Abstract
Electrically conductive and biologically active scaffolds are desirable for enhancing adhesion, proliferation and differentiation of a number of cell types such as neurons. Hence, the incorporation of neuroactive molecules into electroconductive polymers via a specific and stable method is essential for neuronal tissue engineering applications. Traditional conjugation approaches dramatically impair conductivities and/or stabilities of the scaffolds and ligands. In this study, we developed copolymers (PPy-NSE) of N-hydroxyl succinimidyl ester pyrrole and regular pyrrole, which can be immobilized with nerve growth factor (NGF) without significantly hindering electroconductivity. The presence of active ester groups was confirmed using reflectance infrared spectroscopy and X-ray photoelectron spectroscopy (XPS) from the copolymers prepared from different monomer compositions. We selected PPy-NSE50 (polymerized from a 50 : 50 monomer ratio of pyrrole : pyrrole-NSE) for further modification with NGF because this copolymer retains good conductivity (approx. 8 S cm−1) and presents active ester groups for NGF immobilization. We tethered NGF on the PPy-NSE50 surface, and found that PC12 cells extended neurites similarly to cells cultured in NGF-containing medium. XPS and enzyme-linked immunosorbent assay confirmed that NGF immobilized via the active ester on the PPy-NSE50 film was stable for up to 5 days in phosphate-buffered saline solution. Also, application of an external electrical potential to NGF-immobilized PPy films did not cause a significant release of NGF nor reduce their neurotrophic activity. This novel scaffold, providing electroconductive and neurotrophic activities, has potential for neural applications, such as tissue engineering scaffolds and biosensors.
Keywords: polypyrrole, nerve growth factor, PC12 cells, nerve tissue engineering
1. Introduction
Neurons are highly affected by the components of their environment including bioactive molecules such as neurotrophic factors, electrical signals, extracellular matrix molecules and cell-to-cell interactions (Lutolf & Hubbell 2005; Chalfoun et al. 2006; Li & Hoffman-Kim 2008). As a consequence, neuronal tissue engineering has focused on how to produce optimal scaffolds by integrating multiple cues to mimic natural nerve tissue (Serini & Bussolino 2005; Zhang et al. 2005; Silva 2006). In particular, electrical stimulation has been shown to play an important role in inducing cell adhesion and differentiation and reducing inflammation (Schmidt et al. 1997; McCaig et al. 2002; Guimard et al. 2007). Therefore, incorporating neuroactive molecules, such as neurotrophic factors (George et al. 2006; Gomez & Schmidt 2007; Richardson et al. 2007), extracellular matrix components (Collier et al. 2000; Sanghvi et al. 2005; Song et al. 2006) and topographical features for contact guidance (Gomez et al. 2007), into electroconductive materials is a key goal in nerve tissue engineering.
Polypyrrole (PPy) is a conductive polymer that has been widely studied for biomedical applications such as biosensors, drug delivering vehicles and nerve tissue engineering scaffolds. Beneficial properties of PPy, such as biocompatibility, good conductivity and ease of synthesis, make it a promising material to interface with neurons for neuron signal recording or electrical stimulation (Ateh et al. 2006; Guimard et al. 2007). However, the lack of functional groups on PPy has limited the ability to tailor PPy with various biochemical properties (Lee et al. 2006; Song et al. 2006; Guimard et al. 2007). A number of non-covalent modification techniques have been studied, which involve doping and entrapping different molecules, such as hyaluronic acid (Collier et al. 2000), heparin (Li et al. 2005), laminin fragments (Cui et al. 2003; Stauffer & Cui 2006) and nerve growth factor (NGF; Hodgson et al. 1996; Kim et al. 2007) during PPy polymerization. Also, affinity peptides that have specific binding to chloride-doped PPy were used to tether RGD peptides and to promote PC12 adhesion (Sanghvi et al. 2005). These non-covalent coupling techniques, however, require a high concentration of molecules during the synthesis and may not support stable retention of the ligand activity for a sufficient time period for tissue regeneration. Thus, creating covalent links of bioactive molecules to conductive polymers can be considered as an alternative to designing tissue engineering scaffolds (George et al. 2006; Lee et al. 2006; Song et al. 2006; Gomez & Schmidt 2007). However, covalent modification often faces practical hurdles. For example, non-specific covalent reactions such as photocrosslinking may degrade the polymer structure and biomolecules (Scriven 1984; Hermanson 1996; Kapur & Shoichet 2003). Functionalized pyrroles (e.g. amino, carboxylic acid, active ester) have been used to specifically couple chemical compounds, peptides and proteins; however, changes in the pyrrole structure result in a decrease in conductivity by approximately four orders of magnitude (Skotheim et al. 1998; Lee et al. 2006; Guimard et al. 2007). Consequently, incorporation of bioactive molecules should be performed in a controllable and specific way to minimize the reduction in bioactivity and conductivity of the biomaterial.
Neurotrophins modulate neuron survival, differentiation and extracellular signalling (Ebadi et al. 1997; Chao 2003). NGF is the best characterized of the neurotrophins. It plays critical roles in preventing apoptosis, inducing morphogenesis, and maintaining synaptic activities of neurons (Levi-Montalcini 1987; Shooter 2001). In addition, NGF has been reported to be involved in various functions of other cell types, such as angiogenesis in endothelial cells (Nico et al. 2008) and differentiation of immune cells (Aloe et al. 1997). Neurotrophins including NGF hold significant promise in therapeutic use for the treatment of degenerative diseases and injured nerve tissue (Ebadi et al. 1997; Ramer et al. 2000; O'Neill et al. 2005). Injured tissues do not produce sufficient levels of neurotrophins (Boyd & Gordon 2003) so that scaffolds bearing long-term neurotrophic activities are of importance for nerve tissue regeneration. In addition, immobilized NGF has been shown to trigger intracellular cascades and to stimulate neurons without a classical internalization process (Zhang et al. 2000; Kapur & Shoichet 2003; Gomez & Schmidt 2007). Hence, stable and specific chemical conjugation of neurotrophins on conducting polymers is desirable to integrate electrical cues and biochemical cues for improved nerve tissue applications.
In an effort to combine electrical cues with neurotrophic signals, we present a strategy to tailor conductive PPy films with NGF. We synthesized conductive and functionalized electroconductive PPy copolymers with active ester groups for functionalization; these materials show reasonably good conductivity with only one order of magnitude decrease compared with pristine PPy films. The cellular effects of NGF-coupled PPy films were evaluated for neurite formation of PC12 cells in comparison with NGF-containing culture medium. Stabilities of immobilized NGF on the PPy copolymer were studied under physiological conditions and with the application of an external electrical potential.
2. Materials and methods
2.1. Synthesis of pyrrole-NSE
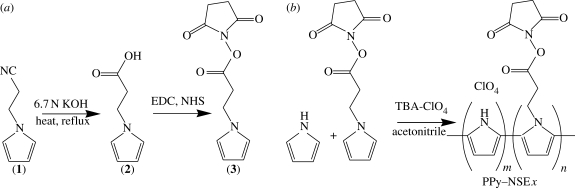
Pyrrole-NSE (3) was synthesized in two steps, based on previously published protocols (Azioune et al. 2004; Bousalem et al. 2004): conversion of 1-(2-cyanoethyl)pyrrole (1) to 1-(2-carboxyethyl)pyrrole (2), followed by the addition of N-hydroxy succinimide (NHS; figure 1 a). First, a solution of 16.5 g 1-(2-cyanoethyl)pyrrole (1; Aldrich) in 50 ml of 6.7 M KOH (Fisher) was refluxed for 20 hours under argon atmosphere until NH3 gas no longer evolved. The reaction mixture was neutralized with 5 N HCl (Fisher) and recrystallized with cold heptane (Fisher). The product, 1-(2-carboxyethyl)pyrrole (2), was characterized by NMR. The 1H NMR (CClD3, ppm) spectrum of (2) showed peaks at 2.81 (t, 2H, CH 2COO), 4.15 (t, 2H, CH 2CH2), 6.11 (dd, 2H, CH α-pyrrole) and 6.62 (dd, 2H, CH β-pyrrole), which were in agreement with other published data (Azioune et al. 2004; Lee et al. 2006).
Figure 1.
(a) Schematic of the pyrrole-NSE synthesis (3). 1-(2-Cyanoethyl)pyrrole (1) was converted to 1-(2-carboxyethyl)pyrrole (2), followed by the NHS substitution at the carboxyl group. (b) Electrochemical synthesis of the copolymers of pyrrole and pyrrole-NSE in acetonitrile with tetrabutylammonium perchlorate (TBA-ClO4) as the dopant. PPy-NSEx indicates the copolymer which is synthesized with x mol% of pyrrole-NSE from a 50 mM total monomer concentration in the polymerizing solution. For example, PPy-NSE75 is prepared in a solution of 12.5 mM pyrrole and 37.5 mM pyrrole-NSE.
Next, pyrrole-NSE (3) was synthesized by the addition of 1.73 g NHS (Sigma) and 3.8 g 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (Sigma) to a solution of 1.4 g 1-(2-caryboxyethyl)pyrrole (2) in 100 ml double deionized (ddI) water. The reaction was carried out for 30 min at room temperature. Insoluble product precipitated from the reaction mixture and was purified by filtration and washing with excess ddI water. The product was dried in a vacuum chamber overnight. The final product was a white powder and was obtained in 80 per cent yield. The structure and purity were confirmed using NMR and IR spectroscopies. 1H NMR (CClD3, ppm) spectrum of (3): 2.87 (m, 4H, CH 2CON); 3.11 (t, 2H, CH 2COO); 4.35 (t, 2H, CH 2N); 6.18 (dd, 2H, CH α-pyrrole); and 6.71 (dd, 2H, CH β-pyrrole). The IR spectra of (3) showed peaks of the succinimidyl ester groups and pyrrolidinone groups at 1740, 1782 and 1814 cm−1. The results were in good agreement with the published literature (Azioune et al. 2004; Bousalem et al. 2004; Khan et al. 2006).
2.2. Polymerization of polypyrrole copolymer films
Polypyrrole copolymers of unmodified regular pyrrole and pyrrole-NSE (3) were electrochemically synthesized on 10×20 mm pieces of either gold-coated glass slides or indium tin oxide-coated glass slides (Delta Technologies). Gold-coated glass slides were prepared by the deposition of 3 nm chromium and 30 nm gold onto glass slides (25×75×1 mm; Fisher) with a thermal evaporator (Denton). Various mixtures of pyrrole (Aldrich) and pyrrole-NSE (3) were prepared with 0.1 M tetrabutylammonium perchlorate (Aldrich) as the dopant in acetonitrile (Fisher). The total concentration was maintained at 50 mM and molar ratios were varied as follows: 100 : 0 (pyrrole : pyrrole-NSE) for PPy-NSE0; 75 : 25 for PPy-NSE25; 50 : 50 for PPy-NSE50; 25 : 75 for PPy-NSE75; and 0 : 100 for PPy-NSE100 (table 1). A constant potential of 1.2 V, versus a saturated calomel electrode (SCE) reference (Fisher), was applied using a Pine Instrument AFRDE5 bipotentiostat for 20 s to synthesize the PPy-NSE films in a three-electrode configuration.
Table 1.
Copolymers synthesized with different initial feed ratios and their conductivities.
| concentration in the solution (mM) | |||
|---|---|---|---|
|
|
|||
| copolymer | pyrrole | pyrrole-NSE | conductivity (S cm−1) |
| PPy-NSE0 | 50.0 | 0.0 (0%) | 6.9×101±7.4 |
| PPy-NSE25 | 37.5 | 12.5 (25%) | 3.0×101±1.3×101 |
| PPy-NSE50 | 25.0 | 25.0 (50%) | 8.1±2.5 |
| PPy-NSE75 | 12.5 | 37.5 (75%) | 5.6×10−1±2.4×10−1 |
| PPy-NSE100 | 0.0 | 50.0 (100%) | 8.5×10−3±6.0×10−3 |
2.3. Conductivity measurements
Conductivities of the PPy copolymer films were measured by the four-point probe method (Runyan 1975). Film thickness was measured by a Veeco profilometer (Dektak 6M Stylus). PPy copolymer films were peeled off and transferred to non-conductive tape (3M). Three different films were prepared on different days, and the thickness and sheet resistivity (R s) were quantified, from which the conductivity was calculated using the following equation:
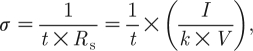 |
where σ is the conductivity (S cm−1, 1 S=1Ω−1); t is the film thickness; R s is the sheet resistance (ohm per square); V is the voltage (V) between the two outer probes; I is the current (A) passing across the two inner probes; and k is a geometric factor for a thin sheet (here, k=π/ln 2=4.532).
2.4. Infrared spectroscopy
Fourier transform infrared spectra were collected using a VeeMax II (Pike Technologies) variable-angle grazing accessory in a dry-air purged Nicolet Magna-IR 860 spectrometer (Welltech) equipped with a liquid-nitrogen-cooled mercury cadmium telluride detector (Krapchetov et al. 2006). An angle of incidence of 78° relative to the substrate normal was used. PPy sample films on gold substrates were characterized by reflectance IR using p-polarized light at a resolution of 2 cm−1. Sixty-four scans at room temperature were performed to obtain the average IR spectra for all samples. Clean gold substrates were used as the background. Baseline was corrected with GRAMS/AI software (Thermo Scientific) in all sample spectra.
2.5. X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy (XPS) was used to confirm active ester groups present on the surface of the PPy copolymers. The spectra were obtained using a physical electronic (PHI model 5700) spectrometer employing a monochromatic Al Kα1,2 source. Calibration of the binding energy was performed by setting C–C/C–H component in the C1s peak at 284.6 eV. Typical operating conditions were 1×10−9 torr in the chamber, and 14 kV and 250 W for the Al X-ray source. High-resolution elemental scans were collected with a pass energy of 11.75 eV at a take-off angle of 45° between the sample and the analyser. Peak deconvolution was performed for high-resolution C1s spectra using XPSpeak software (v. 4.1) to characterize the carbon atoms. Also, conjugation of NGF on the copolymer films was assessed by comparing the spectra and elemental compositions of the copolymer films with those of the control films not treated with NGF.
2.6. Nerve growth factor conjugation
NGF conjugation was accomplished via active ester groups present on the PPy-NSE50 films. First, the synthesized films were incubated in phosphate-buffered saline (PBS) buffer (0.1 M, pH 7.2) overnight to exchange dopants as described in other literature (Azioune et al. 2004; Bousalem et al. 2004). Fifty microlitres of NGF solution (20 μg ml−1 in 0.1 M PBS, pH 7.2) were loaded on the 1 cm2 sample piece. NGF molecules were immobilized on the PPy-NSE50 films by incubation at room temperature for 4 hours. After conjugation, the samples were washed five times to remove unbound NGF using a sterile PBS buffer with 5 min incubation per washing step. For PC12 cell culture experiments, all samples were UV sterilized for 1 hour prior to NGF immobilization, and washed with sterile PBS buffers in a laminar flow hood. Then, the samples were treated with polyallylamine (Sigma) solution (1 mg ml−1 in ddI water) for 2 hours at room temperature to improve cell adhesion and washed two times with sterile ddI water. For XPS experiments, the samples were washed with deionized water two additional times to remove extra phosphate and chloride ions.
2.7. Cell culture
PC12 cells were maintained at 37° C in a humid, 5 per cent CO2 incubator in F-12K culture medium (Sigma) supplemented with 15 per cent heat-inactivated horse serum (Hyclone), 2.5 per cent foetal bovine serum (Hyclone) and 1 per cent penicillin–streptomycin solution (Sigma), and passaged with a 0.25 per cent trypsin–EDTA solution (Sigma) every week. Cell priming was accomplished by culturing in medium containing 50 ng ml−1 NGF one week prior to an experiment. For in vitro assays of neurotrophic activity, the NGF-coupled PPy samples and unmodified PPy samples were transferred to a 24-well culture plate. Then, 2×104 primed cells were inoculated into each well and cultured for 5 days in RPMI-1640 (Gibco) medium containing 10 per cent foetal bovine serum, 5 per cent heat-inactivated horse serum and 1 per cent penicillin–streptomycin solution. Controls consisted of: (i) PPy-NSE0 (non-functionalized PPy) in 50 ng ml−1 NGF medium (positive control), (ii) PPy-NSE50 films in 50 ng ml−1 NGF medium (positive controls), (iii) PPy-NSE0 in NGF-free medium (negative control), and (iv) PPy-NSE50 in NGF-free medium (negative control). In addition, to study the non-specific adsorption of NGF on the PPy films, the PPy-NSE0 films were prepared and treated with NGF in the same way as NGF-conjugated PPy-NSE50 samples for PC12 cell culture designed above.
2.8. Fluorescence microscopy and image analysis
After 5 days in culture, PC12 cells were fixed with 4 per cent paraformaldehyde (Sigma) and 4 per cent sucrose (Sigma) in PBS buffer for 15 min, permeabilized with 0.1 per cent Triton X-100 (Fluka) and 2 per cent bovine serum albumin (BSA; Jackson ImmunoResearch) in PBS buffer for 5 min, and blocked with 2 per cent BSA in PBS buffer for 30 min at room temperature. The PC12 cells were stained with phalloidin–TRITC (Sigma) for 30 min and DAPI (Invitrogen) for 5 min for actin filaments and cell nuclei, respectively. The PC12 cells were then washed three times with PBS buffer, and stored at 4°C until fluorescence images were acquired.
Fluorescence images of cells and neurites were captured using a colour CCD camera (Optronics MagnaFire) attached to a fluorescence microscope (IX-70; Olympus), and analysed using ImageJ (NIH) software. Neurite length was measured from the tip of a neurite to the cell junction. The longest neurite from each cell was measured and counted only when the length was greater than that of the cell body. The number of DAPI-stained nuclei was quantified to obtain the total cell number in each image. The percentage of neurite-bearing PC12 cells was calculated as the number of neurite-bearing cells divided by the total number of cells. Five separate experiments were performed on different days. The averages and standard deviations were obtained and the statistical significance was assessed using a Student's t-test with Origin software.
2.9. Stability of immobilized NGF on the PPy-NSE50 films
To study the stability of NGF immobilized on the PPy-NSE50 films, we incubated the samples in PBS buffer at 37°C for 5 days. NGF stability was measured using two techniques. First, an enzyme-linked immunosorbent assay (ELISA) was used to quantify the released NGF in the medium. A commercial NGF-ELISA kit (Immuno-Max; Promega), with a detection limit of 5 pg ml−1, was used. According to the manufacturer's protocol, the 98-well ELISA plates, hydrochloric acid and sodium carbonate were purchased from Corning, Fisher and Sigma, respectively. Spectroscopic results were measured at 450 nm using a plate reader (ELx808; BioTek). These assays were performed in triplicate. As a second technique to assess NGF stability, XPS analysis was performed to compare the sample spectra and elements on the sample surface after incubation. The ratio of the N1s and C1s peak areas was also calculated to monitor the NGF level on the PPy films. Three samples were analysed for each condition.
Electrical potentials were applied to NGF-immobilized PPy-NSE50 samples to study the stabilities of NGF links and neurotrophic activities of the films according to previous literature (Hodgson et al. 1996; George et al. 2006). A constant reducing potential, −1 V versus SCE, was applied to the NGF-PPy-NSE50 films in PBS buffer for 5 min with a Pt counter electrode. The samples taken at 2 and 5 min were quantified using a previous ELISA method. Also, after washing electrically stimulated NGF-PPy-NSE50 films with PBS buffer (three times), in vitro PC12 cell culture was performed to evaluate neurotrophic activities with unstimulated NGF-PPy-NSE50 (positive), PPy-NSE50 with exogenous NGF (50 ng ml−1, positive) and PPy-NSE50 without exogenous NGF (negative). The PC12 cells were cultured and analysed in terms of neurite formation in an analogous manner as previously described. Three samples per condition were employed (n=3).
3. Results
3.1. Synthesis and characterization of PPy-NSE copolymers
To prepare PPy-NSE copolymer films, regular pyrrole and pyrrole-NSE were polymerized in 0.1 M tetrabutylammonium perchlorate in acetonitrile solution at a potential of 1.2 V (figure 1). We synthesized various PPy copolymers (PPy-NSEx), where x represents the molar per cent of pyrrole-NSE in the total monomer, by varying the monomer feed ratio while keeping the total monomer concentration constant (0.05 M; table 1).
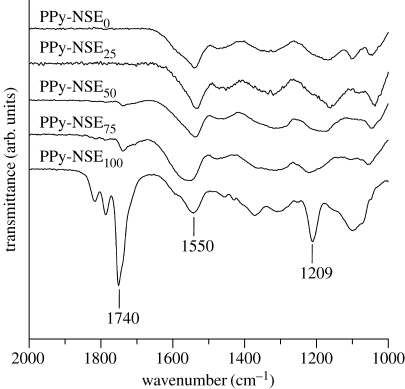
The existence of active ester groups in PPy-NSE copolymers was confirmed using reflectance IR. As shown in figure 2, the characteristic peaks, attributed to C= O stretching vibrations of the succinimidyl ester groups and pyrrolidinone groups, were observed at 1740, 1782 and 1814 cm−1 in PPy-NSE50, PPy-NSE75 and PPy-NSE100, but not in PPy-NSE0 as predicted. Copolymers synthesized with higher pyrrole-NSE ratios in the reaction solution led to the stronger peak at 1740 cm−1 (active ester groups) while showing similar transmittance at 1550 cm−1 (attributed to the C= C stretch in pyrrole rings). These spectra imply that pyrrole-NSE was not favoured in the polymerization reaction compared with unmodified pyrrole, because the incorporation of pyrrole-NSE, which has a branch at the nitrogen atom in the pyrrole ring, may limit the access of the monomer to the growing polymer chain.
Figure 2.
Reflectance IR spectra. PPy-NSE copolymers were synthesized from different pyrrole monomer ratios of pyrrole and pyrrole-NSE. Peaks at 1740, 1782 and 1814 cm−1 represent N-succinimidyl ester and pyrrolidinone, and those at 1550 cm−1 are typical for PPy rings. The peak at 1209 cm−1 is attributed to N–O stretching.
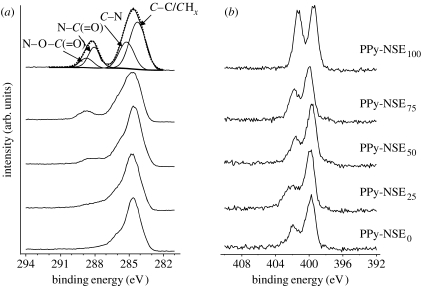
To characterize the surface elements on the PPy copolymers, XPS analysis was performed (figure 3). The peaks at 288.8 and 288.1 eV of the high-resolution C1s spectra are attributed to N–O–C= O and N–C= O, respectively, which resulted from monomeric pyrrole-NSE incorporation into the copolymer films. Similarly, the XPS spectra of N1s indicate distinguishable increases at 402 eV of N–O as the portion of pyrrole-NSE increases. These XPS results indicate that N-succinimidyl ester, which has the capability of forming covalent bonds with nucleophiles such as amines, was present on the PPy-NSE50 film surface.
Figure 3.
High-resolution (a) C1s and (b) N1s XPS spectra obtained from PPy-NSE copolymer films. The N-succinimidyl ester group in PPy (PPy-NSEx) copolymers was recognized by noticeable peaks at 288.8 eV (N–O–C (= O)) and 288.1 eV (N–C (=O)).
We measured the conductivities of each PPy copolymer film using a four-point probe. As the pyrrole-NSE portion in the copolymer increased, the conductivities drastically decreased from 6.9×101 S cm−1 (PPy-NSE0) to below 1×10−2 S cm−1 (PPy-NSE100; table 1). Low conductivity would be a major challenge when using the functionalized pyrrole for biomedical applications such as biosensors or electroconductive scaffolds. Therefore, we selected the PPy-NSE50 films for further protein conjugation because a film of this composition presents significant active functional groups on the surface, and shows a reasonably good conductivity of 8.1±2.5 S cm−1 in the semiconductor range.
3.2. PC12 cell culture for neurite outgrowth studies
PC12 cells, extending neurites in response to NGF, were used as the cell system to assess immobilized NGF activity. NGF immobilization was accomplished simply by loading NGF solution onto the functionalized PPy (PPy-NSE50) presenting N-hydroxy succinimide-activated carboxyl groups to form amide bonds.
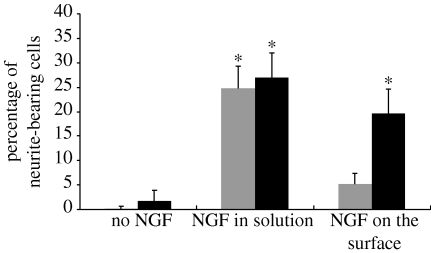
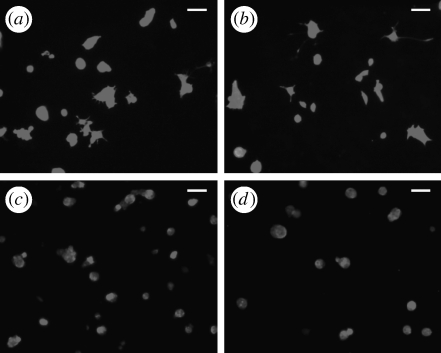
NGF-conjugated PPy-NSE0 (non-functionalized PPy) films, treated with 20 μg ml−1 NGF in the same manner as PPy-NSE50-NGF, were used to assess the effects of non-specific physical adsorption of NGF. Figure 4 shows a significant increase in the number of neurites for cells grown on NGF-conjugated PPy-NSE50 films (20±5%, p<0.001) compared with cells grown on PPy-NSE50 films without NGF (2±2%) and PPy-NSE0 films (0%). Also, the effects were significantly different from the NGF-treated PPy-NSE0 films (5±2%), indicating minimal non-specific binding of NGF. Neurite outgrowth from cells grown on immobilized NGF on PPy-NSE50 was similar to positive controls in which cells were cultured on either PPy-NSE0 (25±5%) or PPy-NSE50 (27±5%) films with the exogenous addition of 50 ng ml−1 of NGF in the culture medium. Figure 5 depicts representative fluorescence images of PC12 cells stained with phalloidin–TRITC after 5 days on various samples. The average neurite length for PC12 cells cultured on NGF-immobilized PPy-NSE50 was not significantly different from those in NGF-containing medium (positive controls).
Figure 4.
Neurite outgrowth of PC12 cells cultured on regular PPy films (grey bars) and on PPy-NSE50 (black bars). The PC12 cells were cultured for 5 days, fixed with 4% paraformaldehyde and stained with phalloidin–TRITC. Without NGF treatment either in the medium or on the surface, there were few cells bearing neurites. For the positive controls, approximately 25–27% of the PC12 cells cultured in NGF-containing medium (50 ng ml−1) extended neurites. NGF-conjugated PPy-NSE50 films (black bars) promoted neurite extension similarly to positive controls, but significantly higher than NGF-treated PPy-NSE0 films (grey bars). Five independent experiments were performed. * p<0.005.
Figure 5.
Immunostaining of PC12 cells cultured in different conditions. After 5 days in culture, phalloidin–TRITC was used to stain the fixed PC12 cells on the PPy films. The PC12 cells cultured (a) in NGF-containing media on the PPy-NSE50 films and (b) on NGF-conjugated PPy-NSE50 films extended neurites, while those cultured (c) without NGF either in solution or on the PPy-NSE50 films and (d) on NGF-treated regular PPy films did not form as many neurites as (a) or (b). Scale bar, 50 μm.
3.3. Stability of immobilized NGF on PPy-NSE50
To assess the stability of NGF coupled via active ester groups, we performed an ELISA assay with a detection limit of 4 pg ml−1 to analyse any NGF released into solution. XPS analysis was also performed to determine the elemental compositions of the substrate surfaces to monitor for loss of NGF. The samples were incubated in sterile PBS solution at 37°C for 5 days and compared with freshly prepared PPy-NSE50 films. NGF bound to the PPy-NSE50 films may be partly attributed to physical adsorption that could be leached into solution. However, there was no detectable NGF leached from NGF-immobilized PPy-NSE50 films after a 5-day incubation.
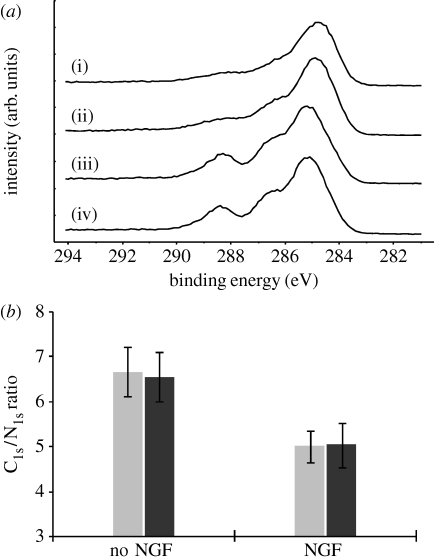
Figure 6 a displays high-resolution C1s XPS spectra of PPy-NSE50 and NGF-PPy-NSE50 samples that were either freshly prepared or incubated for 5 days in PBS buffer. The C1s spectra of the incubated samples were very similar to those prior to incubation. In particular, the characteristic peaks at 288.1 eV, attributed to NGF or links with PPy-NSE50, were retained after the 5-day incubation, suggesting that the NGF moiety was chemically conjugated and stable on the surface. On the other hand, unmodified samples displayed substantially different C1s profiles from NGF-immobilized samples. These findings are further evidence of the NGF-immobilization on PPy-NSE50. Since NGF on films alters the elemental compositions of the surfaces, the atomic ratios of C1s and N1s from the XPS analysis were analysed to quantify the stability of the immobilized NGF (figure 6 b). NGF-immobilized films were found to have significantly lower C1s/N1s values (approx. 5.0) compared with non-treated samples (approx. 6.6). Also, a 5-day incubation of NGF-immobilized samples did not cause substantial change in the C1s/N1s ratio (5.0±0.5) compared with the freshly prepared samples (5.0±0.4).
Figure 6.
XPS analysis was used to assess the stability of NGF-immobilized PPy-NSE50 films. (a) High-resolution C1s spectra of the PPy-NSE50 samples (i) freshly prepared and (ii) incubated, and of the NGF-immobilized PPy-NSE50 samples (iii) freshly prepared and (iv) incubated. Incubation was carried out for 5 days in PBS buffer at 37°C in a cell culture incubator. (b) C1s and N1s ratios of NGF-immobilized PPy-NSE50 films (NGF) and non-treated PPy-NSE50 films (no NGF). After the incubation, the ratios of both incubated films (black bars) were compared with those of freshly prepared films (grey bars). NGF on the film surfaces affects the ratios of C1s and N1s.
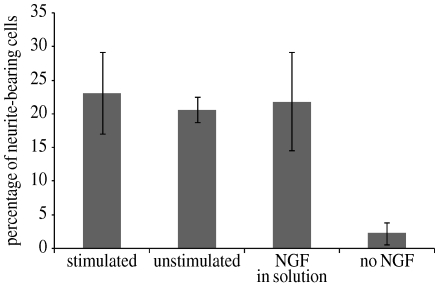
In addition, external electrical potentials were applied to the NGF-PPy-NSE50 films, conditions which were used to release NGF from NGF-doped PPy films as described by others (Hodgson et al. 1996; George et al. 2006). Minimal amount of NGF (less than 15 pg cm−2 film) was detected after applying −1 V for 5 min. Also, as shown in figure 7, the NGF-immobilized films (stimulated) that had a history of exposure to electrical potential induced neurite outgrowth (23±6%) as much as other positive controls, 21±2 per cent and 22±7 per cent for unstimulated NGF-PPy-NSE50 (unstimulated) and PPy-NSE50 with exogenous NGF in medium (NGF in solution), respectively.
Figure 7.
In vitro PC12 cell culture on NGF-immobilized PPy-NSE50 films after applying an external electrical potential. NGF-PPy-NSE50 films were stimulated with an electrical potential of −1.0 V (versus SCE) for 5 min, followed by washing and PC12 cell culture. Neurotrophic activity (percentage of neurite-bearing cells) of the stimulated NGF-PPy-NSE50 was similar to the two positive controls, (i) freshly prepared unstimulated NGF-PPy-NSE50 and (ii) PPy-NSE50 with exogenous NGF (50 ng ml−1 in medium), but significantly different from the negative control of PPy-NSE50 without NGF (p<0.01). Experiments were performed in triplicate.
4. Discussion and conclusion
Conductive films bearing neurotrophic activity would be a desirable platform for neural tissue engineering scaffolds and neural electrode surfaces; combined electrical and biochemical stimulation should yield better regeneration and interfacing with neurons (Zhang et al. 2005; George et al. 2006; Gomez & Schmidt 2007; Guimard et al. 2007). Hence, neurotrophins are required to be stable in/on conducting scaffolds and with electrical potentials. Also, reasonable conductivities of the materials are essential to provide electrical stimulation through the scaffolds. Table 2 summarizes various techniques reported for the production of neurotrophin-modified conducting polymers with their important characteristics including electrical properties. Mostly, non-covalent methods have been developed, which include doping NGF/NT-3 during the electrochemical synthesis of PPy (Hodgson et al. 1996; Kim et al. 2007; Richardson et al. 2007) and coupling biotinylated NGF on avidin-treated biotin-doped PPy films (George et al. 2006). These non-conjugation methods showed good conductivities and controllable NGF release profiles in response to an external electrical stimulation to induce neurite formation from PC12 cells. However, the NGF is released and consumed gradually after successive electrical stimulations.
Table 2.
Various techniques and properties of neurotrophin-incorporated conducting polymers. (Other modification techniques for peptides/proteins could be theoretically used to immobilize neurotrophin(s), such as affinity peptide-mediated modification (Sanghvi et al. 2005), covalent coupling on dopants (Song et al. 2006) and plasma treatment (Kang et al. 1997).)
| techniques | advantages | disadvantages | conductivity a | neurotrophin | reference(s) |
|---|---|---|---|---|---|
| non-covalent techniques | |||||
| doping/entrapping | simpleelectrically controllable release of neurotrophin(s) may be desired for certain applications | requires high concentration of neurotrophin for synthesis | dependent on doping levels of neurotrophinb | NGF | Hodgson et al. (1996) and Kim et al. (2007) |
| biomolecule can be released spontaneously and with electrical stimulation, so that material may not be suitable for long-term use | NT-3 | Richardson et al. (2007) | |||
| affinity binding to a dopant (biotin) | simple specific conjugation | multiple steps | dependent on doping levels of biotinc | biotinylated NGF | George et al. (2006) |
| electrically controllable release of neurotrophin(s) | requires neurotrophin modification | ||||
| biomolecule is consumed with electrical stimulation (not suitable for long-term use) | |||||
| covalent techniques | |||||
| conjugation using radical linkersd | applicable to most organic polymers | complicated, multiple steps | 9.3 S cm−1 | NGF | Gomez & Schmidt (2007) |
| minimized loss of bulk conductivity | non-specific reaction | ||||
| stable and suitable for long-term applications | impairing ligand activities and surface properties of conducting substrates | ||||
| conjugation on functionalized polypyrrole(s) | simple, specific conjugation of ligand | decrease in conductivity depending on polymer compositionrequires synthesis of pyrrole derivatives | 8.1 S cm−1 e | NGF | this study |
| various controllable properties by copolymerization | |||||
| stable links for long-term applications | |||||
Conductivities of regular PPy are in a range of 5–200 S cm−1 depending on dopant types and polymerization conditions (Guimard et al. 2007; Fonner et al. 2008).
Conductivity was not directly measured. Impedances of NGF-doped PPy varied with polymerization time and showed higher impedances compared with regular PPy (Kim et al. 2007). High-level doping of macromolecules is reported to result in a severe conductivity drop (Guimard et al. 2007). For example, hyaluronic acid-doped PPy displayed a conductivity of 3.1×10−3 S cm−1 (Collier et al. 2000).
Electrical property of the materials was not measured.
Azido compound (azido-modified poly(allylamine)) was used as a photocrosslinker to immobilize NGF on PPy doped with PSS.
The value (8.1 S cm−1) was measured from the copolymer of PPy-NSE50, which was doped with perchlorate 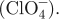 .
.
Therefore, for long-term applications, chemical conjugation approaches appear reasonable because chemically immobilized NGF exhibited neurotrophic activities on various materials, such as polystyrene sulphonate (PSS)-doped PPy films and poly(2-hydroxyethylmethacrylate) (PHEMA) gels coupled with NGF using the arylazido-polyallylamine (azido-PAA) photocrosslinkers (Kapur & Shoichet 2003; Gomez & Schmidt 2007) and glutaraldehyde-activated beads (Naka et al. 2004). Although non-specific photocrosslinkers can be employed to immobilize most organic substrates, this technique can disrupt neurotrophin activities and conductive polymer surfaces as well (Kapur & Shoichet 2003). Thus, the functionalized PPy described here can be a good option to specifically conjugate NGF under relatively simple and mild conditions. One obstacle is that pyrrole derivatives, particularly N-substituted pyrrole derivatives, hinder the structural planarity of π-electron conjugation in PPy, which results in a severe decrease in conductivity (Nalwa 1997; Skotheim et al. 1998; Lee et al. 2006; Guimard et al. 2007). This drastic drop in conductivity of pyrrole derivatives can be mitigated to some extent by synthesizing copolymers of pristine pyrrole and pyrrole derivatives that exhibit a reasonable conductivity and significant reactive functional groups (i.e. N-succinimidyl ester groups) on the surface. In this study, the PPy-NSE50 copolymer still had a reasonably good conductivity comparable with other techniques (table 2) and better than common functionalized PPy and large molecule-doped PPy. Also, bulk conductivities of the PPy can be further improved when synthesizing layer-by-layer structures, where the PPy-NSE copolymer is deposited on top of a more conductive regular PPy layer (i.e. PPy-NSE0).
A copolymer of N-succinimidyl ester pyrrole can be used to simply immobilize biomolecules so that amine-bearing compounds could be directly conjugated onto the PPy surface in physiological conditions without an additional activation step. PPy-NSE polymers and copolymers were reported to bind human serum albumin, BSA and drug molecules (Azioune et al. 2004; Bousalem et al. 2004; Khan et al. 2006).
Our preliminary studies showed that PC12 cells exhibited maximum neurite outgrowth at a concentration of 50 ng ml−1 soluble NGF in medium; this condition was used as a positive control to compare with the effects of NGF-immobilized PPy-NSE50 and PPy-NSE0 sample films (figure 5). The results demonstrate that neurotrophic activity on PPy-NSE50 resulted from NGF immobilized via active ester groups, not as a result of physically adsorbed NGF. NGF-immobilized substrates showed neurotrophic activity similar to exogenous NGF in medium.
Immobilized NGF on PPy-NSE50 showed good stability at physiological conditions and even to electrical potential. We assumed that some of the NGF on the PPy-NSE50 films would be attributed to physical adsorption that might be leached into the medium. However, ELISA analysis indicated that NGF was not detected after 5 days of incubation of NGF-conjugated PPy-NSE50 films in PBS buffer at 37°C. Furthermore, the XPS analysis indicated no significant change in the ratio of C1s and N1s after incubation. These results suggest a negligible release of NGF from the PPy-NSE50 films. Besides leaching and elemental analysis in this paper, cell culture on the incubated sample will be required in future work to confirm the long-term stability of NGF activity on the surface.
Stable activity of immobilized NGF to electrical potentials is also critical to provide neurotrophic activities with electrical cues (George et al. 2006). Non-covalently incorporated neurotrophins were released not only in the manner of passive diffusion into medium but also responding to changes in an external electrical potential (Maddison & Jenden 1992; Guimard et al. 2007; Richardson et al. 2007). Hodgson et al. were able to release NGF in the medium by applying −0.7 or −0.85 V for 3 min, resulting in neurite outgrowth of PC12 cells. Similarly, reduction of NGF-incorporated PPy films caused a quick release of NGF up to 10 μg ml−1 into medium within 150 s. On the other hand, our results show that NGF-PPy-NSE50 films did not release a significant level of NGF into the medium in response to a constant potential (−1 V) for 5 min. More importantly, these electrically stimulated NGF-PPy-NSE50 films maintained the activities to induce neurite formation of PC12 cells as much as non-stimulated films, suggesting that the immobilized NGF on the PPy-NSE50 films was stable on the substrates to electrical potentials and thus useful to provide long-term neurotrophic activities with simultaneous electrical stimulation. Together, these data suggest that chemical coupling with amide bond formation is stable and suitable for long-term applications with electrical stimulation as multifunctional tissue engineering scaffolds.
In conclusion, we successfully introduced neurotrophic factors onto active ester-functionalized PPy polymers and maintained good electrical conductivity. We have shown that polypyrrole films tailored with NGF are stable and thus have the potential to integrate electrical and biochemical cues for nerve tissue engineering scaffolds.
Acknowledgments
The authors would like to thank Tianshen Hu for assistance with the laboratory experiments, Dmitri Krapchetov for infrared microscopy and Jonathan Nickels for helping with the synthesis of the monomer. This work was supported by NIH R01EB004429.
References
- Aloe L., Bracci-Laudiero L., Bonini S., Manni L. 1997. The expanding role of nerve growth factor: from neurotrophic activity to immunologic diseases. Allergy. 52, 883–894. ( 10.1111/j.1398-9995.1997.tb01247.x) [DOI] [PubMed] [Google Scholar]
- Ateh D. D., Navsaria H. A., Vadgama P. 2006. Polypyrrole-based conducting polymers and interactions with biological tissues. J. R. Soc. Interface. 3, 741–752. ( 10.1098/rsif.2006.0141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azioune A., Slimane A. B., Hamou L. A., Pleuvy A., Chehimi M. M., Perruchot C., Armes S. P. 2004. Synthesis and characterization of active ester-functionalized polypyrrole–silica nanoparticles: application to the covalent attachment of proteins. Langmuir. 20, 3350–3356. ( 10.1021/la030407s) [DOI] [PubMed] [Google Scholar]
- Bousalem S., Mangeney C., Chehimi M. M., Basinska T., Miksa B., Slomkowski S. 2004. Synthesis, characterization and potential biomedical applications of N-succinimidyl ester functionalized, polypyrrole-coated polystyrene latex particles. Colloid Polym. Sci. 282, 1301–1307. ( 10.1007/s00396-004-1065-8) [DOI] [Google Scholar]
- Boyd J. G., Gordon T. 2003. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol. Neurobiol. 27, 277–324. ( 10.1385/MN:27:3:277) [DOI] [PubMed] [Google Scholar]
- Chalfoun C. T., Wirth G. A., Evans G. R. 2006. Tissue engineered nerve constructs: where do we stand?. J. Cell. Mol. Med. 10, 309–317. ( 10.1111/j.1582-4934.2006.tb00401.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M. V. 2003. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev. Neurosci. 4, 299–309. ( 10.1038/nrn1078) [DOI] [PubMed] [Google Scholar]
- Collier J. H., Camp J. P., Hudson T. W., Schmidt C. E. 2000. Synthesis and characterization of polypyrrole–hyaluronic acid composite biomaterials for tissue engineering applications. J. Biomed. Mater. Res. 50, 574–584. ( 10.1002/(SICI)1097-4636(20000615)50:4%3C574::AID-JBM13%3E3.0.CO;2-I) [DOI] [PubMed] [Google Scholar]
- Cui X., Wiler J., Dzaman M., Altschuler R. A., Martin D. C. 2003. In vivo studies of polypyrrole/peptide coated neural probes. Biomaterials. 24, 777–787. ( 10.1016/S0142-9612(02)00415-5) [DOI] [PubMed] [Google Scholar]
- Ebadi M., et al. 1997. Neurotrophins and their receptors in nerve injury and repair. Neurochem. Int. 30, 347–374. ( 10.1016/S0197-0186(96)00071-X) [DOI] [PubMed] [Google Scholar]
- Fonner J. M., Forciniti L., Nguyen H., Byrene J., Kou Y., Syeda-Nawaz J., Schmidt C. E. 2008. Biocompatibility implications of polypyrrole synthesis techniques. Biomed. Mater. 3, 034124 ( 10.1088/1748-6041/3/034124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- George P. M., LaVan D. A., Burdick J. A., Chen C. Y., Liang E., Langer R. 2006. Electrically controlled drug delivery from biotin-doped conductive polypyrrole. Adv. Mater. 18, 577–581. ( 10.1002/adma.200501242) [DOI] [Google Scholar]
- Gomez N., Schmidt C. E. 2007. Nerve growth factor-immobilized polypyrrole: bioactive electrically conducting polymer for enhanced neurite extension. J. Biomed. Mater. Res. 81, 135–149. ( 10.1002/jbm.a.31047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez N., Lee J. Y., Nickels J. D., Schmidt C. E. 2007. Micropatterned polypyrrole: combination of electrical and topographical characteristics for stimulation of cells. Adv. Funct. Mater. 17, 1645–1653. ( 10.1002/adfm.200600669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimard N. K., Gomez N., Schmidt C. E. 2007. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 32, 876–921. ( 10.1016/j.progpolymsci.2007.05.012) [DOI] [Google Scholar]
- Hermanson G. T. 1996. Bioconjugate techniques. New York, NY: Academic Press [Google Scholar]
- Hodgson A. J., John M. J., Campbell T., Georgevich A., Woodhouse S., Aoki T., Ogata N., Wallace G. G. 1996. Integration of biocomponents with synthetic structures—use of conducting polymer polyelectrolyte composites. Proc. SPIE Int. Soc. Opt. Eng. 2716, 164–176. ( 10.1117/12.232137) [DOI] [Google Scholar]
- Kang E. T., Neoh K. G., Tan K. L., Loh F. C. 1997. Surface modified and functionalized polyaniline and polypyrrole films. Synth. Met. 84, 59–60. ( 10.1016/S0379-6779(97)80664-9) [DOI] [Google Scholar]
- Kapur T. A., Shoichet M. S. 2003. Chemically-bound nerve growth factor for neural tissue engineering applications. J. Biomater. Sci. Polym. Ed. 14, 383–394. ( 10.1163/156856203321478883) [DOI] [PubMed] [Google Scholar]
- Khan W., Marew T., Kumar N. 2006. Immobilization of drugs and biomolecules on in situ copolymerized active ester polypyrrole coatings for biomedical applications. Biomed. Mater. 1, 235–241. ( 10.1088/1748-6041/1/4/009) [DOI] [PubMed] [Google Scholar]
- Kim D. H., Richardson-Burns S. M., Hendricks J. L., Sequera C., Martin D. C. 2007. Effect of immobilized nerve growth factor on conductive polymers: electrical properties and cellular response. Adv. Funct. Mater. 17, 79–86. ( 10.1002/adfm.200500594) [DOI] [Google Scholar]
- Krapchetov D. A., Ma H., Jen A. K., Fischer D. A., Loo Y. L. 2006. High-sensitivity transmission IR spectroscopy for the chemical identification and structural analysis of conjugated molecules on gallium arsenide surfaces. Langmuir. 22, 9491–9494. ( 10.1021/la0623984) [DOI] [PubMed] [Google Scholar]
- Lee J.-W., Serna F., Nickels J. D., Schmidt C. E. 2006. Carboxylic acid-functionalized conductive polypyrrole as a bioactive platform for cell adhesion. Biomacromolecules. 7, 1692–1695. ( 10.1021/bm060220q) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. 1987. The nerve growth factor 35 years later. Science. 237, 1154–1162. ( 10.1126/science.3306916) [DOI] [PubMed] [Google Scholar]
- Li G. N., Hoffman-Kim D. 2008. Tissue-engineered platforms of axon guidance. Tissue Eng. B. 14, 33–51. ( 10.1089/teb.2007.0181) [DOI] [PubMed] [Google Scholar]
- Li Y., Neoh K. G., Kang E. T. 2005. Controlled release of heparin from polypyrrole–poly(vinyl alcohol) assembly by electrical stimulation. J. Biomed. Mater. Res. 73, 171–181. ( 10.1002/jbm.a.30286) [DOI] [PubMed] [Google Scholar]
- Lutolf M. P., Hubbell J. A. 2005. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 23, 47–55. ( 10.1038/nbt1055) [DOI] [PubMed] [Google Scholar]
- Maddison D. S., Jenden C. K. 1992. Dopant exchange in conducting polypyrrole films. Polym. Int. 27, 231–235. ( 10.1002/pi.4990270306) [DOI] [Google Scholar]
- McCaig C. D., Rajnicek A. M., Song B., Zhao M. 2002. Has electrical growth cone guidance found its potential?. Trends Neurosci. 7, 354–359. ( 10.1016/S0166-2236(02)02174-4) [DOI] [PubMed] [Google Scholar]
- Naka Y., Kitazawa A., Akaishi Y., Shimizu N. 2004. Neurite outgrowths of neurons using neurotrophin-coated nanoscale magnetic beads. J. Biosci. Bioeng. 98, 348–352. ( 10.1016/S1389-1723(04)00294-4) [DOI] [PubMed] [Google Scholar]
- Nalwa H. S. (ed.) 1997. Handbook of organic conductive molecules and polymers, vol. 2 London, UK: Wiley [Google Scholar]
- Nico B., Mangieri D., Benagiano V., Crivellato E., Ribatti D. 2008. Nerve growth factor as an angiogenic factor. Microvasc. Res. 75, 135–141. ( 10.1016/j.mvr.2007.07.004) [DOI] [PubMed] [Google Scholar]
- O'Neill M. J., Murray T. K., Clay M. P., Lindstrom T., Yang C. R., Nisenbaum E. S. 2005. LY503430: pharmacology, pharmacokinetics, and effects in rodent models of Parkinson's disease. CNS Drug Rev. 11, 77–96. ( 10.1111/j.1527-3458.2005.tb00037.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer M. S., Priestley J. V., McMahon S. B. 2000. Functional regeneration of sensory axons into the adult spinal cord. Nature. 403, 312–316. ( 10.1038/35002084) [DOI] [PubMed] [Google Scholar]
- Richardson R. T., et al. 2007. The effect of polypyrrole with incorporated neurotrophin-3 on the promotion of neurite outgrowth from auditory neurons. Biomaterials. 28, 513–523. ( 10.1016/j.biomaterials.2006.09.008) [DOI] [PubMed] [Google Scholar]
- Runyan W. R. 1975. Semiconductor measurements and instrumentation. New York, NY: McGraw-Hill [Google Scholar]
- Sanghvi A. B., Miller K. P., Belcher A. M., Schmidt C. E. 2005. Biomaterials functionalization using a novel peptide that selectively binds to a conducting polymer. Nat. Mater. 4, 496–502. ( 10.1038/nmat1397) [DOI] [PubMed] [Google Scholar]
- Schmidt C. E., Shastri V. R., Vacanti J. P., Langer R. 1997. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc. Natl Acad. Sci. USA. 94, 8948–8953. ( 10.1073/pnas.94.17.8948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriven E. F. V. (ed.) 1984. Azides and nitrenes: reactivity and utility. Orlando, FL: Academic Press [Google Scholar]
- Serini G., Bussolino F. 2005. Common cues in vascular and axon guidance. Physiology. 19, 348–354. ( 10.1152/physiol.00021.2004) [DOI] [PubMed] [Google Scholar]
- Shooter E. M. 2001. Early days of the nerve growth factor proteins. Annu. Rev. Neurosci. 24, 601–629. ( 10.1146/annurev.neuro.24.1.601) [DOI] [PubMed] [Google Scholar]
- Silva G. A. 2006. Neuroscience nanotechnology: progress, opportunities and challenges. Nat. Rev. Neurosci. 7, 65–74. ( 10.1038/nrn1827) [DOI] [PubMed] [Google Scholar]
- Skotheim T. A., Elsenaumer R. L., Reynolds J. R. 1998. Handbook of conducting polymers. New York, NY: Marcel Dekker [Google Scholar]
- Song H. K., Toste B., Ahmann K., Hoffman-Kim D., Palmore G. T. 2006. Micropatterns of positive guidance cues anchored to polypyrrole doped with polyglutamic acid: a new platform for characterizing neurite extension in complex environments. Biomaterials. 27, 473–484. ( 10.1016/j.biomaterials.2005.06.030) [DOI] [PubMed] [Google Scholar]
- Stauffer W. R., Cui X. T. 2006. Polypyrrole doped with 2 peptide sequences from laminin. Biomaterials. 27, 2405–2413. ( 10.1016/j.biomaterials.2005.10.024) [DOI] [PubMed] [Google Scholar]
- Zhang Y., Moheban D. B., Conway B. R., Bhattacharyya A., Segal R. A. 2000. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J. Neurosci. 20, 5671–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Yan H., Wen X. 2005. Tissue-engineering approaches for axonal guidance. Brain Res. Brain Res. Rev. 49, 48–64. ( 10.1016/j.brainresrev.2004.11.002) [DOI] [PubMed] [Google Scholar]