oncol. 2007;25:349–355. doi: 10.1200/JCO.2006.05.6853. [DOI] [PubMed] [Google Scholar]
- 16.Kohli M, Kaushal V, Mehta P. Role of coagulation and fibrinolytic system in prostate cancer. Semin Thromb Hemost. 2003;29:301–308. doi: 10.1055/s-2003-40968. [DOI] [PubMed] [Google Scholar]
- 17.Blackwell K, Haroon Z, Broadwater G, Berry D, Harris L, Iglehart JD, et al. Plasma D-dimer levels in operable breast cancer patients correlate with clinical stage and axillary lymph node status. J Clin Oncol. 2000;18:600–608. doi: 10.1200/JCO.2000.18.3.600. [DOI] [PubMed] [Google Scholar]
- 18.Kim TH, Yang DY, Kim SY, Kim H, Yoo HS, Choi H, et al. Evaluation of the changes of coagulation-fibrinolysis system during transurethral resection of the prostate by thromboelastography. Korean J Urol. 1997;38:1217–1222. [Google Scholar]
- 19.Browder T, Folkman J, Pirie-Shepherd S. The hemostatic system as a regulator of angiogenesis. J Biol Chem. 2000;275:1521–1524. doi: 10.1074/jbc.275.3.1521. [DOI] [PubMed] [Google Scholar]