Abstract
Purpose
The fibroblast growth factor receptor 3 (FGFR3) gene is known to be frequently mutated in noninvasive urothelial carcinomas of the bladder. In this study, we investigated the expression of FGFR3, Ki-67, and p53 in bladder cancers and the effects of expression on tumor recurrence.
Materials and Methods
Fifty-five cases of primary bladder cancer were examined by immunohistochemistry. The relationship of these markers with various clinicopathological factors, including recurrence, was assessed.
Results
Positivity for cytoplasmic FGFR3 (FGFR3-c) was associated with a lower cancer grade (p=0.022) and stage (p=0.011). Recurrence was more frequent in patients with a higher stage, negative FGFR3-c, and high Ki-67 expression. According to univariate analysis, predictors of recurrence-free survival included the following: age, stage, FGFR-c, Ki-67, and p53. However, none of these was independent from the other parameters in multivariate studies.
Conclusions
The immunohistochemical expression of FGFR3 is not only one of the characteristic features of lower-grade and lower-stage urothelial carcinoma but also a possible marker in predicting disease recurrence.
Keywords: Carcinoma, transitional cell; Fibroblast growth factor receptor 3; p53 genes; Recurrence
INTRODUCTION
Most bladder cancers are urothelial carcinomas, of which the majority (75-80%) initially present as non-muscle-invasive tumors. Papillary noninvasive (pTa) and superficially invasive (pT1) tumors can be treated by transurethral resection. However, up to 70% of patients will experience one or more recurrences and about 25% will progress in stage or grade of disease [1]. Therefore, patients require regular monitoring, which is currently performed by periodic cystoscopy and urine cytology. The interval of surveillance varies depending on the specific risk factors of the individual patient. Efforts have been made to clarify the significance of various clinical and histological factors as prognosticators and to develop effective risk assessment tools. Although some conventional factors such as number of tumors, tumor size, grade, stage, and the presence of carcinoma in situ (CIS) have prognostic power on tumor recurrence and progression [2], the ability to estimate each individual's risks of adverse clinical outcome remains limited.
In the ongoing search for new prognostic factors, molecular markers associated with the diagnosis and the prognosis of bladder cancers have been a main subject of study. Fibroblast growth factor receptor 3 (FGFR3) is involved in the regulation of proliferation, differentiation, and apoptosis [3]. The FGFR3 gene is known to be frequently mutated in noninvasive urothelial carcinomas of the bladder [4-6] and might correlate with better clinical outcome [7]. FGFR3 mRNA and protein are also reported to be overexpressed in a significant number of urothelial carcinomas of the bladder [8]. According to Tomlinson et al, the increased expression of FGFR3 protein is associated with mutation [6], and activating mutations are frequently reported and well described in the bladder cancer literature. However, not many studies of FGFR3 protein expression and its clinical significance have been carried out, although immunohistochemical detection of the protein is a much easier and simpler method to apply in the hospital-based setting.
Altered p53 gene and protein status may indicate poorer prognoses and greater recurrences of disease [9-11]. Ki-67, which is strongly associated with cellular proliferation, has been targeted for assessing the proliferative activity of bladder tumor cells and correlating its expression levels with the prognosis of these patients. Many separate studies have shown an association between a high staining index of Ki-67 and an unfavorable disease course [12,13].
In the present study, we examined the protein expression of FGFR3 in urothelial carcinoma of the bladder by immunohistochemistry. The expression levels of p53 and Ki-67 were compared with FGFR3 immunoreactivity, and the relationships among these markers were investigated. Additionally, the correlations between the markers and various clinicopathological factors and between the markers and recurrences in bladder cancers were investigated to study the possible prognostic value of these molecular markers.
MATERIALS AND METHODS
Fifty-five cases of primary non-muscle-invasive urothelial carcinoma (pTa and pT1) of the bladder were included in this study. The patients were diagnosed and treated at one hospital between 2001 and 2007.
H&E slides of all samples were reviewed, and representative formalin-fixed paraffin-embedded tumor blocks were selected. Tumor staging and grading were done according to the TNM staging system and to the 2004 WHO classification criteria for malignant tumors of the urinary tract.
Clinical follow-up data are presented and summarized in Table 1. Patients ranged in age from 33 to 84 years (mean, 67±11.3). They were followed with cystoscopy and urine cytology performed every 3 months for the first 1 year, every 6 months for the second year, and annually thereafter. The mean follow-up period was 26.2 months (range, 3-70 months). Recurrence was defined as cystoscopically visible tumors with histologic confirmation.
TABLE 1.
Clinical and pathological data
PUNLMP: papillary urothelial neoplasm of low malignant potential, a: grading according to the 2004 WHO classification system
For immunohistochemistry, 5 µm deparaffinized and rehydrated sections were treated with 3% hydrogen peroxide and then microwaved for 20 minutes. The primary antibodies were anti-FGFR3 (clone B-9; Santa Cruz Biotechnology, Inc, Santa Cruz, USA; dilution 1:100), anti-p53 (clone DO-7; DAKO, Glostrup, Denmark; dilution 1:100), and anti-Ki-67 (Lab Vision, Fremont, USA; dilution 1:80). We applied anti-FGFR3 overnight in a 4℃ refrigerator and anti-Ki-67 and anti-p53 for 1 hour at room temperature. DAB chromogen was used.
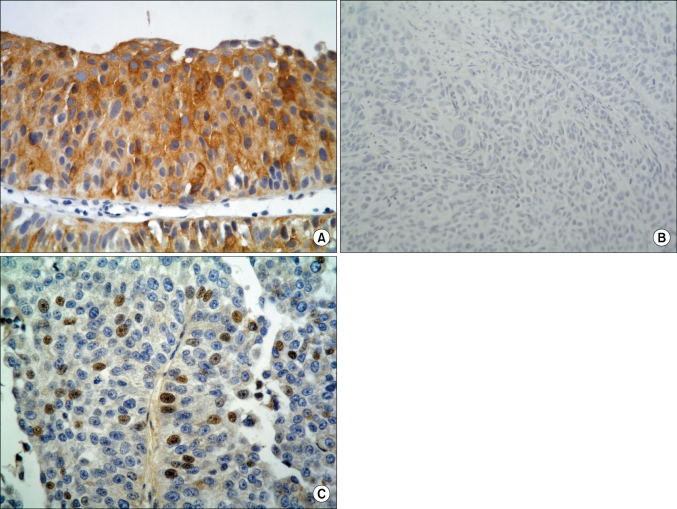
FGFR3 protein was expressed in the tumor cell cytoplasm or nuclei. Cytoplasmic immunoreactivity was assigned scores as follows: 0, negative; 1, weak to moderate; and 2, strong. Nuclear expression was defined as positive when unequivocal staining was observed regardless of extent (Fig. 1). Protein expression levels of Ki-67 and P53 were evaluated by the percentage of positively stained nuclei. Cutoffs were defined at 25% and 10% for Ki-67 [14] and p53 [9,14], respectively.
FIG. 1.
Immunohistochemical expression of FGFR3 in bladder cancer. (A) Strong cytoplasmic expression in tumor cells of low-grade urothelial carcinoma (×400). (B) Negative cytoplasmic FGFR3 reaction in high-grade urothelial carcinoma (×200). (C) Nuclear FGFR3 expression. FGFR3: fibroblast growth factor receptor 3 (×400).
SPSS 12.0 computer software (SPSS Inc., Chicago, USA) was used for data analysis. A finding was considered statistically significant at p<0.05. We examined the following variables: demographic data, macroscopic characteristics (number, size, and growth pattern), and tumor grade and stage. Additionally assessed were the effects of single and combined immunohistochemical markers on disease recurrence. The chi-square test and the two-sided Fisher's exact test were applied to study statistical associations between measured parameters. The Kaplan-Meier method was used to compare curves for the different variables with regard to recurrence-free-survival, with significance evaluated by log-rank test. Patients were censored at the date of first pathological confirmation of recurrent tumor, on the date of last follow-up visit, or at the date of death. Cox's regression analysis was applied to obtain risks of recurrence and to find independent prognostic factors.
RESULTS
1. Molecular markers and clinicopathological parameters
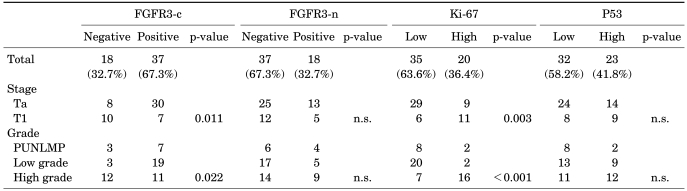
The correlation between the molecular markers and tumor grade and stage is shown in Table 2. Cytoplasmic FGFR3 positivity included immunoreactivity type 1 and 2. The majority (81.3%) of the lower-grade tumors (PUNLMP & low-grade UC) demonstrated cytoplasmic positivity, whereas only 47.8% of high-grade urothelial carcinomas did (p=0.022). We found that 78.9% of pTa tumors showed positive cytoplasmic staining for FGFR3, with 41.2% of pT1 tumors expressing FGFR3 (p=0.011). Nuclear expression was observed in 32.7% of all tumors. There was a tendency for tumors without nuclear reactivity to recur more frequently; among the 37 tumors displaying negative nuclear reactivity, 48.62% showed recurrence, whereas 22.2% of nuclear-positive tumors had recurrences during the follow-up. However, this parameter was not statistically significant. A total of 13 tumors (23.6%) showed both cytoplasmic and nuclear FGFR3 expression. High Ki-67 reactivity was noted in 36.4% and tended to be higher as the stage (p=0.003) and grade (p<0.001) of disease increased. We observed p53 overexpression in 41.8% of the tumors; however, this was not statistically significant with clinicopathological factors.
TABLE 2.
Molecular characteristics related to tumor grade and stage
FGFR3: fibroblast growth factor receptor 3, FGFR3-c: cytoplasmic expression of FGFR3, FGFR3-n: nuclear expression of FGFR3, PUNLMP: papillary urothelial neoplasm of low malignant potential, n.s.: not significant
2. Clinical and molecular characteristics with recurrence within two years
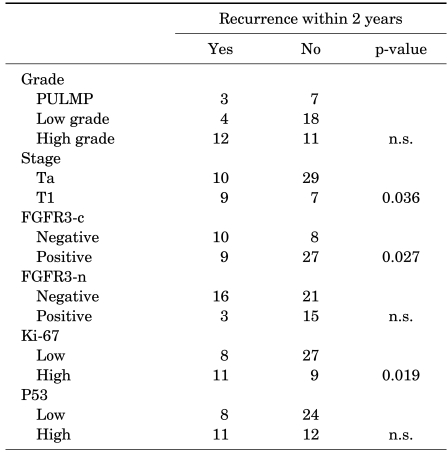
Twenty-two patient cases had disease recurrence in their clinical course and 19 cases (86.4%) among them recurred within 2 years after treatment of primary bladder cancer. We analyzed the relationship between clinical and molecular factors and the presence of 2-year recurrence (Table 3). Tumors with a higher stage, negative cytoplasmic expression of FGFR3, and high Ki-67 expression demonstrated more frequent recurrence. However, tumor grade and p53 overexpression were not significantly associated with recurrence within 2 years.
TABLE 3.
Pathological and molecular characteristics related to recurrence within 2 years
PUNLMP: papillary urothelial neoplasm of low malignant potential, n.s.: not significant, FGFR3: fibroblast growth factor receptor 3, FGFR3-c: cytoplasmic expression of FGFR3, FGFR3-n: nuclear expression of FGFR3
3. Molecular markers and prediction of recurrence
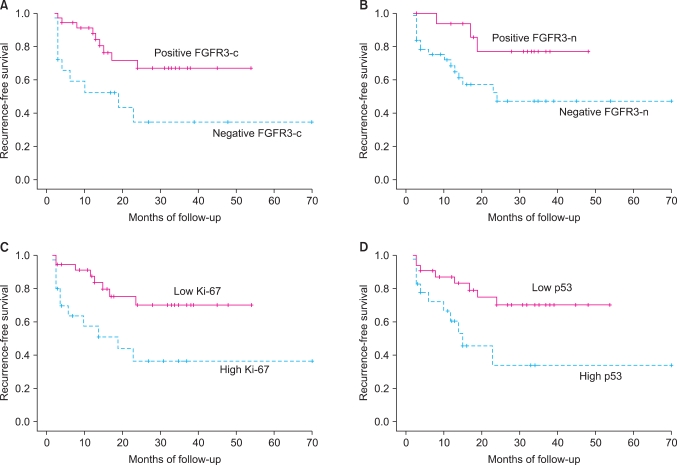
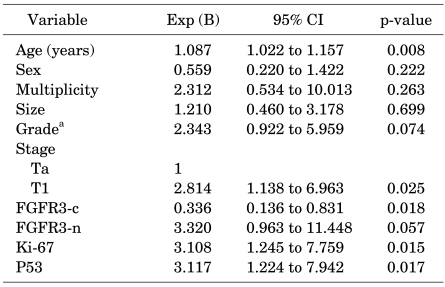
Kaplan-Meier analyses of recurrence-free survival are shown in Fig. 2. Bladder cancer patients with positive cytoplasmic or nuclear FGFR3 had significantly better survival than did patients with FGFR3 negativity. Meanwhile, tumors with high expression of Ki-67 or p53 showed significantly worse survival than did those with low expression. Univariate Cox regression analysis showed that the recurrence-free interval was significantly shorter in older patients and tumors with a higher stage, no cytoplasmic expression of FGFR3, high Ki-67 reactivity, and p53 overexpression (Table 4). However, no parameter proved to be an independent predictor of disease recurrence in the multivariate model.
FIG. 2.
Kaplan-Meier analyses for recurrence-free survival. (A) Cytoplasmic FGFR3 expression (p=0.012). (B) Nuclear FGFR3 expression (p=0.042). (C) Ki-67 (p=0.01). (D) p53 (p=0.011). FGFR3: fibroblast growth factor receptor 3.
TABLE 4.
Univariate Cox regression analysis for recurrence-free survival
Exp (B): exponential, CI: confidence interval, FGFR3: fibroblast growth factor receptor 3, FGFR3-c: cytoplasmic expression of FGFR3, FGFR3-n: nuclear expression of FGFR3, PUNLM: papillary urothelial neoplasm of low malignant potential, a: lower grade (PUNLMP & low grade) vs. higher grade (high grade)
DISCUSSION
Germ-line mutations in FGFR3 are well documented in skeletal dysplasia syndrome. Frequent somatic mutations have been described in multiple myeloma [15], cervical carcinoma [16], and bladder cancer [15,17]. A large proportion (35-60%) of bladder cancers are found to have mutations [14,16], the rate of which was higher in patients with lower-grade and lower-stage tumors. The mutation rate was 75% in low-grade and low-stage cases, but infrequent in muscle-invasive diseases [14].
On the other hand, immunohistochemical expression of FGFR3 protein has not been extensively examined to date and the exact mechanism of cytoplasmic protein expression is still unclear. The expression rate is known to be between 42% and 80.5% in different grades and stages [6,8,18]. Tomlinson et al reported a strong correlation between expression level and mutation status and tumor grade and stage [6]. Two other studies [8,19] using immunohistochemistry reported similar relationships, but one [18] did not. In our study, cytoplasmic FGFR3 was positive in 67.3% of all cases and positivity was associated with lower grade (p=0.022) and lower stage (p=0.011). This was also predictive of recurrence-free survival in the univariate analysis (p=0.018), although it failed to remain an independent predictor in the multivariate model. Barbisan et al reported that strong immunohistochemical expression of FGFR3 was one of the indicators for non-recurrent papillary urothelial neoplasms of low malignant potential [4]. We found no other reports in this regard. In mutation studies, the results are not in agreement. Some authors [19,20] have reported an association between mutation and a higher recurrence rate, whereas others [14,21] have reported the reverse. This discordance is possibly caused by differences in patient populations and in tumor stratification methods.
Interestingly, we noticed frequent nuclear reactivity of FGFR3 protein in the tumor cells. The nuclear expression was observed in 32.7% of the cases and it tended to be observed more frequently in the cases showing recurrences. However, it failed to show prognostic power in the Cox regression analysis. Røtterud et al found nuclear staining in nearly all carcinoma samples and suggested that FGFR3 was translocated from the cytoplasm to the nucleus during the development of bladder cancer [22]. We could find no other reports on the nuclear expression of FGFR3 and its relationship to various clinicopathological factors. The mechanism related to the nuclear location of FGFR3 protein remains unclear. Further research is necessary in this regard.
P53 is one of the most frequently studied tumor suppressor genes. Its overexpression and mutations are known to be associated with poor clinical outcome in various human cancers. In bladder cancer, it appears to be related with invasive, high-grade carcinoma [23,24]; frequent progression; and poor survival [21,25,26]. In the aspect of recurrence, many authors have reported that there is no statistically significant relationship between them, although the recurrence rate tends to be increased in patients with p53 mutation or altered p53 protein expression [25,26]. George et al and Salinas-Sánchez et al did find a significant relationship between these same variables [9,10]. In our study, p53 overexpression was related with a shorter recurrence-free survival in the univariate analyses (p=0.017) but not in the multivariate model. Malats et al said there was a substantial inconsistency in the results on p53 effectiveness to predict recurrence in the meta-analysis [27]. They suggested that the recruitment period, patient selection bias, variability in the immunohistochemical assays, and different cutoff points were responsible factors for these discrepant findings. Differences in study methodology and statistical variables might be included among those factors.
We found that high expression of Ki-67 was related to higher tumor stage (p=0.003), grade (p<0.001), and 2-year recurrence (p=0.019), but did not have an independent prognostic value in terms of tumor recurrence. This result agrees with those of Quintero et al and Yurakh et al, but is in contrast with the findings of other researchers [26,28,29].
Given the heterogeneity of urothelial carcinomas, larger-scale studies of Korean patients are necessary in an ongoing work.
CONCLUSIONS
Molecular markers may provide crucial information in patient management of bladder cancer, which shows highly heterogeneous clinical behavior. The FGFR3 mutation is known to be related to lower grade and stage in bladder cancer and possibly to better patient outcome. Although most of the present study focused on mutation analysis, our work provides evidence that immunohistochemical expression of cytoplasmic FGFR3 is not only one of the characteristic features of low-grade and low-stage urothelial carcinoma, but also of prognostic value for recurrence-free survival, although it was not an independent marker. We suggest that FGFR3 has a high chance of being a useful tool in clinical decision making for patient management.
Footnotes
The authors have nothing to disclose.
This paper was supported by the research fund of Jeju National University Hospital.
References
- 1.Holm C, Rayala S, Jirström K, Stal O, Kumar R, Landberg G. Association between Pak1 expression and subcellular localization and tamoxifen resistance in breast cancer patients. J Natl Cancer Inst. 2006;98:671–680. doi: 10.1093/jnci/djj185. [DOI] [PubMed] [Google Scholar]
- 2.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–475. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 3.L'Hote CG, Knowles MA. Cell responses to FGFR3 signalling: growth, differentiation and apoptosis. Exp Cell Res. 2005;304:417–431. doi: 10.1016/j.yexcr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Barbisan F, Santinelli A, Mazzucchelli R, Lopez-Beltran A, Cheng L, Scarpelli M, et al. Strong immunohistochemical expression of fibroblast growth factor receptor 3, superficial staining pattern of cytokeratin 20, and low proliferative activity define those papillary urothelial neoplasms of low malignant potential that do not recur. Cancer. 2008;112:636–644. doi: 10.1002/cncr.23212. [DOI] [PubMed] [Google Scholar]
- 5.Burger M, van der Aa MN, van Oers JM, Brinkmann A, van der Kwast TH, Steyerberg EC, et al. Prediction of progression of non-muscle-invasive bladder cancer by WHO 1973 and 2004 grading and by FGFR3 mutation status: a prospective study. Eur Urol. 2008;54:835–843. doi: 10.1016/j.eururo.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Tomlinson DC, Baldo O, Harnden P, Knowles MA. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J Pathol. 2007;213:91–98. doi: 10.1002/path.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Rhijn BW, Lurkin I, Radvanyi F, Kirkels WJ, van der Kwast TH, Zwarthoff EC. The fibroblast growth factor receptor 3 (FGFR3) mutation is a strong indicator of superficial bladder cancer with low recurrence rate. Cancer Res. 2001;61:1265–1268. [PubMed] [Google Scholar]
- 8.Gómez-Román JJ, Saenz P, Molina M, Cuevas González J, Escuredo K, Santa Cruz S, et al. Fibroblast growth factor 3 is overexpressed in urinary tract carcinomas and modulates the neoplastic cell growth. Clin Cancer Res. 2005;11:459–465. [PubMed] [Google Scholar]
- 9.George B, Datar RH, Wu L, Cai J, Patten N, Beil SJ, et al. p53 gene and protein status: the role of p53 alterations in predicting outcome in patients with bladder cancer. J Clin Oncol. 2007;25:5352–5358. doi: 10.1200/JCO.2006.10.4125. [DOI] [PubMed] [Google Scholar]
- 10.Salinas-Sánchez AS, Lorenzo-Romero JG, Giménez-Bachs JM, Sánchez-Sánchez F, Donate-Moreno MJ, Rubio-Del-Campo A, et al. Implications of p53 gene mutations on patient survival in transitional cell carcinoma of the bladder: a long-term study. Urol Oncol. 2008;26:620–626. doi: 10.1016/j.urolonc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee SJ, Datar R, Youssefzadeh D, George B, Goebell PJ, Stein JP, et al. Combined effects of p53, p21, and pRb expression in the progression of bladder transitional cell carcinoma. J Clin Oncol. 2004;22:1007–1013. doi: 10.1200/JCO.2004.05.174. [DOI] [PubMed] [Google Scholar]
- 12.Shim JW, Cho KS, Choi YD, Park YW, Lee DW, Han WS, et al. Diagnostic algorithm for papillary urothelial tumors in the urinary bladder. Virchows Arch. 2008;452:353–362. doi: 10.1007/s00428-008-0585-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theodoropoulos VE, Lazaris AC, Kastriotis I, Spiliadi C, Theodoropoulos GE, Tsoukala V, et al. Evaluation of hypoxia-inducible factor 1alpha overexpression as a predictor of tumour recurrence and progression in superficial urothelial bladder carcinoma. BJU Int. 2005;95:425–431. doi: 10.1111/j.1464-410X.2005.05314.x. [DOI] [PubMed] [Google Scholar]
- 14.van Rhijn BW, Vis AN, van der Kwast TH, Kirkels WJ, Radvanyi F, Ooms EC, et al. Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB-1 is superior to pathologic grade for the prediction of clinical outcome. J Clin Oncol. 2003;21:1912–1921. doi: 10.1200/JCO.2003.05.073. [DOI] [PubMed] [Google Scholar]
- 15.Cappellen D, De Oliveira C, Ricol D, de Medina S, Bourdin J, Sastre-Garau X, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23:18–20. doi: 10.1038/12615. [DOI] [PubMed] [Google Scholar]
- 16.Chesi M, Brents LA, Ely SA, Bais C, Robbiani DF, Mesri EA, et al. Activated fibroblast growth factor receptor 3 is an oncogene that contributes to tumor progression in multiple myeloma. Blood. 2001;97:729–736. doi: 10.1182/blood.v97.3.729. [DOI] [PubMed] [Google Scholar]
- 17.van Rhijn BW, van Tilborg AA, Lurkin I, Bonaventure J, de Vries A, Thiery JP, et al. Novel fibroblast growth factor receptor 3 (FGFR3) mutations in bladder cancer previously identified in non-lethal skeletal disorders. Eur J Hum Genet. 2002;10:819–824. doi: 10.1038/sj.ejhg.5200883. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto M, Ohtsuki Y, Ochii K, Seike Y, Iseda N, Sasaki T, et al. Fibroblast growth factor receptor 3 protein expression in urothelial carcinoma of the urinary bladder, exhibiting no association with low-grade and/or non-invasive lesions. Oncol Rep. 2004;12:967–971. [PubMed] [Google Scholar]
- 19.Mhawech-Fauceglia P, Cheney RT, Fischer G, Beck A, Herrmann FR. FGFR3 and p53 protein expressions in patients with pTa and pT1 urothelial bladder cancer. Eur J Surg Oncol. 2006;32:231–237. doi: 10.1016/j.ejso.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 20.van Oers JM, Wild PJ, Burger M, Denzinger S, Stoehr R, Rosskopf E, et al. FGFR3 mutations and a normal CK20 staining pattern define low-grade noninvasive urothelial bladder tumours. Eur Urol. 2007;52:760–768. doi: 10.1016/j.eururo.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Hernández S, López-Knowles E, Lloreta J, Kogevinas M, Amorós A, Tardón A, et al. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J Clin Oncol. 2006;24:3664–3671. doi: 10.1200/JCO.2005.05.1771. [DOI] [PubMed] [Google Scholar]
- 22.Røtterud R, Fossa SD, Nesland JM. Protein networking in bladder cancer: immunoreactivity for FGFR3, EGFR, ERBB2, KAI1, PTEN, and RAS in normal and malignant urothelium. Histol Histopathol. 2007;22:349–363. doi: 10.14670/HH-22.349. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto K, Yamada Y, Okajima E, Kakizoe T, Sasaki H, Sugimura T, et al. Frequent association of p53 gene mutation in invasive bladder cancer. Cancer Res. 1992;52:1393–1398. [PubMed] [Google Scholar]
- 24.Llopis J, Alcaraz A, Ribal MJ, Solé M, Ventura PJ, Barranco MA, et al. P53 expression predicts progression and poor survival in T1 bladder tumours. Eur Urol. 2000;37:644–653. doi: 10.1159/000020232. [DOI] [PubMed] [Google Scholar]
- 25.Ecke TH, Sachs MD, Lenk SV, Loening SA, Schlechte HH. TP53 gene mutations as an independent marker for urinary bladder cancer progression. Int J Mol Med. 2008;21:655–661. [PubMed] [Google Scholar]
- 26.Brunner A, Verdorfer I, Prelog M, Mayeri C, Mikuz G, Tzankov A. Large-scale analysis of cell cycle regulators in urothelial bladder cancer identifies p16 and p27 as potentially useful prognostic markers. Pathobiology. 2008;75:25–33. doi: 10.1159/000113792. [DOI] [PubMed] [Google Scholar]
- 27.Malats N, Bustos A, Nascimento CM, Fernandez F, Rivas M, Puente D, et al. P53 as a prognostic marker for bladder cancer: a meta-analysis and review. Lancet Oncol. 2005;6:678–686. doi: 10.1016/S1470-2045(05)70315-6. [DOI] [PubMed] [Google Scholar]
- 28.Quintero A, Alvarez-Kindelan J, Luque RJ, Gonzalez-Campora R, Requena MJ, Montironi R, et al. Ki-67 MIB1 labelling index and the prognosis of primary TaT1 urothelial cell carcinoma of the bladder. J Clin Pathol. 2006;59:83–88. doi: 10.1136/jcp.2004.022939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yurakh AO, Ramos D, Calabuig-Fariñas S, López-Guerrero JA, Rubio J, Solsona E, et al. Molecular and immunohistochemical analysis of the prognostic value of cell-cycle regulators in urothelial neoplasms of the bladder. Eur Urol. 2006;50:506–515. doi: 10.1016/j.eururo.2006.03.027. [DOI] [PubMed] [Google Scholar]