Abstract
Purpose
We evaluated the efficacy and the availability of laparoscopic orchiopexy to manage a nonpalpable intra-abdominal testis and studied outcomes including the testicular survival rate and associated complications.
Materials and Methods
We retrospectively reviewed the medical records of 67 children (86 testicular units) who underwent laparoscopic orchiopexy for a nonpalpable intra-abdominal testis between 1996 and 2008. The mean patient age was 2.4 years (median, 1 year; range, 0.5-9 years), and the mean follow-up period was 21.8 months (range, 0.3-138.4 months). Testicular viability and orchiopexed positioning were evaluated within 1 month and beyond 3 months.
Results
Of 86 testes, 69 testes were treated with primary laparoscopic orchiopexy (PLO) sparing the internal spermatic vessel, 14 testes were treated with one-stage Fowler-Stephens laparoscopic orchiopexy 1, and 3 testes were treated with two-stage Fowler-Stephens laparoscopic orchiopexy 2. The testicular survival rates were 97.7% (84/86) within 1 month and 93.7% (59/63) beyond 3 months. Of 59 viable testes followed up beyond 3 months, 48 (81.4%) testes were positioned in the lower scrotum and 11 (18.6%) testes in the mid to high scrotum.
Conclusions
Laparoscopic orchiopexy was successful for a nonpalpable intra-abdominal testis with a high testicular survival rate irrespective of the location from the internal ring. However, atrophy of the testis or upward migration of the testis can occur during follow-up, so we suggest watchful, periodic follow-up evaluating the viability and location of orchiopexed testes that are located in the lower scrotum in the immediate postoperative period or during short-term follow-up.
Keywords: Testis, Laparoscopy
INTRODUCTION
An undescended testis is one of the most common clinical disorders of childhood, occurring in approximately 3% of full-term newborns, 21% of premature newborns, and 0.8-1.8% of 1-year-old boys [1-3]. In 80% of these boys, a testis is palpable in the groin, and in 90% of these boys, it is associated with hernia. In these cases, conventional open orchiopexy has been accepted as a standard treatment. In 20% of cases of an undescended testis, a testis is nonpalpable, however, and in 20-50% of nonpalpable testis, the testis is absent. Currently, diagnostic laparoscopy followed by laparoscopic orchiopexy has been accepted as the diagnostic tool of choice to confirm the existence of the nonpalpable testis and to identify the size and location of the testis [3-5]. Since 1976 when Cortesi and associates first described laparoscopic diagnosis of a nonpalpable testis [6], this method for diagnosing a nonpalpable testis has been established as the most reliable method. Since 1992 when the first laparoscopic orchiopexy was reported by Jordan et al [7], laparoscopic orchiopexy has obtained wide popularity with technologic advances in laparoscopic devices. The final goals of orchiopexy are to keep the testis viable and to optimally position the testis. We aimed to evaluate the efficacy and the availability of laparoscopic orchiopexy to manage a nonpalpable intra-abdominal testis by performing a retrospective review of patients' records concerning testicular viability and orchiopexed positioning.
MATERIALS AND METHODS
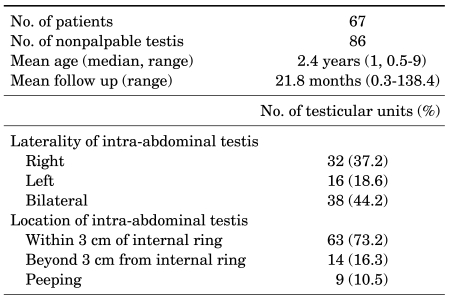
We reviewed the medical records including postoperative clinical results and complications of 67 patients (86 testicular units) who underwent laparoscopic orchiopexy for a nonpalpable intra-abdominal testis between September 1996 and April 2008. The age of the children when undergoing the laparoscopic orchiopexy ranged from 0.5 to 9 years. The mean age of the patients was 2.4±2.2 years and the median age was 1 year. The mean period of clinical follow-up was 21.8±20 months, ranging from 0.3 to 138.4 months. By analyzing the diagnostic laparoscopy, we could classify 67 patients (86 testicular units) who underwent laparoscopic orchiopexy for a nonpalpable intra-abdominal viable testis according to the laterality of the intra-abdominal testis: 48 patients (48 testicular units) had a unilateral nonpalpable intra-abdominal testis, 32 testicular units (37.2%) demonstrated right-sided, 16 testicular units (18.6%) showed left-sided, and 38 testicular units (44.2%) were bilateral nonpalpable intra-abdominal testes (Table 1).
TABLE 1.
Patient characteristics
Of 86 testicular units, 69 testicular units (80.2%) were treated by primary laparoscopic orchiopexy (PLO). The diagnostic laparoscopy showed that 58 testicular units were located within 3 cm of the internal inguinal ring, 2 testicular units were more than 3 cm from the internal inguinal ring, and 9 testicular units were peeping testes. Of 86 testicular units, 17 testicular units (19.8%) were treated by Fowler-Stephens laparoscopic orchiopexy (FSLO). One-stage FSLO was performed in 14 testicular units (16.3%) and two-stage FSLO in 3 testicular units (3.5%) for which the nonpalpable testis was located more than 3 cm from the internal inguinal ring.
All of the laparoscopic orchiopexy procedures were done under general anesthesia. A patient was placed supine in the frog-leg position and in the 30 degrees Trendelenburg position. After general anesthesia was administered, the bladder was emptied with a Nélaton catheter and a nasogastric tube was not inserted. A transverse incision was made 1 cm above or below the umbilicus. Intra-abdominal CO2 was injected by a Veress needle and intra-abdominal pressure was maintained at 10-12 mmHg. A 5 mm port was inserted for the diagnostic laparoscopy and two additional sets of 5 mm working ports were placed symmetrically on the bilateral lower abdomen. The laparoscopic grasper and scissor were used to make up enough lengths of the spermatic cord for a testis to be fixed in the scrotum, and a transverse incision was made on the scrotum, making a subdartos pouch. Looking into the abdominal cavity through the laparoscopy, we perforated the peritoneum on the lateral side of the umbilical ligament and the medial side of the anterior inferior epigastric vessel. The intra-abdominal testis was pulled down into the lowest part of scrotum and fixed there.
The clinical results were evaluated within 1 month after the laparoscopic orchiopexy on the basis of the complications that occurred during the operation and the clinical features at the first postoperative clinical follow-up. All of the 67 patients (86 testicular units) were examined within 1 month. Their clinical results were re-evaluated based on follow-ups that took place at 3 three months after the operation. Of 67 patients (86 testicular units), 48 patients (63 testicular units) were re-examined, excluding 19 patients who did not make the second visit for the postoperative clinical follow-up. Success of the laparoscopic orchiopexy was defined when the orchiopexed testis was palpable in the lower scrotum without evident volume loss during the physical examination by a single surgeon or when no apparent volume loss was shown in the additional ultrasonography compared with the preoperative volume of the testis.
The patients were classified into two different groups according to the testicular location in the scrotum after the laparoscopic orchiopexy, and the SPSS computer software program (version 12.0K for Window) was used to compile statistics on the ages of these two groups. Student's t test was conducted, and p-values of less than 0.05 were considered statistically significant.
RESULTS
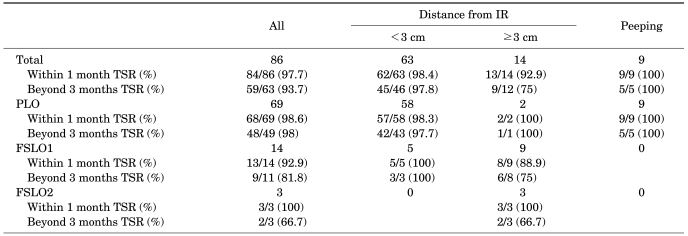
The clinical results within 1 month after the laparoscopic orchiopexy showed that 84 of 86 testicular units survived, giving a testicular survival rate (TSR) within 1 month of 97.7%. Of two testicular units that failed to survive, one testicular unit atrophied within one month and the other testicular unit underwent laparoscopic orchiectomy because of an intra-operative complication. Clinical results beyond 3 months after the laparoscopic orchiopexy were available for only 63 out of 86 testicular units, excluding 19 patients (23 testicular units) who did not make the second visit for the postoperative clinical follow-up. Of these 63 testicular units, 59 testicular units survived, resulting in a TSR beyond 3 months of 93.7%. Among four testicular units that did not survive, two testicular units were the ones that did not survive 1 one month as stated earlier and one testicular unit receiving one-stage FSLO and another receiving two-stage FSLO underwent atrophy (Table 2).
TABLE 2.
Testicular survival outcomes of laparoscopic orchiopexy according to the laparoscopic technique and the location of the nonpalpable intra-abdominal testis
IR: internal ring, TSR: testicular survival rate, PLO: primary laparoscopic orchiopexy, FSLO1: 1 stage Fowler-Stephens laparoscopic orchiopexy, FSLO2: 2 stage Fowler-Stephens laparoscopic orchiopexy
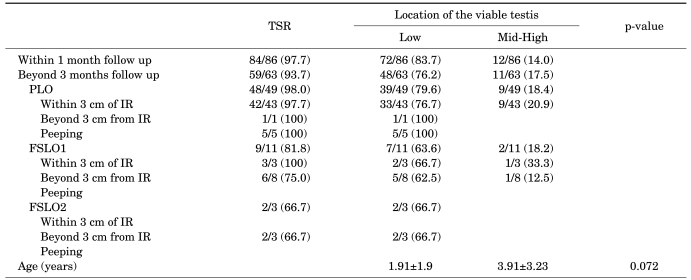
Physical examination of the location of the viable testis within 1 month after the laparoscopic orchiopexy demonstrated that 83.7% were located in the lower scrotum and 14.0% in the upper scrotum; however, when the location was examined beyond 3 months, upward migration of the viable testis was found in some cases. Consequently, we confirmed that 76.2% were located in the lower scrotum and 17.5% in the upper scrotum; therefore, the success rate of the laparoscopic orchiopexy for a nonpalpable intra-abdominal testis was 76.2% (Table 3).
TABLE 3.
Location of the viable testis according to laparoscopic technique
IR: internal ring, PLO: primary laparoscopic orchiopexy, TSR: testicular survival rate, FSLO1: 1 stage Fowler-Stephens laparoscopic orchiopexy, FSLO2: 2 stage Fowler-Stephens laparoscopic orchiopexy
DISCUSSION
With the patient under general anesthesia, we analyzed 86 testicular units that were not palpable by a single surgeon in the physical examination. We examined the diagnostic laparoscopy before the laparoscopic orchiopexy and chose cases of viable testes with well-developed internal spermatic vessels and vas deferens to actually undergo the laparoscopic orchiopexy. The following testicular units were excluded from our analysis. First, we excluded testicular units that had received conventional open orchiopexy by an inguinal incision instead of laparoscopic orchiopexy. In some cases, such units were initially diagnosed as nonpalpable intra-abdominal testes in the physical examination but were palpable under the general anesthesia. Second, some testes did not undergo the orchiopexy or underwent laparoscopic orchiectomy instead of the laparoscopic orchiopexy because they were diagnosed as nubbin testes or vanishing testes in the diagnostic laparoscopy. These two cases were ruled out from this study.
A total of 48 patients (48 testicular units) had a unilateral nonpalpable intra-abdominal testis and 19 patients (38 testicular units) had bilateral nonpalpable intra-abdominal testes. In the physical examination performed under general anesthesia, however, we found that 7 of the cases of bilateral intra-abdominal testes were actually unilateral ones that were palpable in the inguinal area. Therefore, in these 7 cases, laparoscopy was not used and unilateral conventional open orchiopexy via an inguinal incision was performed.
The choice of laparoscopic technique mostly depended on the distance from the internal inguinal ring to the nonpalpable intra-abdominal testis, which could be determined for 67 patients (86 testicular units). A total of 63 testicular units were located within 3 cm of the internal inguinal ring, 14 testicular units were more than 3 cm from the internal inguinal ring, and 9 testicular units showed peeping.
Samadi et al conducted PLO in 70.5% and FSLO in 29.5% of a total of 203 testicular units and reported a success rate of 95%, which was higher than the 76% success rate of open surgery [8]. Lindgren et al did a 6-month clinical follow-up after laparoscopic orchiopexy and reported a success rate of 93% [9]. Lintula et al reported a success rate of 88% for 19 testicular units undergoing laparoscopic orchiopexy and a success rate of 82% for 18 testicular units receiving open surgery, highlighting the excellent surgical outcomes of the laparoscopic orchiopexy [10]. We performed PLO in 80.2% and FSLO in 19.2% of 86 testicular units in this study. In our examination that took place 3 months after the laparoscopic orchiopexy, the TSR was 93.7% and the rate of fixation in the lower scrotum was 76.2%. If classified on the basis of laparoscopic techniques, the PLO showed a fixation rate in the lower scrotum of 79.6%, one-stage FSLO showed a rate of 63.6%, and two-stage FSLO showed a rate of 66.7%. These outcomes were slightly lower than those reported in previous studies, but the successful results in this study confirm the clinical significance of laparoscopic orchiopexy for a nonpalpable intra-abdominal testis. Chang and Franco performed FSLO in 48 testicular units and reported that the success rate of one-stage FSLO was 94.3% and also proved that one-stage FSLO and two-stage FSLO in combination led to a success rate as high as 91.1% [11]. Elder conducted two-stage FSLO in 12 testicular units and reported a success rate of 92% [12]. When judged against these figures, the results of the FSLO in the present study were relatively unsatisfactory, but the results were analyzed in only 17 testicular units. We expect improved results in the near future after accumulating experience with FSLO for nonpalpable intra-abdominal testis.
Two cases developed complications within 1 month after the laparoscopic orchiopexy. In one case, we performed PLO in a patient with bilateral nonpalpable intra-abdominal testes within 3 cm of the internal inguinal ring, but testicular atrophy was detected in the left testis in the physical examination at the 1 month clinical follow-up. In the other case, the patient had bilateral nonpalpable intra-abdominal testes more than 3 cm from the internal inguinal ring, and one-stage FSLO was used to dissect the intra-abdominal testis fixed to the small intestine. However, the vas deferens was injured during the operation, which led to the laparoscopic unilateral orchiectomy. In one case of an intra-operative complication, transient hypercapnia and reactive tachycardia occurred during the PLO; therefore, we had to halt the procedure for 30 minutes and re-started the operation after the heart rate and arterial blood gas analysis were normalized through hyperventilation.
Following are more detailed results of the laparoscopic orchiopexy performed by the authors, highlighting the postoperative location of the viable testis in the scrotum. A total of 79.6% in the PLO group were successfully fixed in the lower scrotum and 64.3% in the FSLO group. When classifying the FSLO into one-stage and two-stage FSLO, the success rate of the one-stage FSLO was 63.6% and that of the two-stage FSLO was 66.7%. When analyzing the ages of patients statistically in the SPSS software program by use of the student's t test, we found that the mean age of the patients whose testes were fixed in the lower scrotum was 1.91±1.9 years and the mean age of the patients whose testes were fixed in the upper scrotum was 3.91±3.23 years. The age of patients whose testes were located in the lower scrotum was therefore younger, but the difference was not significant statistically (p=0.072) (Table 3). When analyzing the postoperative location of the viable testis according to the period of clinical follow-up, 83.7% were fixed in the lower scrotum and 14.0% in the upper scrotum within 1 month; however, 76.2% were located in the lower scrotum and 17.5% in the upper scrotum beyond 3 months. Upward migration of the viable testis occurred in some cases. We conclude that long-term clinical follow-up on the location of the fixed testis is required even if there is a satisfactory result right after or 1 month after the operation.
CONCLUSIONS
Laparoscopic orchiopexy for a nonpalpable intra-abdominal testis shows a high success rate and high survival rate of the testes regardless of the distance from the internal inguinal ring to the nonpalpable intra-abdominal testis. In the follow-up after fixing the viable testes in the lower scrotum, however, atrophy or the upward migration of the testis was detected in some cases. Therefore, long-term and vigilant clinical follow-up is recommended even after successful laparoscopic orchiopexy.
Footnotes
The authors have nothing to disclose.
References
- 1.Scorer CG. The descent of the testis. Arch Dis Child. 1964;39:605–609. doi: 10.1136/adc.39.208.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levitt SB, Kogan SJ, Engel RM, Weiss RM, Martin DC, Ehrlich RM. The impalpable testis: a rational approach to management. J Urol. 1978;120:515–520. doi: 10.1016/s0022-5347(17)57256-0. [DOI] [PubMed] [Google Scholar]
- 3.Choi K, Park T, Kim KS. Laparoscopic orchiopexy for intra-abdominal testis: complications and technical aspects. Korean J Urol. 2000;41:420–424. [Google Scholar]
- 4.Baker LA, Docimo SG, Surer I, Peters C, Cisek L, Diamond DA, et al. A multi-institutional analysis of laparoscopic orchidopexy. BJU Int. 2001;87:484–489. doi: 10.1046/j.1464-410x.2001.00127.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee A, Yun JM, Park MS, Choi H. Therapeutic laparoscopy for impalpable testis. Korean J Urol. 1997;38:848–854. [Google Scholar]
- 6.Cortesi N, Ferrari P, Zambarda E, Manenti A, Baldini A, Morano FP. Diagnosis of bilateral abdominal cryptorchidism by laparoscopy. Endoscopy. 1976;8:33–34. doi: 10.1055/s-0028-1098372. [DOI] [PubMed] [Google Scholar]
- 7.Jordan GH, Robey EL, Winslow BH. Laparoendoscopic surgical management of the abdominal/transinguinal undescended testicle. J Endourol. 1992;6:157–161. [Google Scholar]
- 8.Samadi AA, Palmer LS, Franco I. Laparoscopic orchiopexy: report of 203 cases with review of diagnosis, operative technique, and lessons learned. J Endourol. 2003;17:365–368. doi: 10.1089/089277903767923128. [DOI] [PubMed] [Google Scholar]
- 9.Lindgren BW, Darby EC, Faiella L, Brock WA, Reda EF, Levitt SB, et al. Laparoscopic orchiopexy: procedure of choice for the nonpalpable testis? J Urol. 1998;159:2132–2135. doi: 10.1016/S0022-5347(01)63294-4. [DOI] [PubMed] [Google Scholar]
- 10.Lintula H, Kokki H, Eskelinen M, Vanamo K. Laparoscopic versus open orchidopexy in children with intra-abdominal testes. J Laparoendosc Adv Surg Tech A. 2008;18:449–456. doi: 10.1089/lap.2007.0176. [DOI] [PubMed] [Google Scholar]
- 11.Chang M, Franco I. Laparoscopic Fowler-Stephens orchiopexy: the Westchester Medical Center experience. J Endourol. 2008;22:1315–1319. doi: 10.1089/end.2008.0158. [DOI] [PubMed] [Google Scholar]
- 12.Elder JS. Two-stage Fowler-Stephens orchiopexy in the management of intra-abdominal testes. J Urol. 1992;148:1239–1241. doi: 10.1016/s0022-5347(17)36871-4. [DOI] [PubMed] [Google Scholar]