Abstract
Fingertip forces result from activation of muscles that cross the wrist and muscles whose origins and insertions reside within the hand (extrinsic and intrinsic hand muscles, respectively). Thus, tasks that involve changes in wrist angle affect the moment arm and length, hence the force-producing capabilities, of extrinsic muscles only. If a grasping task requires the exertion of constant fingertip forces, the Central Nervous System (CNS) may respond to changes in wrist angle by modulating the neural drive to extrinsic or intrinsic muscles only or by co-activating both sets of muscles. To distinguish between these scenarios, we recorded electromyographic (EMG) activity of intrinsic and extrinsic muscles of the thumb and index finger as a function of wrist angle during a two-digit object hold task. We hypothesized that changes in wrist angle would elicit EMG amplitude modulation of the extrinsic and intrinsic hand muscles. In one experimental condition we asked subjects to exert the same digit forces at each wrist angle, whereas in a second condition subjects could choose digit forces for holding the object. EMG activity was significantly modulated in both extrinsic and intrinsic muscles as a function of wrist angle (both p < 0.05) but only for the constant force condition. Furthermore, EMG modulation resulted from uniform scaling of EMG amplitude across all muscles. We conclude that the CNS controlled both extrinsic and intrinsic muscles as a muscle synergy. These findings are discussed within the theoretical frameworks of synergies and common neural input across motor nuclei of hand muscles.
Keywords: fingers, synergy, EMG, muscle length
During manipulatory behaviors, fingertip forces at each digit result from the contribution of forces generated by extrinsic and intrinsic hand muscles. Extrinsic muscles cross the wrist, whereas intrinsic muscles do not, i.e., their origins and insertions reside within the hand. Thus, changes in wrist angle alter the length and moment arm of the extrinsic [3], but not the intrinsic, hand muscles [15]. Specifically, the extrinsic flexors have the largest moment arms in flexion with a decreasing trend towards neutral followed by a leveling off or even small increase by larger degrees of extension, whereas the opposite is true for extrinsic extensors, i.e., the largest moment arms are in extension with a decreasing trend towards neutral followed by a leveling off or small increase by larger degrees of flexion [4]. As the ability of a muscle to exert force depends on its length and moment arm [27], changes in wrist angle will affect the force-generating capabilities of the extrinsic hand muscles only. This raises the question of how the Central Nervous System (CNS) coordinates the activity of extrinsic and intrinsic hand muscles underlying the production of a given fingertip force in situations in which wrist angle changes.
It has been shown that the electromyographic (EMG) activity of a muscle changes as a function of its static length [1,7,17]. Therefore, one would expect the exertion of a given fingertip force to elicit a modulation of the neural drive to the extrinsic hand muscles when wrist angle is changed. However, it is not known whether such modulation would be limited to extrinsic muscles only. A second possibility is that intrinsic and extrinsic hand muscles could be controlled as a muscle synergy, i.e., a group of muscles activated together in a fixed spatial and/or temporal relation to one another [6]. If this were the case, the requirement of exerting the same fingertip force regardless of wrist angle would elicit modulation of both extrinsic and intrinsic hand muscles. In a third scenario, the neural drive could be modulated in only the intrinsic muscles to compensate for the change in force-generating capacity of the extrinsic muscles.
The present two-digit grasping study was designed to determine whether changes in wrist angle would elicit a modulation of EMG activity of not only extrinsic, but also intrinsic hand muscles. To address this question, we analyzed hand muscle EMG activity recorded while subjects held an object with the index finger and thumb at different wrist angles. To ensure that any changes in EMG activity of the intrinsic or extrinsic hand muscles were the result of wrist angle and not due to differences in digit normal forces or intrinsic muscle lengths, we required subjects to exert the same forces using the same digit posture at each wrist angle. We hypothesized that changes in wrist angle would cause EMG amplitude modulation of both extrinsic and intrinsic hand muscles.
We also used a second experimental condition in which subjects were allowed to choose the digit forces necessary to hold the object at each wrist angle. We expected that removing the force level constraint would have allowed subjects to explore a range of digit forces across wrist angles. Therefore we hypothesized that subjects would employ significantly different digit forces as a function of wrist angle. Preliminary results of this study have been published as an abstract [18] and as part of a book chapter [12].
Eight subjects (5 males; mean age 29 years, range 22-45 years) took part in the experiments. The experimental procedures were approved by the Institutional Review Board at Arizona State University. All subjects gave their informed consent prior to each recording session. Subjects were asked to hold a grip device (Fig. 1A,C; mass: 0.125 Kg) vertically against gravity with the tips of the thumb and index finger at three different wrist angles, i.e., neutral, flexion and extension (Fig. 1C). Subjects were required to maintain the same digit posture at all wrist angles to prevent changes in intrinsic muscle length. The target wrist angles were defined for each subject by asking them to hold the grip device with a natural, comfortable wrist angle, defined as the neutral angle. Then, while still holding the device, we asked subjects to maximally flex and then maximally extend their wrist without reaching discomfort. The target flexion and extension wrist angles were defined as 50% of the angle between neutral and the maximum flexion and extension angles, respectively.
Figure 1. Experimental setup.
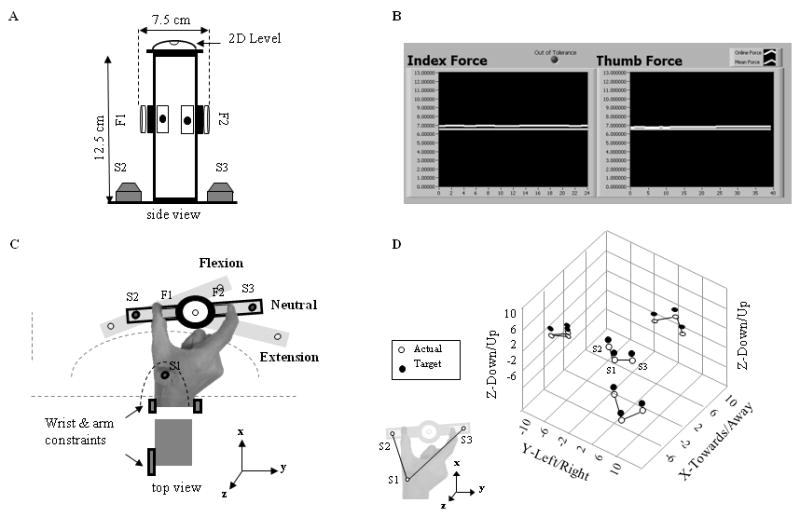
A: plexiglass object fitted at mid-height with two force/torque sensors (F1 and F2; Nano17/SI-25-250; 17 mm diameter; ATI Industrial Automation, Apex, NC). Two position tracking sensors (S2 and S3) were placed on the bottom of the object and a two-dimensional (2D) level was positioned on the top. B: feedback of normal digit forces. C: relative wrist angles: 50% maximum flexion, neutral, and 50% maximum extension. Location of wrist and arm constraints, F1-F2, and S1-S3 (with corresponding arcs) are shown together with the coordinate system. D: mismatch between actual and target digit configurations (open and filled circles, respectively). Data in the center denote 3D positions of S1, S2, and S3. Left, top, and bottom data denote projections of S1-S3 onto each axis.
Three six-dimensional position/orientation (P/O) sensors (Polhemus Fastrak, Colchester, VT) were used to define the target wrist angles and digit postures (Fig. 1C) and to provide 3-D visual feedback (Fig. 1D) to the subjects about their performance. The first P/O sensor (S1) was placed on the back of the hand between the first and second metacarpals (Fig. 1C). The second and third sensors (S2 and S3) were placed at the base of the object below the thumb and index finger, respectively (Fig. 1A,C) and were used to ensure consistent digit postures, thus intrinsic muscle lengths, across wrist angles and trials. The S1 sensor was used to determine the three target wrist angles and the sensor positions associated with each wrist angle. For the digit posture to remain constant, the relative position of S1, S2, and S3 had to remain invariant at all wrist angles. Thus, the distances between all the target positions (S1, S2 and S3) were determined for the neutral angle and subjects were required to maintain the same distances for each target wrist angle. This requirement ensures the adoption of the same thumb and index finger postures (Fig. 1D) as long as the point of fingertip-force sensor contact (see below) remained consistent. This was controlled for by marking this contact-point on the fingertips. The target wrist angles and the sensors' position at each wrist angle were calculated as a rotation of the relative positions of the three P/O sensors measured at the neutral wrist angle (see inset, Fig. 1D) derived from the angular deviations of S1 in the xy plane.
Subjects were given a “set” period at each wrist angle to match their performance with the target wrist angle and digit posture (target S1, S2 and S3 positions; Fig. 1D). To facilitate this task, the location of the S1 target in the visual display was fixed relative to the 3-D workspace, while the locations of the S2 and S3 targets were rotated in the xy plane to indicate the different wrist angles. Providing visual feedback of the actual and target S2 and S3 positions allowed subjects to maintain both a constant position and orientation of the object, and hence thumb and index finger posture, with respect to the P/O sensor placed on the hand (S1), across wrist angles. Variations in the digit posture were determined by changes in the horizontal and/or vertical distance between the actual P/O sensors and were indicated as mismatches between the feedback display of the target vs. actual hand and object positions (Fig. 1D). Subjects were required to maintain a wrist angle (rotation of S1) within ±2° from the target wrist angle in the xy plane. Subjects' difficulty in maintaining digit posture closely aligned with the target posture, requiring the use of prolonged “set” periods, indicated that the low tolerance thresholds (on average, 7, 6 and 6 mm for the x, y and z dimensions, respectively), used to ensure reproducibility of digit posture, were very strict.
Subjects were asked to hold the grip device at each wrist angle under three experimental conditions: (1) 10% and (2) 20% of their maximum voluntary force (MVF; Fig. 1B) and (3) as light as possible while preventing object slip (‘ALAP’ condition) and maintaining a consistent digit posture. To determine MVF, each subject was asked, before the start of the experiment, to squeeze the grip device with their thumb and index finger as strongly as possible for 5 seconds while holding the device vertical. The MVF value was obtained by using the larger of two trials and averaging the largest normal forces exerted by the two digits. For the first two conditions, subjects matched the target force with the actual force from each digit (Fig. 1B) within a tolerance level of ±10%, after which force and EMG recording began. During the third condition subjects received no visual feedback of the digit forces.
The experimental session consisted of five trials per condition. For each trial, subjects held the grip device for 5 seconds at each of the three wrist angles. Prior to recording the forces and EMG at each wrist angle, subjects used the “set” period to match the actual and target digit posture, wrist angle and digit forces. During data collection, if subjects failed to maintain any of the above position or force measures within the appropriate tolerance level throughout the entire 5 s recording period, that wrist angle recording was repeated. On average, ∼20% of the wrist angles had to be repeated. As only 4 of the 8 subjects were able to successfully perform the 20% MVF condition, analyses are presented only for the 10% MVF and the ALAP conditions. The order of presentation of wrist angles was randomized across trials within each force condition. Force conditions were blocked and their order of presentation was counterbalanced across subjects.
Multi-unit EMG activity was recorded through wire electrodes from three extrinsic and two intrinsic hand muscles. The extrinsic muscles examined were the Flexor Pollicis Longus (FPL) and the index finger compartments of the Flexor Digitorum Profundus (FDP2) and Extensor Digitorum Communis (ED2). The intrinsic muscles recorded from were the First Dorsal Interosseous (FDI) and First Palmar Interosseous (FPI). A detailed description of wire electrode preparation, insertion, and verification of electrode placement in the target muscles is presented elsewhere ([13,25,26]). After the experimental session, we recorded EMG during isometric maximum voluntary contraction (MVC) of each muscle. For each muscle, we positioned the subject's distal phalange of the thumb or the index finger against a fixed resistance such that the muscle of interest could be contracted along its primary line of action (flexion for FPL and FDP2; extension for ED2; adduction or abduction at the index metacarpophalangeal joint for FPI and FDI, respectively). EMG was recorded during two three-second periods with a one minute rest between trials. We used the largest value of consecutive 0.5 s averages of the EMG recorded for the MVC of that muscle.
EMG and forces were sampled at 2 and 1 kHz, respectively. The EMG signals were amplified (×1000) and band-pass filtered (1–1000 Hz; Grass Instruments). Position data were recorded at ∼25 Hz.
The mean EMG amplitude for each muscle was subtracted from the raw data which was then full-wave rectified (Fig. 2A,B). For each trial, the average EMG amplitude of each muscle was expressed as a percentage of its MVC (%EMG). For analysis of the coordination of multi-muscle EMG activity we constructed an n-dimensional vector (n = 5 muscles) defined by the %EMG amplitude of each muscle (termed muscle activation pattern [25]; MAP). A unit MAP vector was computed for each trial and then averaged across trials within subject, wrist angles and conditions [4,20,21]. The length of the MAP vector was quantified as the square root of the sum of the squares of its components for each trial and then averaged over the five trials within each subject, wrist angle and condition.
Figure 2. Muscle activation patterns.
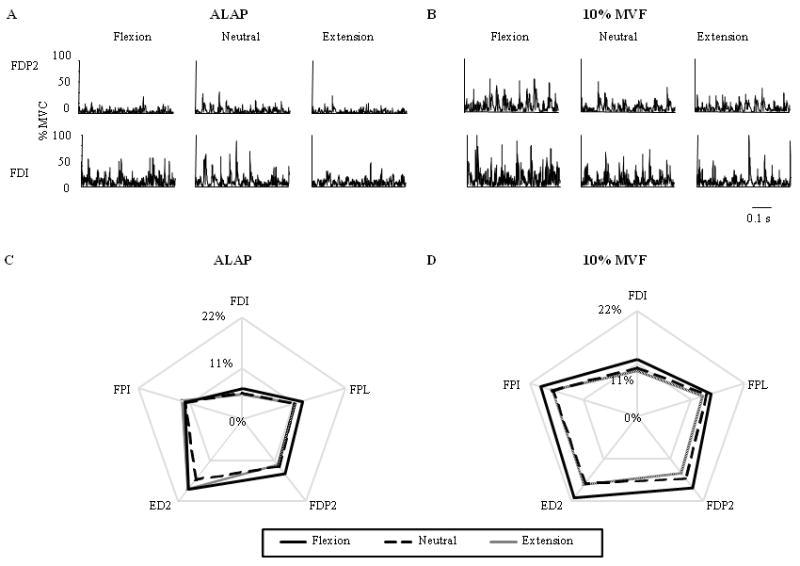
%EMG activity from an extrinsic (FDP2) and intrinsic (FDI) muscle at each wrist angle during the ALAP and 10% MVF conditions (A and B, respectively). %EMG from each muscle and wrist angle averaged across all subjects are shown for the C) ALAP and D) 10% MVF conditions. Flexor Pollicis Longus: FPL; index compartment of Flexor Digitorum Profundus and Extensor Digitorum Communis: FDP2 and ED2, respectively; First Palmar and Dorsal Interossi: FPI and FDI, respectively.
To determine the effect of wrist angle and condition on digit forces and force direction, we performed repeated measures ANOVAs on the (1) average sum of the normal forces with Condition (2 levels) and Wrist angle (3 levels) as within-subject factors, and (2) normal-to-tangential (N/T) force ratios with Condition, Wrist angle and Digit (2 levels) as the within-subject factors. Contrary to our second hypothesis, digit normal forces in the ALAP condition were not affected by wrist angle. Hence potential differences in %EMG could now be uniquely ascribed to changes in wrist angle, thus allowing for a comparison of the %EMG modulation at each wrist angle between the two conditions. Thus, we performed two separate repeated measures ANOVAs with Condition, Wrist angle, and Muscle (3 levels for extrinsic and 2 levels for intrinsic muscles). To further explore the differences observed between the two conditions, the above ANOVAs were performed separately for the 10% MVF and ALAP condition. Note that in two subjects, we lost the EMG signal of one extrinsic muscle during the experiment (FPL in one subject and FDP2 in another). In these cases, the %EMG values were averaged across all subjects for a given experimental and wrist angle condition and were substituted for the missing value.
The degree of similarity in %EMG coordination patterns between wrist angles was assessed by calculating the correlation coefficient between unit MAP vectors for each pair of wrist angles [21]. Scaling of MAP vector magnitude as a function of wrist angle was assessed by separate repeated-measures ANOVAs for each condition. For all ANOVAs, post hoc pair-wise comparisons were made using Least Significant Difference (LSD) adjustments when a significant effect (p ≤ 0.05) was found.
We found that subjects used significantly smaller normal forces during the ALAP (∼6% MVF) than the 10% MVF condition (mean ± S.E.: 6.6 ± 0.7 N and 12.6 ± 1.7 N, respectively; F(1,7) = 11.798, p < 0.05). However, changes in wrist angle did not significantly affect the normal forces or N/T ratios in either condition.
Figure 2C and D show the muscle activation patterns across wrist angles and conditions. ANOVA revealed a main effect of Condition (F(1,7) = 9.76; p < 0.05) and Wrist angle (F(2,14) = 4.80; p < 0.05), but not of Muscle on the %EMG amplitudes of the extrinsic hand muscles, with flexion and neutral wrist angles being significantly different from each other (p < 0.01). Similar results were found for the intrinsic hand muscles, i.e., main effects of Condition and Wrist angle (F(1,7) = 7.63 and F(2,14) = 4.39, respectively; both p < 0.05), but not of Muscle, with the post hoc analysis revealing significant differences between flexion and neutral wrist angles (p < 0.05).
Figure 2C and D suggests a clearer and more systematic scaling of %EMG amplitudes in 10% MVF than in the ALAP condition. Specifically, in the 10% MVF condition, %EMG amplitude was larger for wrist flexion than either neutral or extension. Most importantly, this pattern was exhibited not only by extrinsic muscles, as predicted based on changes in the moment arms of these muscles, but also for intrinsic muscles. This was confirmed by the ANOVA performed separately on the 10% MVC condition revealing main effects of Wrist angle on %EMG amplitudes for both extrinsic (F(2,14) = 7.44; p < 0.01) and intrinsic muscles (F(2,14) = 4.44; p < 0.05). Post hoc analysis revealed significant differences between (1) flexion and extension wrist angles (p < 0.05) for the intrinsic hand muscles and (2) flexion and both neutral and extension wrist angles (both at p < 0.05) for the extrinsic hand muscles. In contrast, changes in wrist angle did not cause significant changes in %EMG amplitude for either intrinsic or extrinsic muscles during the ALAP condition. These results may be due to the consistent scaling of %EMG amplitude with changes in wrist angle in all subjects for the 10% MVF condition and the larger between-subject variability in the ALAP condition.
Figure 2D also suggests a uniform scaling of the %EMG amplitudes across all intrinsic and extrinsic muscles in the 10% MVF condition. Correlation analysis on the unit MAP vectors for each pair of wrist angles (all r > 0.96) indicates that there were no directional differences, i.e., the relative levels of muscle activation in the MAP vector were similar between postures. Additionally, the lengths of the MAP vectors indicate that the pattern of muscle activation was scaled between wrist postures. Together, these findings support our hypothesis that the neural drive to both extrinsic and intrinsic hand muscles is modulated with changes in wrist angle, however, this modulation may be dependent on the amount force produced. Studies of lower limb muscles have revealed that EMG amplitudes are modulated as a function of their length, suggesting that the neural drive may be altered depending on the muscle's ability to generate force at different points in its length-tension curve [1,5,17]. Only one study has examined the modulation of EMG activity during grasping in response to changes in wrist angle [23]. In that study, FDI exhibited a similar modulation with wrist angle as found in our 10% MVC condition in which both force amplitude and digit posture were controlled. However, neither grip forces nor digit postures were controlled for in that study. These methodological differences prevent a direct comparison between the present results and those of that study. Furthermore, Werremeyer and Cole [23] did not examine the relations between the intrinsic and extrinsic muscle activity, nor did they control for digit posture or forces. Thus, the present findings extend their work in important ways by quantifying the degree to which the intrinsic and extrinsic hand muscles were coordinated and modulated to changes in the length and moment arm of the extrinsic muscles only, hence revealing that these muscles were controlled as a muscle synergy.
The notion that the CNS uses functional units of behavior to reduce the number of control variables has been supported by several studies revealing muscle synergies that scale both in time and amplitude to produce jumping, swimming and walking [2,6,8]. Other studies have shown that muscle synergies scale with force amplitude [4,20], but differ with force direction [4,11]. In terms of hand control, relations have also been identified between the activity of motor cortical cells and distinct functional muscle synergies in the hand and arms of monkeys during manipulatory behaviors [10]. Furthermore, researchers have described a small number of muscle synergies in the hand during finger spelling and hand shaping [22].
The present work differs from these studies in that in our tasks subjects were asked to exert static forces (however see [20, 21]). When the same digit forces were exerted at all wrist angles, we identified one muscle synergy which was scaled only in amplitude with respect to wrist angle. The less consistent modulation of EMG amplitude to wrist angle in the ALAP condition could have been due to a force threshold above which muscle synergies are recruited or 6% MVF may fall on a point on the force-length curve that does not require a modulation of the neural drive to exert the same force. Note that previous research showing an effect of force amplitude on the scaling of muscle synergies has not examined force amplitudes as low as 6% MVF [21].
Previous studies of single motor units of hand muscles have revealed correlated neural drive to motor neuron pools of intrinsic [16] and extrinsic [9,12,13,14,24,25] hand muscles as well as to both sets of hand muscles [26]. Analysis of correlated variability in multi-unit EMG amplitude of hand muscles has further revealed diffused, broad co-activation of intrinsic and extrinsic hand muscles [19]. Therefore, our results are compatible with a theoretical framework for grasp control based on neural inputs that favor diffused, rather than focal, activation to motor pools of hand muscles. Furthermore, the EMG amplitude modulation of intrinsic muscles as a function of changes in extrinsic muscle length points to the involvement of proprioception (i.e., from muscle length and/or force receptors) in the modulation of neural drive to motor nuclei of both intrinsic and extrinsic hand muscles. Further work, however, is needed to understand the neural mechanisms underlying the synergistic control of hand muscles and their task-dependency.
Acknowledgments
We thank Dr. Mark Jesunathadas for his comments on the manuscript. This publication was made possible by grant number 2R01 AR47301 from the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not represent the official views of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Babault N, Pousson M, Michaut A, Van Hoecke J. Effect of quadriceps femoris muscle length on neural activation during isometric and concentric contractions. J Appl Physiol. 2003;94:983–990. doi: 10.1152/japplphysiol.00717.2002. [DOI] [PubMed] [Google Scholar]
- 2.Bizzi E, Cheung VC, d'Avella A, Saltiel P, Tresch M. Combining modules for movement. Brain Res Rev. 2008;57:125–133. doi: 10.1016/j.brainresrev.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brand PW, Hollister A. Clinical Mechanics of the Hand. 3rd. St. Louis, MO: Mosby; 1999. [Google Scholar]
- 4.Buchanan TS, Almdale DP, Lewis JL, Rymer WZ. Characteristics of synergic relations during isometric contractions of human elbow muscles. J Neurophysiol. 1986;56:1225–1241. doi: 10.1152/jn.1986.56.5.1225. [DOI] [PubMed] [Google Scholar]
- 5.Cresswell AG, Loscher WN, Thorstensson A. Influence of gastrocnemius muscle length on triceps surae torque development and electromyographic activity in man. Exp Brain Res. 1995;105:283–290. doi: 10.1007/BF00240964. [DOI] [PubMed] [Google Scholar]
- 6.d'Avella A, Saltiel P, Bizzi E. Combinations of muscle synergies in the construction of a natural motor behaviour. Nat Neurosci. 2003;6:300–308. doi: 10.1038/nn1010. [DOI] [PubMed] [Google Scholar]
- 7.Gillard DM, Yakovenko S, Cameron T, Prochazka A. Isometric muscle length-tension curves do not predict angle-torque curves of human wrist in continuous active movements. J Biomech. 2000;33:1341–1348. doi: 10.1016/s0021-9290(00)00127-5. [DOI] [PubMed] [Google Scholar]
- 8.Giszter SF, Mussa-Ivaldi FA, Bizzi E. Convergent force fields organized in the frog's spinal cord. J Neurosci. 1993;13:467–491. doi: 10.1523/JNEUROSCI.13-02-00467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hockensmith GB, Lowell SY, Fuglevand AJ. Common input across motor nuclei mediating precision grip in humans. J Neurosci. 2005;25:4560–4564. doi: 10.1523/JNEUROSCI.0046-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holdefer RN, Miller LE. Primary motor cortical neurons encode functional muscle synergies. Exp Brain Res. 2002;146:233–243. doi: 10.1007/s00221-002-1166-x. [DOI] [PubMed] [Google Scholar]
- 11.Johanson ME, Valero-Cuevas FJ, Hentz VR. Activation patterns of the thumb muscles during stable and unstable pinch tasks. J Hand Surg [Am] 2001;26:698–705. doi: 10.1053/jhsu.2001.26188. [DOI] [PubMed] [Google Scholar]
- 12.Johnston JA, Winges SA, Santello M. Neural control of hand muscles during prehension. Adv Exp Med Biol. 2009;629:577–596. doi: 10.1007/978-0-387-77064-2_31. [DOI] [PubMed] [Google Scholar]
- 13.Johnston JA, Winges SA, Santello M. Periodic modulation of motor-unit activity in extrinsic hand muscles during multidigit grasping. J Neurophysiol. 2005;94:206–218. doi: 10.1152/jn.01134.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keen DA, Fuglevand AJ. Common input to motor neurons innervating the same and different compartments of the human extensor digitorum. J Neurophysiol. 2004;91:57–62. doi: 10.1152/jn.00650.2003. [DOI] [PubMed] [Google Scholar]
- 15.Knutson JE, Kilgor KL, Mansour JM, Crago PE. Intrinsic and extrinsic contributions to the passive moment at the metacarpophalangeal joint. J Biomech. 2000;33:1675–1681. doi: 10.1016/s0021-9290(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 16.McIsaac TL, Fuglevand AJ. Common synaptic input across motor nuclei supplying intrinsic muscles involved in the precision grip. Exp Brain Res. 2008;188:159–164. doi: 10.1007/s00221-008-1432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onishi H, Yagi R, Oyama M, Akasaka K, Ihashi K, Handa Y. EMG-angle relationship of the hamstring muscles during maximum knee flexion. J Electromyogr Kinesiol. 2002;12:399–406. doi: 10.1016/s1050-6411(02)00033-0. [DOI] [PubMed] [Google Scholar]
- 18.Raleigh LM, Johnston JA, Santello M. Relations between EMG activity of intrinsic and extrinsic hand muscles as a function of wrist posture during two-digit grasping. Soc Neurosci Abstr. 2006;147(2) [Google Scholar]
- 19.Valero-Cuevas FJ, Venkadesan M, Todorov E. Structured variability of muscle activations supports the minimal intervention principle of motor control. J Neurophysiol. 2009;102:59–68. doi: 10.1152/jn.90324.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valero-Cuevas FJ, Zajac FE, Burgar CG. Large index-fingertip forces are produced by subject-independent patterns of muscle excitation. J Biomech. 1998;31:693–703. doi: 10.1016/s0021-9290(98)00082-7. [DOI] [PubMed] [Google Scholar]
- 21.Valero-Cuevas FJ. Predictive modulation of muscle coordination pattern magnitude scales fingertip force magnitude over the voluntary range. J Neurophysiol. 2000;83:1469–1479. doi: 10.1152/jn.2000.83.3.1469. [DOI] [PubMed] [Google Scholar]
- 22.Weiss EJ, Flanders M. Muscular and postural synergies of the human hand. J Neurophysiol. 2004;92:523–535. doi: 10.1152/jn.01265.2003. [DOI] [PubMed] [Google Scholar]
- 23.Werremeyer MM, Cole KJ. Wrist action affects precision grip force. J Neurophysiol. 1997;78:271–280. doi: 10.1152/jn.1997.78.1.271. [DOI] [PubMed] [Google Scholar]
- 24.Winges SA, Santello M. Common input to motor units of digit flexors during multi-digit grasping. J Neurophysiol. 2004;92:3210–3220. doi: 10.1152/jn.00516.2004. [DOI] [PubMed] [Google Scholar]
- 25.Winges SA, Johnston JA, Santello M. Muscle-pair specific distribution and grip type modulation of neural common input to extrinsic digit flexors. J Neurophysiol. 2006;96:1258–1266. doi: 10.1152/jn.00327.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winges SA, Kornatz KW, Santello M. Common input to motor units of intrinsic and extrinsic hand muscles during two-digit object hold. J Neurophysiol. 2008;99:1119–1126. doi: 10.1152/jn.01059.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zajac FE. Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng. 1989;17:359–411. [PubMed] [Google Scholar]


