Abstract
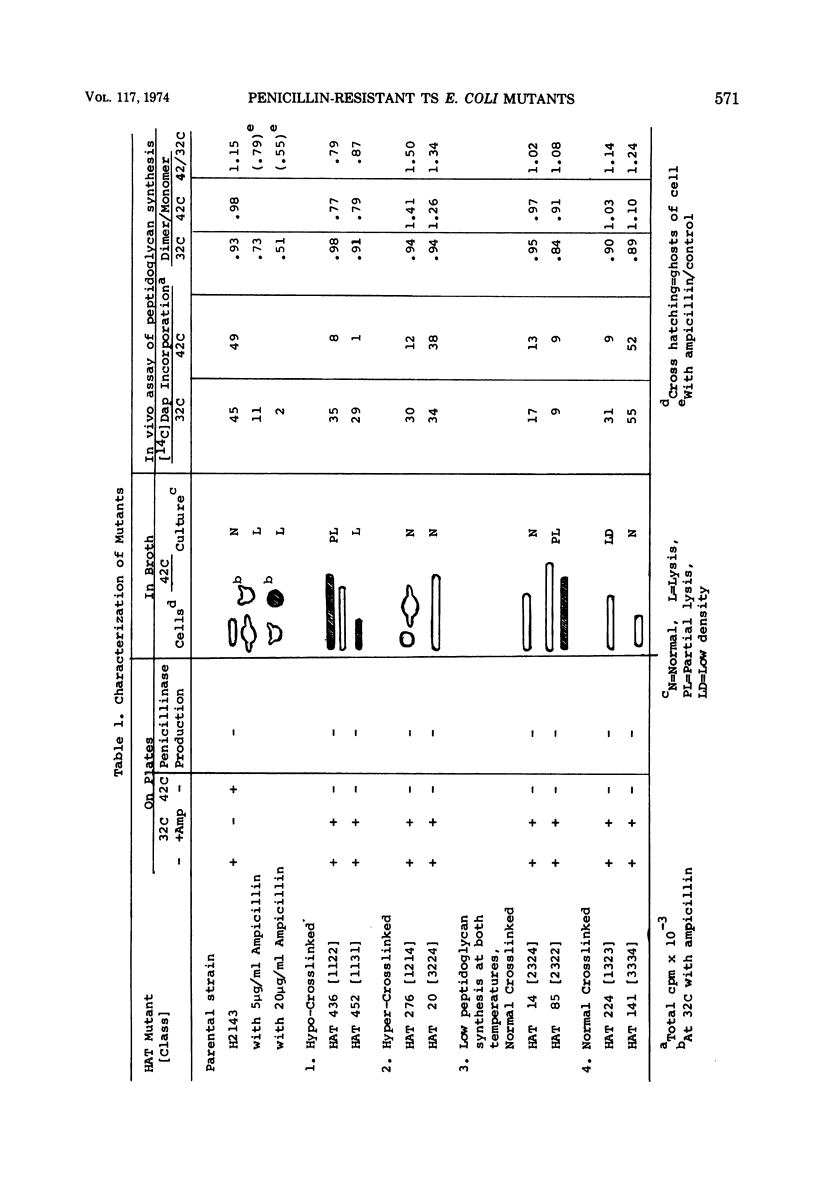
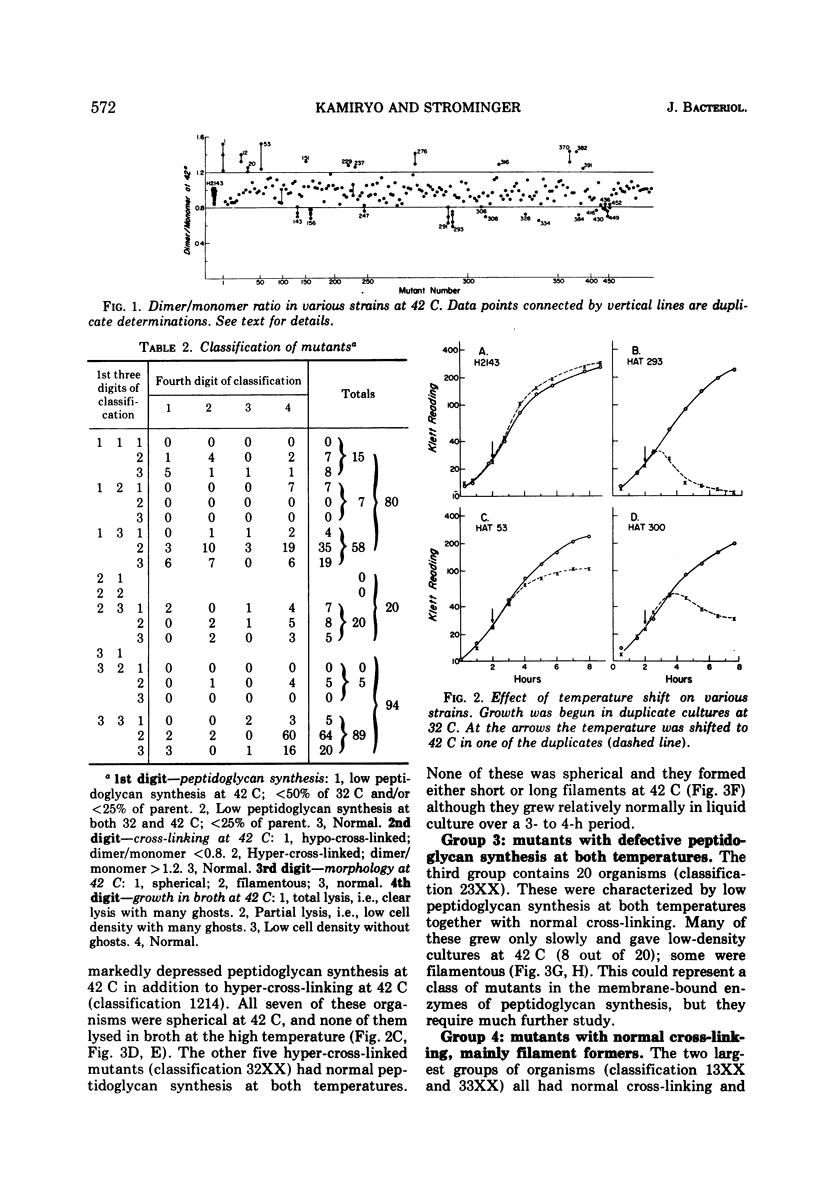
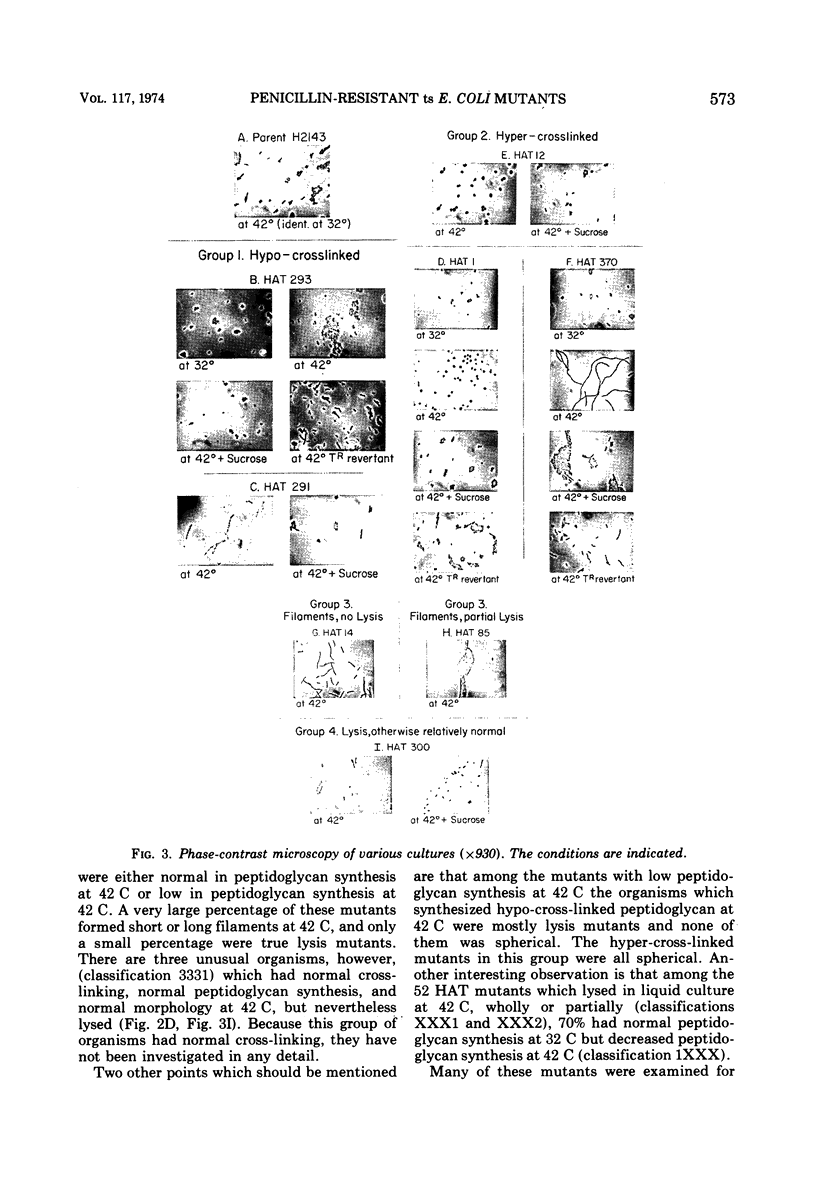
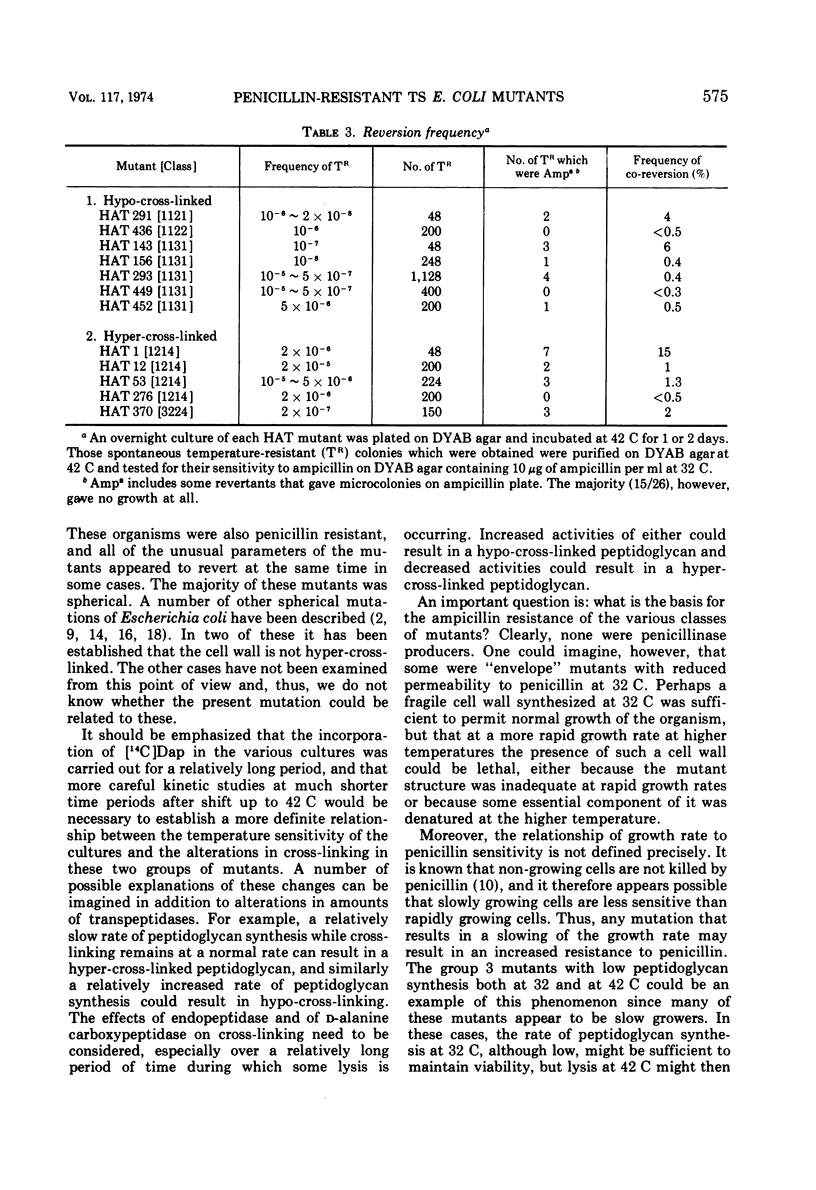
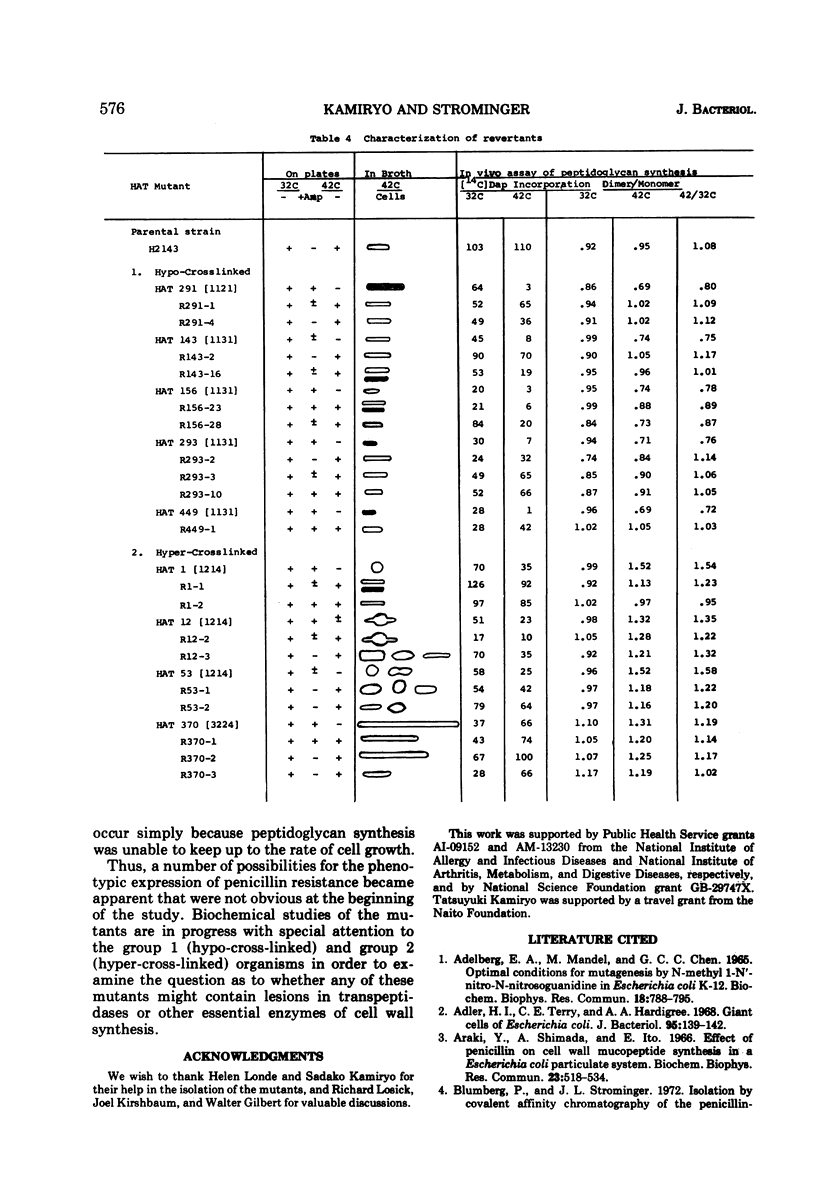
A group of Escherichia coli mutants which are ampicillin resistant at 32 C and which either are unable to grow or lyse at 42 C has been selected. These mutants have been classified by a number of characteristics: total peptidoglycan synthesis measured by [14C]diaminopimelic acid incorporation, extent of cross-linking of the peptidoglycan which is synthesized, growth characteristics at the two temperatures, and morphology. Two especially interesting groups of mutants have been described. In one of these, a hypo-cross-linked peptidoglycan was synthesized at the nonpermissive temperature. Most of these organisms lysed at 42 C. In another group, the peptidoglycan synthesized at 42 C was hyper-cross-linked. Many of these organisms were spherical. Studies of revertants indicated that ampicillin resistance, temperature sensitivity, cross-linking, growth characteristics, and morphological changes may be related to a single mutational event in both of these groups.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Terry C. E., Hardigree A. A. Giant cells of Escherichia coli. J Bacteriol. 1968 Jan;95(1):139–142. doi: 10.1128/jb.95.1.139-142.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y., Shimada A., Ito E. Effect of penicillin on cell wall mucopeptide synthesis in a Escherichia coli particulate system. Biochem Biophys Res Commun. 1966 May 25;23(4):518–525. doi: 10.1016/0006-291x(66)90760-1. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Five penicillin-binding components occur in Bacillus subtilis membranes. J Biol Chem. 1972 Dec 25;247(24):8107–8113. [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Isolation by covalent affinity chromatography of the penicillin-binding components from membranes of Bacillus subtilis. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3751–3755. doi: 10.1073/pnas.69.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricas E., Dezélée P. Sur l'idenité de la mucoendopeptidase et de la carboxy-peptidase I d'Escherichia coli, eneymes hydrolysant des liaisons de configuration D-D et inhibées par la pénicilline. C R Acad Sci Hebd Seances Acad Sci D. 1969;269(3):390–393. [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G., Boman H. G., Jansson J. A., Thorén S. Resistance of Escherichia coli to Penicillins I. Genetic Study of Some Ampicillin-Resistant Mutants. J Bacteriol. 1965 Jul;90(1):54–62. doi: 10.1128/jb.90.1.54-62.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R., Höltje J. V., Schwarz U. Targets of penicillin action in Escherichia coli. Nature. 1972 Feb 25;235(5339):426–429. doi: 10.1038/235426a0. [DOI] [PubMed] [Google Scholar]
- Henning U., Rehn K., Braun V., Höhn B. Cell envelope and shape of Escherichia coli K12. Properties of a temperature-sensitive rod mutant. Eur J Biochem. 1972 Apr 24;26(4):570–586. doi: 10.1111/j.1432-1033.1972.tb01800.x. [DOI] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Glycopeptide transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Proc Natl Acad Sci U S A. 1966 Mar;55(3):656–663. doi: 10.1073/pnas.55.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XIV. Purification and properties of two D-alanine carboxypeptidases from Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3193–3201. [PubMed] [Google Scholar]
- Lazdunski C., Shaprio B. M. Relationship between permeability, cell division, and murein metabolism in a mutant of Escherichia coli. J Bacteriol. 1972 Aug;111(2):499–509. doi: 10.1128/jb.111.2.499-509.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg J. Gene Recombination and Linked Segregations in Escherichia Coli. Genetics. 1947 Sep;32(5):505–525. doi: 10.1093/genetics/32.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi S., Kamiryo T., Blumberg P. M., Linnett P., Willoughby E., Strominger J. L. Mechanism of action and development of resistance to a new amidino penicillin. J Bacteriol. 1974 Feb;117(2):578–587. doi: 10.1128/jb.117.2.578-587.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P., RICHMOND M. H. NATURE AND INTERACTIONS OF THE GENETIC ELEMENTS GOVERNING PENICILLINASE SYNTHESIS IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Aug;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Burman L. G., Eriksson-Grennberg K. G. Resistance of Escherichia coli to penicillins. 8. Physiology of a class II ampicillin-resistant mutant. J Bacteriol. 1970 Mar;101(3):659–668. doi: 10.1128/jb.101.3.659-668.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S. Mutation in Escherichia coli K-12 mediating spherelike envelopes and changes tolerance to ultraviolet irradiation and some antibiotics. J Bacteriol. 1969 Jun;98(3):1274–1277. doi: 10.1128/jb.98.3.1274-1277.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock J. J., Ghuysen J. M., Linder R., Salton M. R., Perkins H. R., Nieto M., Leyh-Bouille M., Frere J. M., Johnson K. Transpeptidase activity of Streptomyces D-alanyl-D carboxypeptidases. Proc Natl Acad Sci U S A. 1972 Mar;69(3):662–666. doi: 10.1073/pnas.69.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W., Willoughby E., Strominger J. L. Further studies of the D-aspartic acid-activating enzyme of Streptococcus faecalis and its attachment to the membrane. J Biol Chem. 1972 Sep 10;247(17):5289–5296. [PubMed] [Google Scholar]
- Strominger J. L., Blumberg P. M., Suginaka H., Umbreit J., Wickus G. G. How penicillin kills bacteria: progress and problems. Proc R Soc Lond B Biol Sci. 1971 Dec 31;179(1057):369–383. doi: 10.1098/rspb.1971.0103. [DOI] [PubMed] [Google Scholar]




