Abstract
Severe psychological stress in the first trimester of pregnancy increases the risk of schizophrenia in the offspring. To begin to investigate the role of glucocorticoid receptors in this association, we determined the effects of the glucocorticoid dexamethasone (2 mg/kg), administered to pregnant rats on gestation days 6–8, on maternal behaviors and schizophrenia-relevant behaviors in the offspring. Dams receiving dexamethasone exhibited increased milk ejection bouts during nursing. Offspring of dexamethasone-treated dams (DEX) showed decreased juvenile social play and a blunted acoustic startle reflex in adolescence and adulthood, effects that were predicted by frequency of milk ejections in the dams. DEX offspring also showed increased prepulse inhibition of startle and reduced amphetamine-induced motor activity, effects not correlated with maternal behavior. It is postulated that over-stimulation of receptors targeted by glucocorticoids in the placenta or other maternal tissues during early gestation can lead to psychomotor and social behavioral deficits in the offspring. Moreover, some of these deficits may be mediated by alterations in postnatal maternal behavior and physiology produced by early gestational exposure to excess glucocorticoids.
Keywords: hypothalamic–pituitary–adrenal axis, stress hormones, dexamethasone, prenatal stress, schizophrenia, autism, acoustic startle response, social play, psychomotor development, sensorimotor gating, rat
Introduction
Psychosocial stress during pregnancy increases the risk of psychiatric illness in offspring, including the risk of schizophrenia, ADHD, autism, and mood disorders (Beversdorf et al., 2005; Holmes & Seckl, 2006; Khashan et al., 2008; Kinney, Miller, Crowley, Huang, & Gerber, 2008; Laplante et al., 2004; Malaspina et al., 2008; Susser & Lin, 1992). Moreover, the effects of prenatal exposure to stressors appear to depend critically on the timing of the exposure. For example, experience of severe stress by the mother significantly increases the risk for schizophrenia in the offspring only if it occurs in the first trimester of pregnancy (Khashan et al., 2008; Malaspina et al., 2008; Susser & Lin, 1992). Studies in animals have also shown that exposures to stressors or stress-related hormone analogues during pregnancy produce psychophysiological, cognitive and other behavioral abnormalities in the offspring (Estanislau & Morato, 2006; Lobel et al., 2008; Maccari & Morley-Fletcher, 2007; Nagano, Ozawa, & Suzuki, 2008; O'Regan, Kenyon, Seckl, & Holmes, 2008; Seckl, 2008). Schizophrenia-related behavioral phenotypes produced by prenatal exposure to stressors or glucocorticoids include changes in acoustic startle reflex and prepulse inhibition (PPI) of startle, social play behavior, and spontaneous and amphetamine-induced motor activity (Hauser, Feldon, & Pryce, 2006; Hougaard et al., 2005; Lee, Brady, Shapiro, Dorsa, & Koenig, 2007).
Given the multiple, gestational stage-dependent effects of glucocorticoid receptor stimulation on embryonic development, this mechanism may in part underlie the stage-dependent effects of maternal stress on behavior of the offspring (Kapoor, Dunn, Kostaki, Andrews, & Matthews, 2006; Michael & Papageorghiou, 2008; Seckl, 2008). One approach to investigating this possibility is to administer synthetic glucocorticoids, such as dexamethasone (DEX), to pregnant rodents at specific periods during gestation. Administration of DEX to pregnant rat dams during mid- to late gestation produces alterations in hypothalamic–pituitary–adrenal axis function, brain serotonin and dopamine neurotransmission, and behavior in the offspring (Hauser et al., 2006; Hossain et al., 2008; Hougaard et al., 2005; Nagano et al., 2008; Slotkin, Kreider, Tate, & Seidler, 2006). There are very few studies in animal models in which maternal stress or glucocorticoid exposure has been limited to early gestation (Hauser et al., 2007; Kranendonk et al., 2006; Mueller & Bale, 2008; Woods & Weeks, 2005), but where effects of early and late gestational exposure have been compared, they have been found to differ.
Determining the effects of early gestational glucocorticoid exposure might inform studies on the association between severe psychosocial stress in early pregnancy and mental illness in the offspring (Malaspina et al., 2008). Moreover, such data are important for understanding potential effects of treatments for congenital hyperplasia (Lajic, Nordenstrom, & Hirvikoski, 2008; Meyer-Bahlburg et al., 2004) and infertility (Michael & Papageorghiou, 2008), which involve administration of synthetic glucocorticoids during early pregnancy. Thus, we examined in the rat the effects of early gestational exposure of the pregnant dam to DEX on the behavior in the offspring. Offspring were assessed at juvenile, adolescent, and adult ages (Spear, 2000). We report that early gestational DEX treatment leads to psychomotor and social behavioral abnormalities in the offspring, some of which may be mediated by effects of this exposure on maternal behavior.
Methods
Animals
The experiment was approved by the Institutional Animal Care and Use Committees at the New York State Psychiatric Institute and Columbia University. Twenty Wistar females (Hilltop, Scottdale, PA) were time-mated at approximately 80 days of age and shipped to the laboratory on gestation day (GD) 2.
Prenatal Treatment and Postnatal Culling and Weaning of Litters
On GDs 6, 7, and 8, ten pregnant rats were injected (i.p.) with dexamethasone (DEX; Sigma, St. Louis, MO), prepared in isotonic saline at a concentration of 2.0 mg/ml and injected at a dose of 2.0 mg/kg. Controls (CON; n = 10) were injected with 1.0 ml/kg of isotonic saline. Once the dams gave birth, the number of pups was counted, and each litter was culled to eight pups. Litters with fewer than eight pups were not included in the study. Birth weights of all of the pups were measured, and all pups used in the study were kept with their birth mothers. The pups from each cohort were weaned from their mothers on postnatal day (PD) 23, and pair- or triple-housed with a same-sex littermate except as noted below.
Maternal Behavior
Maternal behavior was recorded on PDs 8 and 9. The observations were conducted during thirty 10-s periods during which the examiner noted the following: mother's location (in nest vs. out of nest); one of three nursing postures (arched, flat on top of pups, or on her side); milk ejection (evidenced by more dramatic arch in mother's back and activation of pups); licking (anogenital or other).
Play Behavior in Offspring
After weaning, two females and two males from each litter were randomly selected to be tested for social play behavior. These animals were singly housed from postnatal day (PD) 25 until the completion of play testing on PD 29. Test chambers consisted of 17.5″ square × 25″ (h) poly(methyl methacrylate) boxes lined with standard chip bedding (Beta Chip, Nepco, Warrensburg, NY) that were located in a small, sound-attenuated, lighting- and temperature-controlled room. Animals were habituated and tested during their light cycle, but under red-light conditions. Same-sex pairs of littermates were used for play testing; each pair was assigned to a specific test chamber which remained in the same location/orientation and contained the same bedding throughout the experiment. On PDs 26 and 27, animals were individually habituated to their assigned test chamber for 10–15min. On PDs 28 and 29, pairs were placed in their testing cages and allowed to interact for 10 min. During this time, animals were filmed with no experimenter in the room. Films were scored by experimenters blinded to the prenatal treatment of the animals. Anogenital/flank sniffing, walkovers, rears, and pins were identified according to the previously published criteria (Panksepp, 1981; Pellis, Pellis, & Whishaw, 1992); frequencies of these behaviors were recorded with the aid of the time-stamping shareware Stopwatch+© (Center for Behavioral Neuroscience [www.cbn-atl.org], David Brown, designer).
Acoustic Startle Reflex and Prepulse Inhibition of Startle in Offspring
Offspring were tested on PDs 48–52, then again in adulthood (PDs 74–78) for the acoustic startle reflex and PPI of startle. All stimuli were delivered and startle responses were measured in startle monitoring systems by Hamilton-Kinder (SM100, SM1001) with custom-made enclosure inserts for juvenile rats (Scientific Design, Pittsburgh, PA). Rats were placed individually into poly(methyl) methacrylate enclosures with the adjustable lids lowered to about 1/8″ above the highest point on the rat's dorsal surface. The size of the chamber allowed the rat to turn but not rear. The enclosure was, in turn, mounted in a sound-attenuating chamber on a platform which measures the force produced by the startle response. During each session, rats were habituated to a background noise level 60 dB for 5 min, after which an ascending then descending series of 40 ms noise bursts ranging from 88 to 120 dB separated by varying intertrial intervals (ITIs) (15 ± 6 s) was used to determine acoustic startle threshold and habituate the rats to the startle stimulus. Following this block of stimuli, average baseline acoustic startle response and PPI were measured with a pseudo-randomized presentation of “startle-only” and “prepulse-startle” trials, as follows: In one-quarter of the trials, the 110 dB startle stimulus was presented alone; on the other 75% of the trials, a “prepulse” (20 ms noise burst with loudness at 2, 4, 8, 12, or 16 dB above background) preceded the startle tone with an interstimulus interval (ISI) of 100 ms. Displacement of the animal was measured for 200 ms after the onset of the startle stimulus. Data from 8 to 12 post-habituation trials of each of the above-described prepulse-startle and startle-stimulus-alone trial types were averaged. The baseline acoustic response was calculated as the average displacement on startle stimulus-only trials. PPI of startle was quantified as a percentage decrease from the baseline acoustic startle response (1 − [PPx/ST], where PP = mean response on prepulse-startle trials at the prepulse loudness “x,” and ST = mean response on startle-only trials interspersed among prepulse-startle trials).
Spontaneous and Amphetamine-Induced Motor Activity
Spontaneous and amphetamine-induced motor activity was tested in adult offspring DEX and CON offspring at ages PDs 80–90. Three males and three females from the six control and five DEX litters were tested. Each male and female within a litter was randomly assigned to one of three dose groups: saline, amphetamine 1.0 mg/kg, or amphetamine 5.0 mg/kg. Rats were acclimated to handling and to the testing room prior to the experiment. The apparatus consisted of 17.5″ square × 25″ (h) poly(methyl) methacrylate boxes; horizontal was estimated from breaks in infrared beams (two 17″ × 17″ grids placed at heights of 2″ and 5″ from the floor; VersaMax System, Accuscan, Columbus, OH). Rats were naïve to the test chamber. Rats were placed in the chamber and activity was recorded for 30 min. Rats were then injected with saline, 1.0 or 5.0 mg/kg amphetamine (i.p.) and activity was measured for an additional 80 min. Data were expressed as ambulation or stereotypy averages for the three 10-min bins during habituation and eight 10-min bins following injection.
Statistical Analyses
Statistical analyses were performed using SPSS (SPSS, Inc., Chicago, IL) or SYSTAT (San Jose, CA) statistical softwares. Except as noted below, to avoid confounding litter effects with the factors of interest, data from same-sex littermates were averaged. For all experiments, offspring of both sexes were included; however, because sex effects were not the focus of this study, where no significant effects of sex were found, the final analyses did not include sex as a factor. However, we also note that because we did not monitor ovarian cycle in the female offspring, we may have missed sex differences detectable only during specific stages of the cycle.
The effects of prenatal treatment on maternal body weights, litter size, and litter averages of birth, weaning weights of the pups and each of the maternal behaviors scored were analyzed with Student's t-tests. For play behavior, the frequencies of each behavior were averaged across same-sex littermates, and the effects of prenatal treatment and sex on each outcome were analyzed with univariate ANOVAs.
For the acoustic startle response and PPI of startle, data were averaged across three males and across three females from each litter at each testing age. Acoustic startle and PPI of startle were analyzed separately with repeated-measures ANOVAs using startle stimulus (startle or prepulse) loudness and testing age as the repeated or within-litter measures, and prenatal treatment and sex as between-subjects factors. Significant main effects of prenatal treatment or interaction of prenatal treatment with other factors prompted analyses of the effects of the significant factors on previously defined characteristics of the startle reflex amplitude or PPI functions (Martin-Iverson & Stevenson, 2005). Briefly, individual startle amplitude and PPI curves, plotted as a function of startle stimulus loudness or prepulse loudness, respectively, were fitted to sigmoidal functions. The best fit was obtained by setting the maximal response to the individual animals' maximal response, determined empirically. The following characteristics of the curve were compared across groups: maximal response (an indicator of response capacity), the stimulus salience level at which the half-maximal response occurred (a measure of stimulus potency), and the slope of the dynamic range of the curve (a measure of response efficiency) (Martin-Iverson & Stevenson, 2005). The dynamic range is bounded by the stimulus levels associated with the greatest rates of change in the slope of the function. For all animals this was at approximately 32 and 50 dB above background for the startle stimulus and 4 and 12 dB above background for prepulse stimuli. Student's t-tests or univariate ANOVAs were used to analyze the effects of prenatal DEX treatment or its interaction with other factors on these startle and PPI characteristics.
For analysis of spontaneous locomotor activity and habituation to the testing chamber, data were averaged for the three males and three females for each litter across the three 10-min time blocks following placement of the rats in the test chamber. A mixed ANOVA was then used in which time block was the within subjects factor and prenatal treatment and sex were between subjects factors. For the post-injection period, ambulation and stereotypy were analyzed separately. For ambulation, the response to the drug was isolated and summarized for each subject by averaging activity from 20 to 60 min post-injection (after which locomotion rapidly declined to baseline levels). For stereotypy (a response that persists longer than ambulation), the average post-injection response from 20 to 80 min post-injection was used. These averages were analyzed separately with mixed ANOVAs including dose as a within-litter factor, and prenatal treatment and sex as between-subjects factors.
Results
As noted in the Methods Section, where initial analyses found no significant effects of sex or interactions of sex with prenatal treatment, this factor was not included the final analyses.
Weight Changes in Dams during Pregnancy
Of those dams whose litters were included in all postnatal analyses, DEX-treated dams lost 10.8 ± 1.4 g (mean ± SEM), between gestational day 6 (before the first DEX injection) and day 8. In contrast, saline-injected (CON) dams gained 7.8 ± 1.2 g over this period (t[9] = 9.86, p < .001). However, the mean weight gain from gestational day 6 to day 20 of gestation was similar between DEX-injected (126 ± 7 g) and CON dams (128 ± 4 g) (p > .8), and just before birth, these groups showed no significant difference in mean body weight (CON mean weight of 374 ± 6 g; DEX mean weight 361 ± 6 g) (t[9] = 1.52, p = .16).
Litter Sizes and Birth Weights
Of the total of 20 time-mated females, 8 DEX-treated and 9 CON dams delivered litters. All animals delivered either late on Day 22 or on Day 23 of gestation. The average litter size for the DEX-treated dams was 9.5 ± 2.0 (mean ± SE) which did not differ significantly from the litter size of CON dams (11.3 ± 1.0, t[14] .84, p = .42). However, we note that whereas no CON dam gave birth to less than eight pups, three of the eight DEX dams had litter sizes of less than 6. There was also a trend for DEX litters to have a lower percentage of males (53.2 ± 5.4 for CON litters vs. 37.2 ± 7.4 for DEX litters; t[14] = 1.75, p = .10). There was no effect of prenatal treatment on the average birth weights of male (CON: 7.1 ± .2 g; DEX 7.6 ± .2 g, p > .20) or female (CON: 6.8 ± .4 g, DEX 6.9 ± .4 g, p > .60) offspring. On the day of delivery, litters were culled to a minimum litter size of eight healthy pups. This resulted in elimination of the three smaller DEX litters described above and one CON litter in which one of the eight pups was runted. In addition, since all remaining DEX litters had at least four males, one CON litter with only three males was removed from the study. This yielded a total of six CON and five DEX-treated litters for further analysis. In these remaining litters, there was no difference between CON and DEX litters in the birth weights of males (CON: 30.4 ± 2.2 g, DEX 28.5 ± .9 g, p > .4) or females (CON: 24.1 ± 1.4 g, DEX 26.6 ± .9 g) (p > .5).
Maternal Behavior
A total of sixty 10-s observations of maternal behavior were made on PDs 8 and 9. There were no significant group differences in the probability of finding the dam in the nest (mean (%) ± SEM; CON: 80 ± 7, DEX: 80 ± 7), or the relative frequencies of arched-back nursing postures and passive (lying on side or back) postures, or on bouts of pup licking (Tab. 1). However, the number of times mothers were seen in the full-arch posture coinciding with pup activation—a combination of maternal and pup behavior which is associated with milk letdown and ejection—was greater in the DEX mothers (t[9] = 2.94, p < .02; Tab. 1).
Table 1. Frequencies of Maternal Behaviors in CON and DEX-Treated Dams.
| Frequenciesa (Mean ± SEM) | ||
|---|---|---|
| Behavior | CON | DEX |
| Passive nursing | 26.8 ± 4.0 | 22.6 ± 4.0 |
| Arched back nursing | 18.0 ± 3.6 | 25.4 ± 2.8 |
| Milk ejection behavior | 3.8 ± .9 | 7.4 ± .7* |
| Pup licking | 7.0 ± 2.9 | 8.6 ± 1.8 |
Out of a total of 60 observations.
p < .05.
Weights at Weaning
At weaning on PD 23, there were no significant differences in body weights between DEX and CON offspring (means (in grams) ± SEM,: CON males 61 ± 3; CON females 61 ± 2; DEX males 63 ± 3, DEX females 60 ± 2) (t[9] = .114, p = .911).
Social Play Behavior
Based on initial analyses, showing no systematic differences in play across test days, data were averaged across days and littermates for the final analysis of the effect of prenatal DEX on play. Univariate analyses showed a significant decrease in walkovers (F[1,9] = 5.2, p < .05; Fig. 1A) and a weak trend for a reduction in pins (p = .16; Fig. 1B) in DEX pups. There were no significant effects of prenatal treatment on rearing or anogenital sniffing (data not shown).
FIGURE 1.
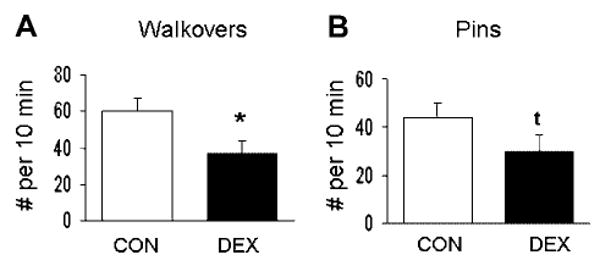
Social play behaviors in juvenile offspring of dams treated with saline (CON; n = 6; white bars) or DEX (n = 5; black bars). Bars show litter means (±SEM) for the number of walkovers (A) and pins (B) per a 10-min period of interaction between two same-sexed littermates. DEX offspring showed decreases in play behavior. *p < .05, tp = .16.
Acoustic Startle Reflex and Prepulse Inhibition of Startle
Acoustic Startle Response
Figure 2 shows the amplitude of the acoustic startle response as a function of startle stimulus loudness in CON and DEX offspring tested during the peripubertal period (Fig. 2A) or in adulthood (Fig. 2B). As expected from the literature, the startle response increased significantly with increasing loudness of the stimulus (F[5,90] = 164, p < .001). There was also main effect of testing age (F[1,18] = 106, p < .001) and interactions of prenatal treatment with other factors. There were significant two-way interactions between prenatal treatment and startle stimulus loudness (F[5,90] = 4.6, p < .01) and testing age and startle stimulus loudness (F[5,90] = 86, p < .001). Moreover, there was a significant three-way interaction among prenatal treatment, age, and stimulus loudness (F[5,100] = 3.1, p < .02). The three-way interaction was evaluated by analyzing the overall and testing age-specific effects of prenatal DEX treatment on characteristics of the startle amplitude function. These analyses revealed that maximum startle amplitude was significantly decreased in DEX offspring (main effect of DEX: F[1,40] = 6.1, p < .05), an effect produced by a weak trend for group differences in adolescence (t[20] = 1.5, p = .15; Fig. 2A) that reached significance in adulthood (t[20] = 2.2, p < .05; Fig. 2B).
FIGURE 2.
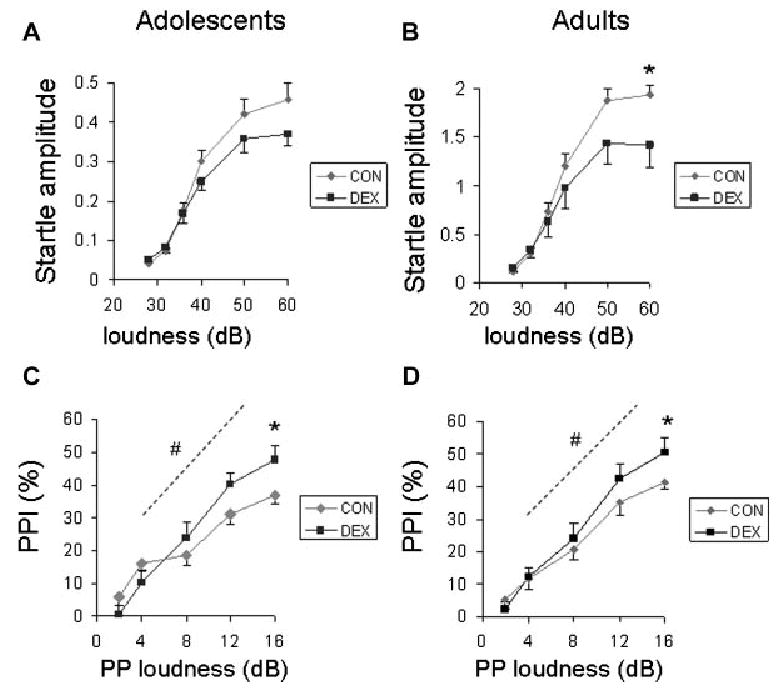
Acoustic startle reflex (A and B) and prepulse inhibition of startle (PPI) (C and D) in offspring of dams treated with saline (CON; gray symbols) or DEX (black symbols) during early gestation. Offspring were tested during late adolescence (A and C) or in adulthood (B and D) (see text). Panels A and B show the increase in the startle reflex (Newtons) as a function of startle stimulus loudness (dB; difference from background of 60 dB). Maximum startle amplitude (reflex capacity) was significantly decreased in DEX offspring. Panels C and D show increasing inhibition of the startle reflex as a function of the loudness (dB, difference from background) of the prepulse stimulus delivered 100 ms prior to the startle stimulus. DEX offspring showed a significant increase in the maximal level of prepulse inhibition and in the efficiency of PPI, defined as the slope within the dynamic range of the prepulse loudness-reflex inhibition function (see the Methods Section). *p < .05, main effect of prenatal DEX on maximum response; #p < .05, main effect of prenatal DEX on slope.
Prepulse Inhibition of Startle
The ANOVA revealed that PPI increased significantly as a function of the loudness of the prepulse stimulus (main effect, F[4,72] = 190, p < .001; Fig. 2C and D). There was also a significant interaction between prenatal treatment and prepulse loudness (F[4,72] = 6.8, p < 0,001). Two characteristics of the prepulse salience-PPI sigmoidal function were found to be different in DEX offspring. DEX offspring showed a significant increase in maximum PPI (F[1,39] = 9.1, p < .01) and a trend for an increase in PPI efficiency (the slope of the dynamic range of the function; F[1,39] = 3.0, p = .09); these effects did not differ as a function of testing age (Fig. 2C and D). The overall analysis also revealed a three-way interaction among testing age, sex and prepulse loudness (F[4,72] = 2.7, p < .05); however, no pairwise comparisons relevant to this interaction were significant.
Spontaneous and Amphetamine-Induced Motor Activity
Data from males of one litter were not included due to procedural abnormalities during the test at one of the doses.
Pre-Injection Habituation
For the habituation period, the mixed ANOVA showed a highly significant decrease in activity over the three 10-min blocks (F[2,19] = 219, p < .001), indicative of habituation to the chamber. There was no significant main effect of prenatal treatment or interaction of prenatal treatment with time block.
Ambulation Following Amphetamine
The ANOVA revealed main effects of prenatal treatment (F[1,17] = 4.6, p < .05), sex (F[1,17] = 7.4, p < .05), and a dose-dependent effect of amphetamine (F[2,34] = 54.0, p < .001) on motor activity, but no interaction among these factors. Ambulation was decreased in DEX offspring (Fig. 3A) and lower in males than in females (grand mean (in cm) ± SEM; males: 770 ± 220; females: 1101 ± 207). Post hoc comparisons of different dose levels (corrected with a modified Bonferonni method) revealed that ambulation levels following 1.0 or 5.0 mg/kg were higher than ambulation following saline injection (p's < .05), but were significantly decreased at 5.0 mg/kg relative to the lower dose (p < .05) (Fig. 3A and B).
FIGURE 3.
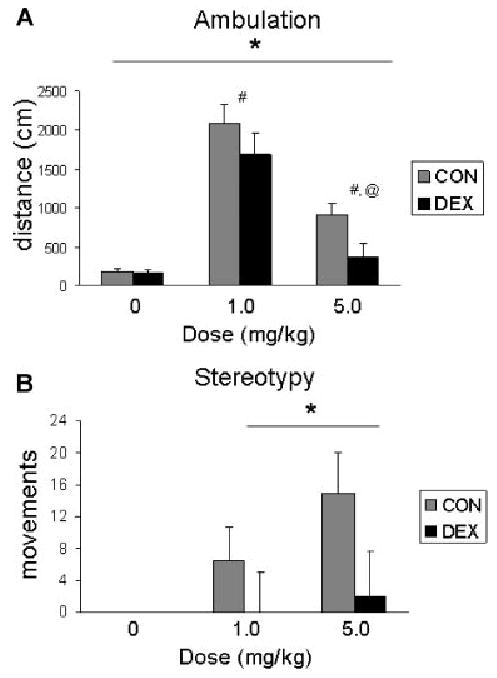
Average ambulation (A) and stereotypy (B) following injection of .0 (saline), 1.0 or 5.0 mg/kg amphetamine. DEX offspring show decreases in these measures of motor activity. For main effect of prenatal DEX treatment: *p < .05. For dose comparisons: #p < .05 relative to saline, @p < .05 relative to 1.0 mg/kg dose.
Stereotypy
No stereotypy was observed following injection of saline (all values = 0). Thus, the two doses of amphetamine were included in a mixed ANOVA. This analysis revealed significantly reduced levels of amphetamine-induced stereotypy in DEX-treated offspring (main effect of prenatal treatment (F[1,17] = 4.8, p < .05; Fig. 3B) and a trend for sex differences, with females showing lower levels than males (grand mean (in movements) ± SEM; males: 10.0 ± 7.2; females: 2.3 ± 6.8; F[1,17] = 3.5, p = .08).
Interaction of Milk Ejection Frequency With Effects of DEX on the Offspring
The significant increase in milk ejection during the neonatal period in the DEX-treated dams raised the possibility that this maternal behavior might have acted as an intervening variable in the effects of prenatal DEX treatment on behavior in the offspring. Although this question can be addressed only with cross-fostering experimental designs with appropriate controls, additional statistical analyses of the current data can provide preliminary evidence for a relationship between the effects of DEX on this maternal behavior and the behavior of the offspring. Thus, the effects of prenatal DEX were reanalyzed adding milk ejection as a covariate in the ANOVA.
Milk ejection frequency interacted significantly with the effects of DEX on play behavior and the acoustic startle reflex. For play behavior, the analysis revealed a highly significant negative correlation between milk ejection frequency and walkovers (partial correlation r[8] = −.77; F[1,1,8] = 11.7; p < .001) and no residual effect of DEX treatment (p > .9). Similarly, milk ejections correlated with startle reflex capacity (maximum startle response) (partial correlation r[20] = −.52, F[1,1,19] = 8.4, p < .01), and after accounting for effects of the covariate, there were no residual effects of DEX treatment on this characteristic in the offspring (p > .5). Including milk ejection as a covariate unexpectedly revealed an effect of prenatal DEX treatment on ambulation during the habituation period of the motor activity test. There was a significant negative correlation of milk ejection with ambulation (partial correlation r[18] = −.63; F[1,1,18] = 11.6, p < .01). Accounting for this source of variance revealed a significant residual effect of prenatal DEX treatment (F[1,1,16] = 7.2, p < .05) with the corrected average ambulation being greater in DEX offspring than in controls (estimated means (in cm) ± SEM for DEX: 1259 ± 95; for CON, 884 ± 79).
On the other hand, the increases in maximal PPI and PPI efficiency in DEX offspring were not correlated with milk ejection during the neonatal period (partial correlations: with max PPI r = .13; with PPI efficiency r = .14; p's > .35), and the residual main effects of prenatal DEX treatment were significant for both measures (max: F[1,1,38] = 3.4, p < .05; PPI efficiency: F[1,1,38] = 3.2, p < .05). Similarly, milk ejections did not interact significantly with the effects of DEX treatment on post-injection ambulation or stereotypy in the offspring (Pearson correlation p values >.5).
Discussion
The goal of the present experiments was to begin to examine whether glucocorticoid receptor over-activation during early gestation, as may occur with some forms of stress, can lead to behavioral phenotypes in the offspring that would, in turn, support the hypothesis of a prenatal stress diathesis model of schizophrenia and related disorders. We found that administration of the glucocorticoid receptor agonist DEX to pregnant rat dams on Days 6–8 of gestation leads to deficits in social play behavior, a blunting of the acoustic startle response, increased PPI of startle, and reduced amphetamine-induced motor activity in the offspring. Importantly, we also observed changes in maternal behavior and physiology that may mediate a specific subset of the behavioral phenotypes in the offspring.
Based on data from the GD9.5 embryo, the earliest age for which fetal GR expression has been reported to our knowledge (Speirs, Seckl, & Brown, 2004), we postulate that fetal GR expression during the period of DEX exposure in this study is likely to be very low. Thus, the effects of DEX observed in this study may not be due to activation of fetal GR receptors. However, there are a number of other potential sites at which GR over-stimulation during very early gestation (GDs 6–8) could affect the programming of physiology and behavior in the offspring. In particular, DEX could have effects on fetal development by affecting placental function or postnatally, through DEX-mediated changes in maternal physiology and behavior. Circulating glucocorticoid levels change during gestation and the placenta is a major target of these hormones (Heller, Orti, & De Nicola, 1986; Mark, Augustus, Lewis, Hewitt, & Waddell, 2009; Meaney, Szyf, & Seckl, 2007; Michael & Papageorghiou, 2008; Seckl, 2008). Glucocorticoid levels within the placenta, their transport across the placental barrier, and actions at specific cell types are regulated by the enzymes 11-beta-hydroxysteroid dehydrogenase 2 and P-glycoprotein which are, in turn, regulated by hormones and other factors that fluctuate as a function of gestational stage (Mark et al., 2009; Meaney et al., 2007; Michael & Papageorghiou, 2008; Seckl, 2008). Glucocorticoid receptors are expressed in the placenta early in gestation and increase thereafter (Heller et al., 1986). Moreover, glucocorticoids have multiple actions on the placenta and fetus, each of which varies systematically across tissue compartment and gestational period (Michael & Papageorghiou, 2008; Seckl, 2008). The actions of glucocorticoids on maternal peripheral tissues and, importantly, in the brain, also affect energy metabolism, cardiovascular function, and multiple hormonal and behavioral responses in the dam that can also have persistent effects on the offspring (Budge, Stephenson, & Symonds, 2007; Seckl, 2008; Smith, Seckl, Evans, Costall, & Smythe, 2004; Smith & Waddell, 2003; Woods & Weeks, 2005). The DEX dose and treatment in the present study was chosen to model the multiple actions of glucocorticoid receptors during early gestation. Unlike endogenous glucocorticoids, DEX is not metabolized by 11-beta-hydroxysteroid dehydrogenase 2 (Woods & Weeks, 2005). Moreover, the dose of DEX used in the current experiments exceeds doses shown to achieve significant (up to 90%) occupancy at central glucocorticoid receptors in the adult rat (Reul, van den Bosch, & de Kloet, 1987; Spencer, Young, Choo, & McEwen, 1990). Thus, probable targets of the DEX in this study include glucocorticoid receptors in the maternal periphery, the maternal brain, the placenta, and the fetus. Importantly, this may model glucocorticoid receptor over-stimulation by the markedly increased levels of glucocorticoids produced by some forms of maternal stress. For example, Takahashi, Turner, and Kalin (1998) reported that daily tail shock increases corticosterone in both the pregnant rat dam and the fetus, and these responses are correlated. Interestingly, the gestational stage at which stress-induced corticosterone levels in the dam differed maximally from non-stressed levels was GDs 6–8.
The current findings indicate that early gestational exposure to DEX decreases social play, a peer-directed social behavior characteristic of the late juvenile and adolescent period in the rat (Panksepp, 1981; Spear, 2000). These results are consistent with studies showing that early gestational administration of exogenous glucocorticoids to marmosets (DEX) (Hauser et al., 2007) or pig sows (hydrocortisone) (Kranendonk et al., 2006) reduces social affiliative behavior in the offspring. Although we could not find parallel studies in rats specifically addressing the effects of glucocorticoid (or stress) exposure limited to early gestation, variable or inescapable shock-induced stress applied for the last half or trimester of gestation also leads to social play deficits in the offspring (Lee et al., 2007; Morley-Fletcher, Rea, Maccari, & Laviola, 2003). While the specific neural circuits involved in the play deficits were not determined in this study, there is considerable overlap in the limbic cortico-striatal and limbic thalamic circuits that mediate sensorimotor gating, the psychomotor response to amphetamine, behavioral domains also affected by early gestational DEX exposure (Swerdlow, Braff, & Geyer, 2000; Vanderschuren, Niesink, & Van Ree, 1997). Decreased social play behavior in prepubertal rodents might provide a model for understanding the disturbances in neural systems that may underlie the decreased social function often observed in adolescents at risk for schizophrenia (Cannon et al., 2008).
We found that that early gestational DEX treatment also resulted in lower acoustic startle responses in the offspring. This reflex is mediated by the caudal pedunculopontine reticular nucleus via its outputs to the facial, cranial, and spinal motor neurons (Plappert & Pilz, 2001) and is modulated by noradrenergic projections from the locus ceoruleus (Adams & Geyer, 1981). Acoustic startle has been shown to be decreased in schizophrenia patients and individuals diagnosed as prodromal for schizophrenia and is related to symptom severity in these patients (Braff, Grillon, & Geyer, 1992; Quednow, Frommann, Berning, Maier, & Wagner, 2008). Notably, unlike the effects of early gestational DEX exposure, administration of DEX or cold stress to pregnant rats during mid-late gestation has been shown to increase acoustic startle amplitudes in the offspring, a response that may be secondary to increased anxiety (Hougaard et al., 2005; Tazumi et al., 2005). Thus, the timing within gestation of an exposure to excess glucocorticoids may be critical in determining the effects of this exposure on the fetus.
Across a number of species, including humans, PPI is thought to represent the gating of sensory input by the neural circuitry activated by the prepulse (Swerdlow et al., 2000). Although not specific to schizophrenia-related disorders, deficits in PPI have been demonstrated in people with schizophrenia, schizotypal personality disorder without psychosis, and in asymptomatic first-degree relatives of people with schizophrenia (Braff, Geyer, & Swerdlow, 2001; Quednow et al., 2008). Offspring of dams treated with DEX in early gestation showed increased PPI. Importantly, it is unlikely that this effect was secondary to the decreased acoustic startle reflex also observed in DEX offspring since in a subset of DEX and control offspring matched for baseline startle, the increase in PPI was still present (data not shown). Thus, it is likely the increased PPI represents a change in the forebrain circuits that mediate sensory gating. These include dopaminergic, inputs to the ventral striatum and prefrontal cortex, and the hippocampus, a brain region particularly high in glucocorticoid receptors (Meaney et al., 2007; Swerdlow et al., 2000).
In rodents, amphetamine-induced ambulation has been shown to be correlated with, and dependent upon, increases in ventromedial striatal dopamine efflux (Kelly, Seviour, & Iversen, 1975; Moore & Robbins, 2008). As with PPI, amphetamine-induced ambulation is regulated by the hippocampal inputs to the striatum and is altered in adulthood following pre- or perinatal disruptions of limbic circuits that regulate stress responses (Meaney et al., 2007; Moore & Robbins, 2008). Recently O'Regan et al. (2008) reported that maternal DEX exposure during late gestation leads to lower spontaneous activity and blood pressure in the home cage, but a greater hypertensive response to stress and amphetamine. Although we did not conduct home cage observations in the present study, we found that motor activity induced by the stress of i.p. injection and/or amphetamine was lower in the early gestational DEX-treated pups. Further study is necessary to determine the mechanisms underlying the context-dependent decreases in motor activity produced by early or late gestational glucocorticoid exposure. Although baseline PPI and the response to amphetamine are not always correlated, the increased PPI and decreased response to amphetamine lead us to speculate that early gestation glucocorticoid over-exposure may alter limbic cortico-striatal and/or dopaminergic circuits in a manner that leads to a blunting of behaviors normally driven by mesostriatal DA (Hadamitzky, Harich, Koch, & Schwabe, 2007; Kelly et al., 1975). This underlying phenotype might, in turn, be expected to contribute disproportionately to negative symptoms observed in schizophrenia-related disorders.
Early gestational DEX treatment affected the physiology and behavior of the dams during and after gestation. DEX produced a decrease in body weight of the dams during the 3-day period of drug administration which was normalized by the end of gestation and was not associated with differences in the birth weight of the pups. Our finding replicates a previous report that administration of DEX (.1 mg/kg) during GDs 1–10 reduced food intake and body weight of the dam during of the exposure, but that food intake increased such that total weight gain during gestation and the birth weight of pups was normal (Woods & Weeks, 2005). However, this and other studies also showed that administration of the same dose of DEX during late gestation produced a marked decrease in dam body weight from which dams did not recover and led to significant decreases in the birthweight, cardiovascular function, and seizure susceptibility of the offspring (Woods & Weeks, 2005; Young, Teskey, Henry, & Edwards, 2006). Thus, pathways regulating energy metabolism in the pregnant female should continue to be explored as a possible intervening mechanisms in the effects of gestational stress or glucocorticoid responses, with particular attention paid to the gestational timing of the exposure (Hawkins et al., 2000; McMillen, Edwards, Duffield, & Muhlhausler, 2006).
Early gestational DEX exposure also led to significant increases in milk letdown events in DEX-exposed dams. We observed a similar increase in arched-back nursing that did not reach statistical significance. Interestingly, these changes in maternal behavior may be opposite of the effects of uncontrollable stress applied during the last semester of gestation (Smith et al., 2004). The increased frequency of milk ejection bouts in DEX-treated dams may have compensated for less milk let-down per bout, or may represent increased caloric intake in the DEX pups required to maintain their body weights which did not differ from CON offspring. Importantly, milk ejection frequency was significantly correlated with subsequent abnormalities in the pups' social play behavior, the acoustic startle reflex and locomotor habituation. This may reflect parallel effects of early gestational DEX treatment on maternal and offspring behavior. Alternatively, it is tempting to speculate that this behavioral change in the DEX-treated dam (and the associated sequelae) acted as an intervening variable in determining the behavioral abnormalities in the DEX offspring. Differences in maternal behavior have potent and persistent effects on the behavior of the offspring that are mediated via multiple epigenetic mechanisms (Meaney et al., 2007; Weaver et al., 2004). For example, adolescent male offspring of dams exhibiting naturally occurring or brief separation-induced high levels of pup licking and grooming show lower levels or “feminization” of social play (Arnold & Siviy, 2002; Parent & Meaney, 2008). However, the impact of maternal behavior on the long-term effects of prenatal or neonatal stress in the offspring is not straightforward, and may depend on both the maternal behavior and target behavioral outcome in the offspring (Baker et al., 2008; Barros et al., 2006; Champagne & Meaney, 2006). For example, unlike what we observed with DEX exposure, Lee et al. (2007) reported that the decreases in social behavior in the offspring of prenatally stress dams are unaffected by cross-fostering.
The observed effects of early gestational treatment with DEX complement findings in a recent study showing that pregnant rats who received chronic variable stress in early gestation delivered male offspring showing anhedonia, altered responses to tail-suspension or forced swim stress, and an increased sensitivity to selective serotonin reuptake inhibitor treatment (Mueller & Bale, 2008). Taken together, these studies support the notion that exposure of the fetus to the effects of maternal stress early in gestation leads to a blunting of the acoustic startle reflex, psychomotor slowing and decreased motivation, as well as deficits in other affective and social behaviors. A higher risk for these psychological phenotypes might also be predicted to occur with high-dose glucocorticoid treatment early in pregnancy. Determining the mechanisms underlying these effects will lead to a greater understanding of the role of “maternal programming” of the fetus in the health of the offspring (Meaney et al., 2007; Seckl, 2008).
Acknowledgments
This work was funded in part by an award from the Sackler Institute for Developmental Psychobiology at Columbia University (M.M.M.), a T32 Fellowship in Developmental Neuroscience T32 MH018264 (T.S.C.), The Lieber Center for Schizophrenia Research at Columbia University (D.M., H.M.), PHS MH 66171 (H.M.), 2-K24 MH01699 (D.M.).
Contract grant sponsor: Sackler Institute for Developmental Psychobiology at Columbia University
Contract grant sponsor: The Lieber Center for Schizophrenia Research at Columbia University
Contract grant sponsor: The National Institutes of Healh
Contract grant numbers: PHS MH 66171, 2-K24 MH01699
References
- Adams LA, Geyer MA. Effects of 6-hydroxydopamine lesions of locus coeruleus on startle in rats. Psychopharmacology. 1981;73(4):394–398. doi: 10.1007/BF00426474. [DOI] [PubMed] [Google Scholar]
- Arnold JL, Siviy SM. Effects of neonatal handling and maternal separation on rough-and-tumble play in the rat. Developmental Psychobiology. 2002;41(3):205–215. doi: 10.1002/dev.10069. [DOI] [PubMed] [Google Scholar]
- Baker S, Chebli M, Rees S, Lemarec N, Godbout R, Bielajew C. Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain Research. 2008;1213:98–110. doi: 10.1016/j.brainres.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Barros VG, Rodriguez P, Martijena ID, Perez A, Molina VA, Antonelli MC. Prenatal stress and early adoption effects on benzodiazepine receptors and anxiogenic behavior in the adult rat brain. Synapse. 2006;60(8):609–618. doi: 10.1002/syn.20336. [DOI] [PubMed] [Google Scholar]
- Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE, et al. Timing of prenatal stressors and autism. Journal of Autism and Developmental Disorders. 2005;35(4):471–478. doi: 10.1007/s10803-005-5037-8. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: Normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156(2–3):234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Archives of General Psychiatry. 1992;49(3):206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Budge H, Stephenson T, Symonds ME. Maternal nutrient restriction is not equivalent to maternal biological stress. Current Drug Targets. 2007;8(8):888–893. doi: 10.2174/138945007781386839. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. Prediction of psychosis in youth at high clinical risk: A multisite longitudinal study in North America. Archives of General Psychiatry. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biological Psychiatry. 2006;59(12):1227–1235. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Estanislau C, Morato S. Behavior ontogeny in the elevated plus-maze: Prenatal stress effects. International Journal of Developmental Neuroscience. 2006;24(4):255–262. doi: 10.1016/j.ijdevneu.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Hadamitzky M, Harich S, Koch M, Schwabe K. Deficient prepulse inhibition induced by selective breeding of rats can be restored by the dopamine D2 antagonist haloperidol. Behavioural Brain Research. 2007;177(2):364–367. doi: 10.1016/j.bbr.2006.11.037. [DOI] [PubMed] [Google Scholar]
- Hauser J, Dettling-Artho A, Pilloud S, Maier C, Knapman A, Feldon J, et al. Effects of prenatal dexamethasone treatment on postnatal physical, endocrine, and social development in the common marmoset monkey. Endocrinology. 2007;148(4):1813–1822. doi: 10.1210/en.2006-1306. [DOI] [PubMed] [Google Scholar]
- Hauser J, Feldon J, Pryce CR. Prenatal dexamethasone exposure, postnatal development, and adulthood prepulse inhibition and latent inhibition in Wistar rats. Behavioural Brain Research. 2006;175(1):51–61. doi: 10.1016/j.bbr.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Hawkins P, Steyn C, McGarrigle HH, Calder NA, Saito T, Stratford LL, et al. Cardiovascular and hypothalamic-pituitary-adrenal axis development in late gestation fetal sheep and young lambs following modest maternal nutrient restriction in early gestation. Reproduction, Fertility, and Development. 2000;12(7–8):443–456. doi: 10.1071/rd99071. [DOI] [PubMed] [Google Scholar]
- Heller CL, Orti E, De Nicola AF. Regulatory factors of glucocorticoid binding in early and term rat placenta. Journal of Steroid Biochemistry. 1986;25(1):53–58. doi: 10.1016/0022-4731(86)90280-3. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Seckl JR. The role of 11beta-hydroxysteroid dehydrogenases in the brain. Molecular and Cellular Endocrinology. 2006;248(1–2):9–14. doi: 10.1016/j.mce.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Hossain A, Hajman K, Charitidi K, Erhardt S, Zimmermann U, Knipper M, Canlon B. Prenatal dexamethasone impairs behavior and the activation of the BDNF Exon IV promoter in the PVN in adult offspring. Endocrinology. 2008;149:6356–6365. doi: 10.1210/en.2008-0388. [DOI] [PubMed] [Google Scholar]
- Hougaard KS, Andersen MB, Kjaer SL, Hansen AM, Werge T, Lund SP. Prenatal stress may increase vulnerability to life events: Comparison with the effects of prenatal dexamethasone. Brain Research Developmental Brain Research. 2005;159(1):55–63. doi: 10.1016/j.devbrainres.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: Prenatal stress and glucocorticoids. Journal of Physiology. 2006;572(Pt 1):31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Research. 1975;94(3):507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, et al. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Archives of General Psychiatry. 2008;65(2):146–152. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- Kinney DK, Miller AM, Crowley DJ, Huang E, Gerber E. Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. Journal of Autism and Developmental Disorders. 2008;38(3):481–488. doi: 10.1007/s10803-007-0414-0. [DOI] [PubMed] [Google Scholar]
- Kranendonk G, Hopster H, Fillerup M, Ekkel ED, Mulder EJH, Taverne MAM. Cortisol administration to pregnant sows affects novelty-induced locomotion, aggressive behaviour, and blunts gender differences in their offspring. Hormones & Behavior. 2006;49(5):663–672. doi: 10.1016/j.yhbeh.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Lajic S, Nordenstrom A, Hirvikoski T. Long-term outcome of prenatal treatment of congenital adrenal hyperplasia. Endocrine Development. 2008;13:82–98. doi: 10.1159/000134827. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Barr RG, Brunet A, Galbaud du Fort G, Meaney ML, Saucier JF, et al. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatric Research. 2004;56(3):400–410. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: Reversal by oxytocin. Brain Research. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychology. 2008;27(5):604–615. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- Maccari S, Morley-Fletcher S. Effects of prenatal restraint stress on the hypothalamus-pituitary-adrenal axis and related behavioural and neurobiological alterations. Psychoneuroendocrinology. 2007;32(Suppl 1):S10–S15. doi: 10.1016/j.psyneuen.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Corcoran C, Kleinhaus KR, Perrin MC, Fennig S, Nahon D, et al. Acute maternal stress in pregnancy and schizophrenia in offspring: A cohort prospective study. BMC Psychiatry. 2008;8:71. doi: 10.1186/1471-244X-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark PJ, Augustus S, Lewis JL, Hewitt DP, Waddell BJ. Changes in the placental glucocorticoid barrier during rat pregnancy: Impact on placental corticosterone levels and regulation by progesterone. Biology of Reproduction. 2009;80:1209–1215. doi: 10.1095/biolreprod.108.073650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Iverson MT, Stevenson KN. Apomorphine effects on emotional modulation of the startle reflex in rats. Psychopharmacology (Berl) 2005;181:60–70. doi: 10.1007/s00213-005-2217-3. [DOI] [PubMed] [Google Scholar]
- McMillen IC, Edwards LJ, Duffield J, Muhlhausler BS. Regulation of leptin synthesis and secretion before birth: Implications for the early programming of adult obesity. Reproduction. 2006;131(3):415–427. doi: 10.1530/rep.1.00303. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends in Molecular Medicine. 2007;13(7):269–277. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg HFL, Dolezal C, Baker SW, Carlson AD, Obeid JS, New MI. Cognitive and motor development of children with and without congenital adrenal hyperplasia after early-prenatal dexamethasone. Journal of Clinical Endocrinology & Metabolism. 2004;89(2):610–614. doi: 10.1210/jc.2002-021129. [DOI] [PubMed] [Google Scholar]
- Michael AE, Papageorghiou AT. Potential significance of physiological and pharmacological glucocorticoids in early pregnancy. Human Reproduction Update. 2008;14(5):497–517. doi: 10.1093/humupd/dmn021. [DOI] [PubMed] [Google Scholar]
- Moore H, Robbins TW. Modeling psychiatric disordersin experimental animals. In: Tasman JKA, Lieberman JA, First MB, Maj M, editors. Psychiatry. West Sussex: John Wiley & Sons; 2008. pp. 275–288. [Google Scholar]
- Morley-Fletcher S, Rea M, Maccari S, Laviola G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. European Journal of Neuroscience. 2003;18(12):3367–3374. doi: 10.1111/j.1460-9568.2003.03070.x. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. Journal of Neuroscience. 2008;28(36):9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Ozawa H, Suzuki H. Prenatal dexamethasone exposure affects anxiety-like behaviour and neuroendocrine systems in an age-dependent manner. Neuroscience Research. 2008;60(4):364–371. doi: 10.1016/j.neures.2007.12.005. [DOI] [PubMed] [Google Scholar]
- O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Prenatal dexamethasone ‘programmes’ hypotension, but stress-induced hypertension in adult offspring. Journal of Endocrinology. 2008;196(2):343–352. doi: 10.1677/JOE-07-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. The ontogeny of play in rats. Developmental Psychobiology. 1981;14(4):327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Parent CI, Meaney MJ. The influence of natural variations in maternal care on play fighting in the rat. Developmental Psychobiology. 2008;50(8):767–776. doi: 10.1002/dev.20342. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC, Whishaw IQ. The role of the cortex in play fighting by rats: Developmental and evolutionary implications. Brain, Behavior and Evolution. 1992;39(5):270–284. doi: 10.1159/000114124. [DOI] [PubMed] [Google Scholar]
- Plappert CF, Pilz PK. The acoustic startle response as an effective model for elucidating the effect of genes on the neural mechanism of behavior in mice. Behavioural Brain Research. 2001;125(1–2):183–188. doi: 10.1016/s0166-4328(01)00299-6. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Frommann I, Berning J, Maier KU, Wagner M. Impaired sensorimotor gating of the acoustic startle response in the prodrome of schizophrenia. Biological Psychiatry. 2008;64:766–773. doi: 10.1016/j.biopsych.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Reul JM, van den Bosch FR, de Kloet ER. Relative occupation of type-I and type-II corticosteroid receptors in rat brain following stress and dexamethasone treatment: Functional implications. Journal of Endocrinology. 1987;115(3):459–467. doi: 10.1677/joe.0.1150459. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoids, developmental ‘programming’ and the risk of affective dysfunction. Progress in Brain Research. 2008;167:17–34. doi: 10.1016/S0079-6123(07)67002-2. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Kreider ML, Tate CA, Seidler FJ. Critical prenatal and postnatal periods for persistent effects of dexamethasone on serotonergic and dopaminergic systems. Neuropsychopharmacology. 2006;31(5):904–911. doi: 10.1038/sj.npp.1300892. [DOI] [PubMed] [Google Scholar]
- Smith JT, Waddell BJ. Leptin distribution and metabolism in the pregnant rat: Transplacental leptin passage increases in late gestation but is reduced by excess glucocorticoids. Endocrinology. 2003;144(7):3024–3030. doi: 10.1210/en.2003-0145. [DOI] [PubMed] [Google Scholar]
- Smith JW, Seckl JR, Evans AT, Costall B, Smythe JW. Gestational stress induces post-partum depression-like behaviour and alters maternal care in rats. Psychoneuroendocrinology. 2004;29(2):227–244. doi: 10.1016/s0306-4530(03)00025-8. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Speirs HJ, Seckl JR, Brown RW. Ontogeny of glucocorticoid receptor and 11beta-hydroxysteroid dehydrogenase type-1 gene expression identifies potential critical periods of glucocorticoid susceptibility during development. Journal of Endocrinology. 2004;181(1):105–116. doi: 10.1677/joe.0.1810105. [DOI] [PubMed] [Google Scholar]
- Spencer RL, Young EA, Choo PH, McEwen BS. Adrenal steroid type I and type II receptor binding: Estimates of in vivo receptor number, occupancy, and activation with varying level of steroid. Brain Research. 1990;514(1):37–48. doi: 10.1016/0006-8993(90)90433-c. [DOI] [PubMed] [Google Scholar]
- Susser ES, Lin SP. Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944–1945. Archives of General Psychiatry. 1992;49(12):983–988. doi: 10.1001/archpsyc.1992.01820120071010. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Animal models of deficient sensorimotor gating: What we know, what we think we know, and what we hope to know soon. Behavioural Pharmacology. 2000;11(3–4):185–204. doi: 10.1097/00008877-200006000-00002. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Turner JG, Kalin NH. Prolonged stress-induced elevation in plasma corticosterone during pregnancy in the rat: Implications for prenatal stress studies. Psychoneuroendocrinology. 1998;23(6):571–581. doi: 10.1016/s0306-4530(98)00024-9. [DOI] [PubMed] [Google Scholar]
- Tazumi T, Hori E, Uwano T, Umeno K, Tanebe K, Tabuchi E, et al. Effects of prenatal maternal stress by repeated cold environment on behavioral and emotional development in the rat offspring. Behavioural Brain Research. 2005;162(1):153–160. doi: 10.1016/j.bbr.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neuroscience and Biobehavioral Reviews. 1997;21(3):309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior [see comment] Nature Neuroscience. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Woods LL, Weeks DA. Prenatal programming of adult blood pressure: Role of maternal corticosteroids. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2005;289(4):R955–R962. doi: 10.1152/ajpregu.00455.2004. [DOI] [PubMed] [Google Scholar]
- Young NA, Teskey GC, Henry LC, Edwards HE. Exogenous antenatal glucocorticoid treatment reduces susceptibility for hippocampal kindled and maximal electroconvulsive seizures in infant rats. Experimental Neurology. 2006;198(2):303–312. doi: 10.1016/j.expneurol.2005.11.013. [DOI] [PubMed] [Google Scholar]


