Abstract
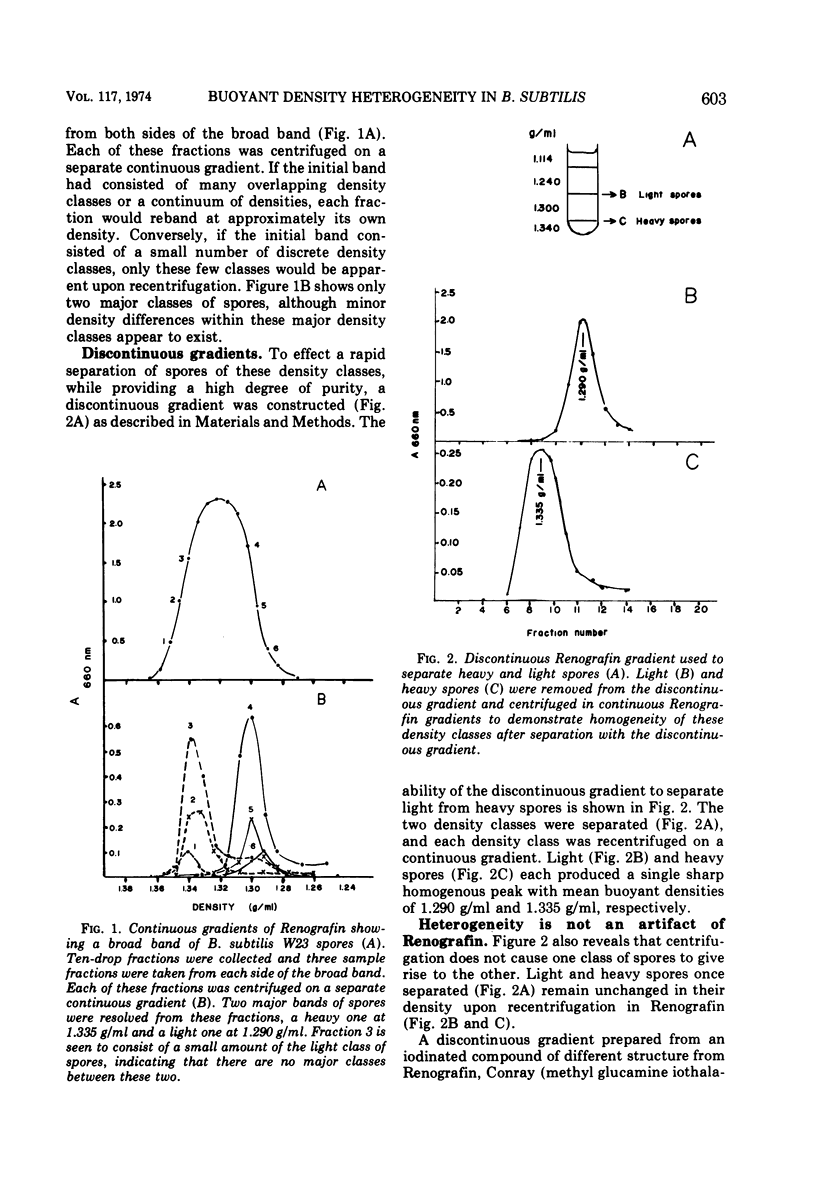
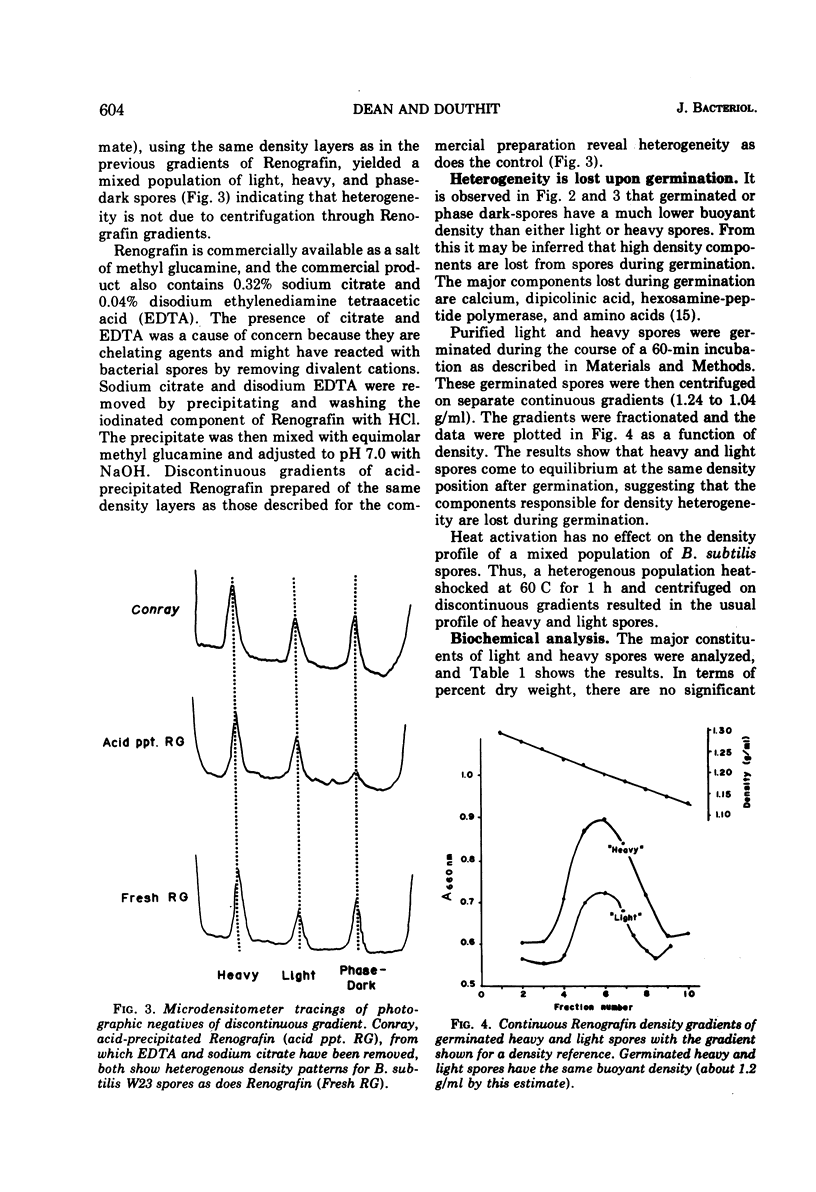
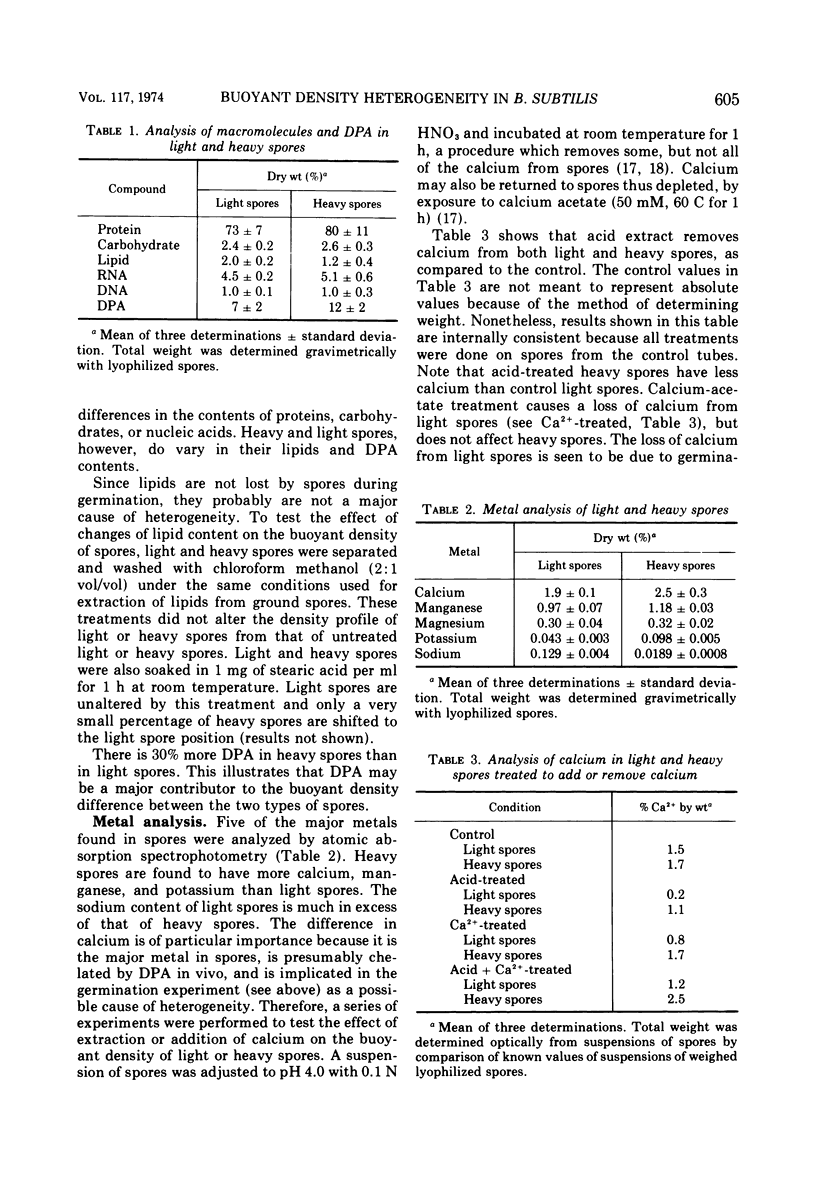
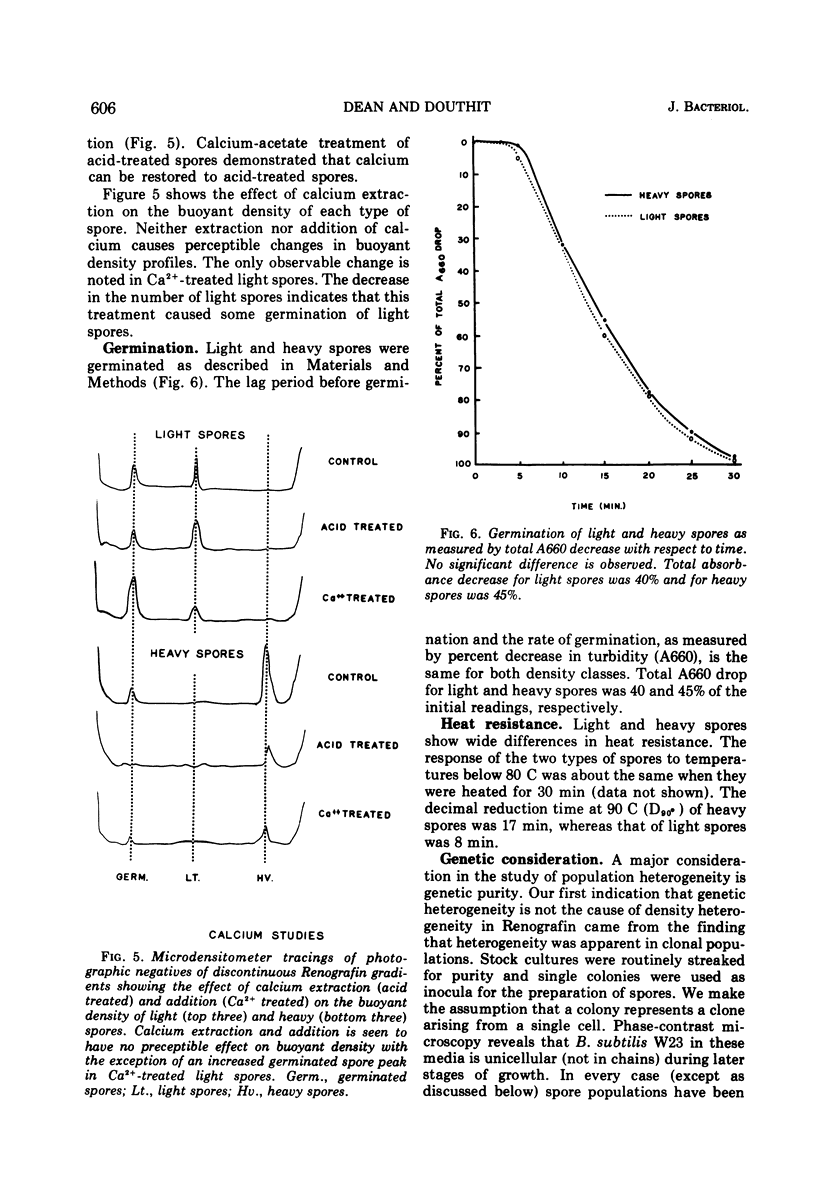
The biochemical and physiological basis of density heterogeneity in Renografin of Bacillus subtilis W23 spores was determined by analysis of metals, macromolecules, and dipicolinic acid in the two density classes of the population. Germination rate and heat resistance were measured in both density classes. Atomic absorption spectrophotometry revealed that heavy spores (density = 1.335 g/ml) have 30% more calcium than light spores (density = 1.290 g/ml). Other metals found in greater amounts in heavy spores were manganese and potassium. However, light spores had more sodium than heavy spores. The amounts of carbohydrates, nucleic acids, and proteins were the same in both types of spores, but light spores contained more lipids, whereas heavy spores had 30% more dipicolinic acid than light spores. Calcium and lipid were excluded as causes of the heterogeneity in density in that alteration of their contents in spores did not detectably affect the density of these spores. Spores of two densities were genetically similar. Furthermore, light density spores arose earlier during sporulation than heavy spores as determined by releasing refractile forespores at various times during sporulation. We concluded that light spores represent an incomplete stage in development because they became heavy when reinoculated into spent sporulation medium. This must involve the additional accretion or synthesis of dipicolinic acid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R. L., Sueoka N. Phase transitions in ribonucleic acid synthesis during germination of Bacillus subtilis spores. Proc Natl Acad Sci U S A. 1968 Jan;59(1):153–160. doi: 10.1073/pnas.59.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHURCH B. D., HALVORSON H., HARTMAN R. S., RAMSEY D. S. Population heterogeneity in the resistance of aerobic spores to ethylene oxide. J Bacteriol. 1956 Aug;72(2):242–247. doi: 10.1128/jb.72.2.242-247.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- POWELL J. F., STRANGE R. E. Biochemical changes occurring during the germination of bacterial spores. Biochem J. 1953 May;54(2):205–209. doi: 10.1042/bj0540205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice G. A., Wolfe F. H., Clegg L. F. The use of density gradient centrifugation for the separation of germinated from nongerminated spores. J Appl Bacteriol. 1972 Jun;35(2):345–349. doi: 10.1111/j.1365-2672.1972.tb03706.x. [DOI] [PubMed] [Google Scholar]
- Rode L. J., Foster J. W. Influence of exchangeable ions on germinability of bacterial spores. J Bacteriol. 1966 Apr;91(4):1582–1588. doi: 10.1128/jb.91.4.1582-1588.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode L. J., Foster J. W. Quantitative aspects of exchangeable calcium in spores of Bacillus megaterium. J Bacteriol. 1966 Apr;91(4):1589–1593. doi: 10.1128/jb.91.4.1589-1593.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir H., Gilvarg C. Density gradient centrifugation for the separation of sporulating forms of bacteria. J Biol Chem. 1966 Mar 10;241(5):1085–1090. [PubMed] [Google Scholar]


